Abstract
Background
Pain related to ultraviolet B radiation (UVR) induced sunburn is an established, simple, acute pain model. One of the major criticisms is related to the potential dermal adverse events caused by the UVR exposure. This study tried to validate the model for oral and topical drugs and to define the minimum required UVR exposure.
Methods
This subject- and observer-blinded, placebo-controlled, crossover study evaluated 600 mg oral ibuprofen (IB) and topical hydrocortisone-21-acetate (HC) twice daily (bid) in 24 healthy volunteers. Treatment started immediately after irradiation and again at 12 hours, 24 hours, and 36 hours post-UVR. Assessment of hyperalgesia to heat and signs of inflammation (erythema, skin temperature) for all areas was performed after UVR and again at 6, 12, 24, 36, and 48 hours. Subjects returned within 4–11 days to the study site for the second period of the study. As in the first period, subjects received HC at one side and topical placebo on the other side, but oral treatment was crossed-over.
Results
The primary analysis failed to show the expected superiority of the IB-group vs the placebo group in period 1 of the study. Evaluating period 2 alone clearly showed the expected treatment effects of IB for erythema and heat pain threshold. The results were less pronounced for skin temperature. In contrast to IB vs oral placebo, there were no differences in treatment response between HC and topical placebo. UVR at all dosages induced profound erythema and reduction of heat pain threshold without causing blisters or other unexpected discomfort to the subjects. The changes were almost linear between 1 and 2 minimal erythema doses (MED), whereas the change from 2 to 3 MED was less pronounced.
Conclusion
Use of 2 MED in upcoming studies seems to be reasonable to limit subjects’ UVB exposure. The following procedural changes are suggested:
Intensified training sessions before randomization to treatment
Increase in sample size if they are crossover studies
Simplification in design (either oral or topical treatment)
Keywords: ibuprofen, hydrocortisone-21-acetate, pain, inflammation, UV radiation, validation
Introduction
Pain related to UVR-induced sunburn is an established, simple, acute pain model.1,2 One of the major criticisms related to this model is the potential dermal adverse events caused by the UVR exposure.
The main cause of sunburn in human skin is UVR of wavelengths from 290 nm to 320 nm. Erythema begins to develop approximately 3–5 hours after UVR exposure, reaches a maximum at 12–24 hours, and fades over 72 hours.3 Skin UVR is followed by a series of biochemical and immunologic events that cause inflammation. Histologically, it is characterized by dyskeratotic and vacuolated keratinocytes known as sunburn cells, mild epidermal spongiosis, depletion of Langerhans cells, dermal edema, endothelial cell enlargement, and later a neutrophilic dermal infiltrate.4 Some of the substances that are thought to be inflammatory mediators include prostaglandins, lipoxygenase products, cytokines (eg, tumor necrosis factor-alpha), adhesion molecules, reactive oxygen radicals, and mast cell-derived mediators, such as histamine, and substance P.5
Inflammation and pain related to UVR-induced sunburn was introduced by Bickel et al1 for the evaluation of analgesic effects and is now more broadly used as a simple pain model using three times the minimal erythema dose (MED) to evaluate the efficacy of NSAIDs but can also be used for corticosteroids.6–8
The study was designed to establish a test model for therapeutic interventions using UVR-induced pain and inflammation as a surrogate and to define the minimum required UVR exposure. Those effects were studied at various degrees of severity of UVR-induced inflammation and pain (1–3 times MED). For reference in future studies, this study evaluated and compared the effects of an orally administered NSAID (ibuprofen) and a topical corticosteroid with known effects on UVR-induced pain and inflammation. Subjects were randomized to receive ibuprofen (IB) or oral placebo during the first period and vice versa during the second period (crossover design).
Methods
This was a single-center, randomized, subject- and observer-blinded, placebo-controlled, crossover study of the effect of oral IB and topical hydrocortisone-21-acetate (HC) in healthy volunteers, conducted in two periods, using an intra-individual comparison of application areas. The study was approved by the national authorities (BfArM) and the independent ethics committee of the Landesärztekammer Bayern.
Subjects
Healthy subjects with a target age of 18–55 years and Fitzpatrick skin types I, II, or III9 who signed the informed consent were screened for study participation. Patients meeting any of the following exclusion criteria were not included into the trial: skin lesions; dermatological diseases or tattoo in the treatment areas; known hypersensitivity or allergy (including photoallergy) to any ingredient of the study medication; history of photosensitivity disease; sunburn, excessive tan, uneven skin tones or blemishes of the test areas or use of tanning lamps or tanning beds within 3 months before enrolment; conditions or analgesic medication which might interfere with pain rating during the study; and contraindications and warnings listed in the summary of product information of IB. Systemic or topical drugs that might affect responses to UVR or interfere with responses to the investigational medical product including corticosteroids, thiazides, tetracyclines, NSAIDs, and drugs with potential dermatologic adverse events defined by the respective summary of product characteristics (eg, tetracyclines, gyrase inhibitors) had to be washed out over a period of 5 times half-life, prior to the first irradiation, and were not taken throughout the study.
Subjects were also excluded if they showed poor repeatability of heat pain threshold determination in the training session.
Treatments
Immediately after irradiation, subjects were randomized to receive either IB (Ibuhexal®; Hexal AG, Holzkirchen, Germany) 600 mg film tablet (IB) or oral placebo. In addition to the oral medication, the test areas for topical treatment were randomized to receive either HC (Soventol® HydroCort 0.25%, Medice Pharma GmbH, Iserlohn, Germany) or topical placebo 15 μL/cm2 applied to three treatment areas each, on one side of the back.
Medication was administered twice daily (bid) at 12-hour intervals during site visits.
Treatment allocation was crossed over in period 2. Subjects who received IB in period 1 received oral placebo in period 2 and vice versa. Topical treatment was the same as in period 1 but new treatment areas on the back were selected (and irradiated) and side allocation was switched.
Study procedures
Irradiation was performed using a UVB system (UV 109 B; Waldmann, Villingen-Schwenningen, Germany). Irradiance was measured on each (protected) individual test area before irradiation using a broadband UVB detector connected to a radiometer (UV Meter for UV 21; Waldmann, Villingen- Schwenningen, Germany). The number of test areas that were irradiated at one step was dependent on the homogeneity of the irradiance (±15% of 2.5 mW/cm2 allowed).
To define the MED, skin areas with 2 cm diameter were irradiated with the following defined doses: 20, 50, 80, 110, 140, 170, 200, and 230 mJ/cm2. Readout was performed 24 ± 2 hours after UVR under standardized lighting conditions, using a 75 W blue light bulb. The individual MED corresponded to the dose level inducing an erythema assessment score of 2 on a 7-point categorical scale (mild erythema with clearly defined borders).
For the evaluation of effects on pain and inflammation, four skin areas (diameter of 2 cm) were defined on each side of the back (different from the areas of MED determination). One of the four areas on one side remained nonirradiated, one area on the contralateral side was irradiated with 3 MED but did not receive topical treatment. The other three areas were irradiated with 1, 2, or 3 MED, respectively, on both sides for topical treatment.
For the detection of heat pain threshold, heat stimuli were delivered to the skin from a thermode (9 × 9 mm; Somedic AB, Stockholm, Sweden) with feedback controlled by a thermocouple mounted in the surface of this thermode. The upper range of the thermode was limited to 52°C to protect from skin damage. The temperature of the thermode was slowly increased by 0.5°C per second from an adapting temperature of 32°C. Subjects were instructed to terminate heating by pressing a button as soon as the heat produced by the thermode became painful. The heat pain threshold was evaluated with a series of five consecutive determinations for each test area. For determination of the heat pain threshold temperature, the mean of the last three values was calculated.
In order to accustom subjects to the experimental equipment and procedures, they underwent a training session before randomization to treatment. Heat pain thresholds were measured at areas used for MED reading and nonirradiated control areas. Ten assessments (five on one normal skin area and five on the test area of the area that was rated 1 MED) were performed. Upon poor repeatability of thresholds (last three values for each test area must not be more than 3°C apart) subjects were excluded from the study. Data acquired in the training session were recorded but not included in the analysis.
The degree of erythema was evaluated visually using a 7-point categorical scale (0 = no erythema response, 6 = intense erythema with sharp borders and edema).
Skin temperature at the test areas was evaluated as a surrogate marker for an inflammatory response using an infrared thermometer (PCE-FIT 10, 149 × 77 × 43 mm; PCE Group, Meschede, Germany). Measurement distance between thermometer and skin was 5 cm. Measurements were done in duplicate at ambient temperature after 15 minutes of adaptation.
During both periods of the study, rating was done immediately after UVR but before the first application of medication. Subsequent ratings for all test areas were performed before application of the drug at 6 hours and at 12 hours, 24 hours, and 36 hours, and then again at 48 hours/final visit.
Efficacy parameters were always evaluated in the following sequence: (1) degree of erythema; (2) skin temperature; (3) hyperalgesia to heat.
Statistics
The sample size of 24 was chosen with reference to Sycha et al and our own previous data.2,7 For the evaluation of the study endpoints a two-period crossover-analysis Wilcoxon test (2-sided test for difference) for sums, differences, and crossover differences was performed.9
Due to the fact that the results of the primary efficacy analysis showed potential carryover effects, separate analyses were performed using nonparametric testing.
The analysis was performed using the intention-to-treat population.
Results
For study participation, 28 subjects were screened. Four of the subjects screened were not included. The reasons for screening failure were: three subjects showed poor repeatability of heat pain threshold determination in the training session (exclusion criterion) and one subject withdrew informed consent. As planned, 24 subjects were randomized. All subjects completed the study; there were no dropouts.
The analysis of the primary endpoint (hyperalgesia to heat) comparing the IB group with the oral placebo group, using the area irradiated with 3 MED and without topical treatment, showed no statistically significant treatment effects (P = 0.4502, see Table 1). However, in contrast to the assumptions made during preparation of the study, significant carryover effects could be demonstrated for the comparison of period 1 and 2 (P = 0.0386). Similarly, the comparison of the topical products (HC vs placebo) at 3 MED showed no statistical significant treatment effects, but showed carryover effects. For erythema, statistically significant treatment effects could be shown (P = 0.0233) without a significant carryover effect (P = 0.1574), while skin temperature showed pronounced carryover effects (P = 0.0052) without evidence of treatment effects.
Table 1.
Descriptive statistics (mean ± standard deviation) and results of the two-period crossover-analysis of treatment and carryover effects comparing ibuprofen to placebo (3 MED)
| Parameter | Ibuprofen | Placebo | Treatment | Carryover |
|---|---|---|---|---|
| HPT | 270.8 ± 15.8 | 267.2 ± 17.1 | ns | P = 0.0386 |
| Erythema | 14.7 ± 2.0 | 16.5 ± 1.8 | P = 0.0233 | ns |
| Temperature | 202.2 ± 4.1 | 203.3 ± 3.3 | ns | P = 0.0052 |
Note: Evaluation of sum scores HPT, erythema, and skin temperature.
Abbreviations: MED, minimal erythema dose; HPT, heat pain threshold; ns, not significant.
Due to the fact that the primary analysis showed substantial carryover effects, separate analyses were performed for the first and the second period of the study with a focus on the comparison of heat pain threshold and erythema of the IB group.
IB failed to show significant effects on all parameters in period 1 of the study. In contrast, during period 2 the expected treatment effects could be shown for IB as compared to placebo (see Table 2).
Table 2.
Descriptive statistics (mean ± standard deviation) and results of Wilcoxon–Mann–Whitney analysis comparing ibuprofen to placebo (P value 1) and hydrocortisone to placebo (P value 2) at various MED levels for period 2
| Parameter | MED level | Placebo | Ibuprofen | P value 1 | Hydrocortisone | P value 2 |
|---|---|---|---|---|---|---|
| HPT | 0 | 289.4 ± 11.8 | ||||
| 1 | 274.5 ± 16.7 | 287.4 ± 8.5 | 0.0430 | 278.0 ± 13.8 | >0.1 | |
| 2 | 268.4 ± 18.2 | 282.0 ± 11.2 | 0.0357 | 268.1 ± 17.9 | >0.1 | |
| 3 | 264.7 ± 14.4 | 278.9 ± 13.1 | 0.0513 | 265.2 ± 20.8 | >0.1 | |
| Erythema | 0 | 0 | ||||
| 1 | 10.3 ± 2.5 | 6.3 ± 2.9 | 0.0094 | 10.4 ± 2.9 | >0.1 | |
| 2 | 16.1 ± 2.1 | 12.2 ± 2.7 | 0.0044 | 16.0 ± 2.3 | >0.1 | |
| 3 | 17.3 ± 1.9 | 15.0 ± 2.3 | 0.0944 | 17.8 ± 1.9 | >0.1 | |
| Temperature | 0 | 199.4 ± 3.6 | ||||
| 1 | 201.9 ± 3.7 | 200.0 ± 3.8 | >0.1 | 203.0 ± 2.6 | >0.1 | |
| 2 | 204.4 ± 2.9 | 200.2 ± 3.7 | 0.0037 | 204.0 ± 2.9 | >0.1 | |
| 3 | 205.0 ± 2.4 | 201.7 ± 3.8 | 0.0716 | 204.8 ± 3.0 | >0.1 |
Note: Evaluation of sum scores for heat pain threshold (HPT), erythema, and skin temperature.
Abbreviations: MED, minimal erythema dose; HPT, heat pain threshold; ns, not significant.
A detailed analysis for the various treatment groups and MED levels within period 2 showed at 3 MED that IB is superior to placebo for both erythema and hyperalgesia to heat (P = 0.0944 and P = 0.0513, respectively) with a large effect size (Mann–Whitney estimator = 0.843 and 0.7686, respectively). Also, skin temperature showed a trend in favor of IB. The differences were even more pronounced at 2 MED (erythema: P = 0.0044, hyperalgesia to heat: P = 0.0357, skin temperature: P = 0.0037). At 1 MED, the differences were again statistically significant in favor of IB for erythema and hyperalgesia, but not for skin temperature (erythema: P = 0.0094, hyperalgesia: P = 0.0430, skin temperature: P = 0.396).
There were no differences in treatment response between HC and placebo for any strength of irradiation (1–3 MED) and any criteria observed (erythema, hyperalgesia, skin temperature).
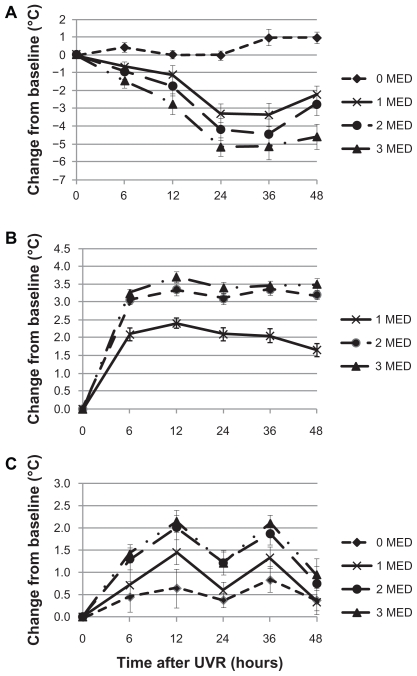
The kinetics of the UVR-induced changes indicated a radiation dose-related decrease of the heat pain threshold starting 6 hours after UV exposure (see Figure 1A). The changes are most pronounced 24–36 hours after UVR and start returning to baseline levels at 48 hours post-UVR. For erythema, 1 and 2 MED produced a profound increase in the erythema score. The scoring did not increase substantially further for 3 MED as compared to 2 MED (Figure 1B). Skin temperature shows a circadian rhythm with higher temperature in the evening (12 hours, 36 hours) and UVR dose-dependent increase for 1 and 2 MED with again no substantial further increase for 3 MED (Figure 2C).
Figure 1.
Kinetics of UVR-induced changes (Mean ± S EM) of heat pain threshold (A), erythema (B) and skin temperature (C) for nonirradiated skin (0 MED) and increasing levels of UVR (1–3 MED).
Abbreviations: UVR, ultraviolet B radiation; SEM, standard error in the mean; MED, minimal erythema dose.
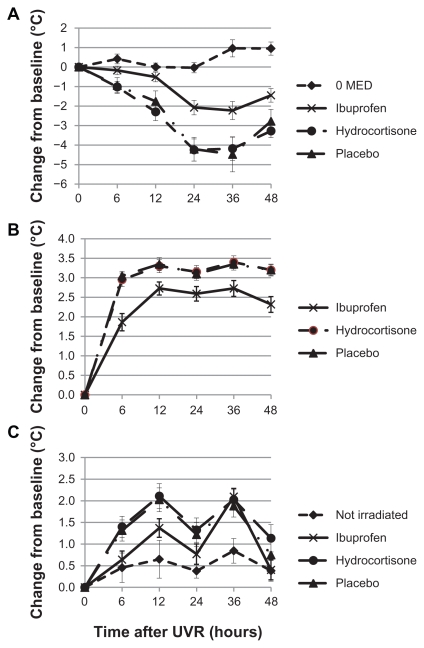
Figure 2.
Kinetics of UVR-induced changes (Mean ± S EM) of heat pain threshold (A), erythema (B) and skin temperature for skin irradiated at 2 MED and treated with ibuprofen or hydrocortisone as compared to nonirradiated skin (0 MED).
Abbreviations: UVR, ultraviolet B radiation; SEM, standard error in the mean; MED, minimal erythema dose.
Since 3 MED did not seem to provide improved assay sensitivity as compared to 2 MED, the kinetics of UVR-induced changes between IB, HC, and placebo were compared at 2 MED.
IB produced profound treatment-induced improvements for all outcome criteria (see Figure 2A–C) with differences least pronounced for skin temperature. HC failed to show a differentiation from placebo for all criteria.
Sixteen adverse effects (AE) were observed in 12 (50%) of the 24 subjects treated. All AEs were of mild intensity. Only one AE (from 16) was judged as related to IMP: “erythema at test area”. No severe AEs occurred.
No clinically relevant changes in laboratory parameters or vital signs were observed.
All IMP were very well tolerated. The study procedures (UVR) did not cause unexpected discomfort to the subjects.
Discussion
The analysis of the primary endpoint (hyperalgesia to heat) for the crossover design failed to show the expected superiority of the IB group vs the OP group as reported by others.2 In line with the study of Sycha et al,2 we found substantial differences between study periods. But in contrast to this study, our design had more complexity due to the additional topical treatment. This may be one explanation for the lack of statistical significance for the primary hypothesis. Substantial differences in heat pain threshold evaluations between the first and follow-up session were also reported by Yarnitzky et al.10 Reasons given for the bias include practice effects. One of the important practice effects is training-related change in reaction times which are of particular importance to the evaluation of heat pain threshold. But practice effects may not only involve the subjects but also the study personnel. Since evaluating period 2 alone clearly showed the expected pharmacological effects, we suggest the following changes for follow-up studies: intensified training sessions before randomization to treatment; increase in sample size for crossover studies; simplification in design (either oral or topical treatment); use of a larger thermode (eg, 18 × 18 mm) to recruit more pain receptors for more consistent results.
If period 2 was analyzed alone, the predicted effects of IB were shown consistently across different degrees of inflammation (1–3 MED) for erythema and heat pain threshold. The results were less pronounced for skin temperature.
In contrast to IB, there were no differences in treatment response between HC and PG for any strength of irradiation (1–3 MED) and any criteria observed (erythema, hyperalgesia, skin temperature). There is contradictory evidence for the effects of corticosteroids for the treatment of UVR-induced skin reactions. Mid- or high-potency steroids showed reduction of acute sunburn symptoms when applied before UVR exposure but not when applied 6 hours or later after UVR exposure.6,8 The lack of effects seen in this study may either be related to the fact that the first treatment was applied immediately after UVB radiation and not before, or because of the low potency of hydrocortisone per se or as used in this study. The evaluation of low potency steroids may also require larger sample sizes.11
UVR at up to 3 MED induced profound reduction in heat pain threshold, increase of skin temperature and caused profound erythema. The changes were almost linear between 1 and 2 MED, whereas the change from 2 to 3 MED was less pronounced, in particular for erythema and skin temperature. This is in line with the findings of Harrison et al.12 One of the reasons claimed for this observation is first evidence of edematous changes at 3 MED. Jocher et al suggested the use of 1.5 MED on the basis of just evaluating the effect on erythema.11 Since we found similar pharmacological effects between 2 and 3 MED, use of 2 MED in upcoming studies seems to be reasonable to limit UVB exposure to subjects. In order to allow precise determination of the UV dosages only subjects with skin type I–III according to Fitzpatrick13 were enrolled and individual sensitivity to UV was determined.
A variety of experimental pain models for the evaluation of NSAID efficacy involving healthy subjects have been reported.1–14 Nevertheless, UVR-induced pain models offer several advantages over other pain models in healthy volunteers. Inflammation and hyperalgesia are constant for several hours, representing an advantage not only over freezing, burning, or chemically induced inflammation, but also over painful clinical disorders which are often of episodic nature. UVR models are therefore able to test the effects of drugs with low variability. In addition, the upper heating limit of the thermode can be defined in order to avoid skin damage, and the discontinuation of the heating procedure is under the subject’s control at any time. Hence, compared with freezing, burning, or chemically induced inflammation and hyperalgesia, UVR-induced pain models should be better tolerated by the volunteers.
All study medications were very well tolerated. The study procedures (UVR) did not cause blisters or other unexpected discomfort to the subjects.
Conclusion
Use of 2 MED in upcoming studies seems to be reasonable to limit UVR exposure to subjects. The following procedural changes are suggested: Intensified training sessions before randomization to treatment; increase in sample size for crossover studies; simplification in design (either oral or topical treatment).
Acknowledgments
Dipl stat Marion Ocak, MD Research Munich, provided assistance with statistical analysis. Dr Franziska Puosi, X-pert Med GmbH, was involved in data collection and project management.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bickel A, Dorfs S, Schmelz M, Forster C, Uhl W, Handwerker HO. Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain. 1998;76(3):317–325. doi: 10.1016/S0304-3959(98)00062-1. [DOI] [PubMed] [Google Scholar]
- 2.Sycha T, Gustorff B, Lehr S, Tanew A, Eichler HG, Schmetterer L. A simple pain model for the evaluation of analgesic effects of NSAIDs in healthy subjects. Br J Clin Pharmacol. 2003;56(2):165–172. doi: 10.1046/j.0306-5251.2003.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll MS, Wagner RF., Jr Clinical management of the acute sunburn reaction. Cutis. 2000;66(1):53–58. [PubMed] [Google Scholar]
- 4.Gilchrest BA, Soter NA, Stoff JS, Mihm MC., Jr The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol. 1981;5(4):411–422. doi: 10.1016/s0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- 5.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100(1):35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 6.Faurschou A, Wulf HC. Topical corticosteroids in the treatment of acute sunburn: a randomized, double-blind clinical trial. Arch Dermatol. 2008;144(5):620–624. doi: 10.1001/archderm.144.5.620. [DOI] [PubMed] [Google Scholar]
- 7.Rother M, Schmelz M, Lehmann J, Grossmann M. Clinical trial design to study the effects of pharmacological intervention on different models of skin pain and inflammation – methodology and first results. Exp Dermatol. 2004;13(9):578. [Google Scholar]
- 8.Duteil L, Queille-Roussel C, Lorenz B, Thieroff-Ekerdt R, Ortonne JP. A randomized, controlled study of the safety and efficacy of topical corticosteroid treatments of sunburn in healthy volunteers. Clin Exp Dermatol. 2002;27(4):314–318. doi: 10.1046/j.1365-2230.2002.01033.x. [DOI] [PubMed] [Google Scholar]
- 9.Koch G. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972;28(2):577–584. [PubMed] [Google Scholar]
- 10.Yarnitzky D, Sprecher E, Zaslansky R, Hemli JA. Multiple session experimental pain measurement. Pain. 1996;67(2–3):327–333. doi: 10.1016/0304-3959(96)03110-7. [DOI] [PubMed] [Google Scholar]
- 11.Jocher A, Kessler S, Hornstein S, Schulte Mönting J, Schempp CM. The UV erythema test as a model to investigate the anti-inflammatory potency of topical preparations – reevaluation and optimization of the method. Skin Pharmacol Physiol. 2005;18(5):234–240. doi: 10.1159/000086669. [DOI] [PubMed] [Google Scholar]
- 12.Harrison GI, Young AR, McMahon SB. Ultraviolet radiation-induced inflammation as a model for cutaneous hyperalgesia. J Invest Dermatol. 2004;122(1):183–189. doi: 10.1046/j.0022-202X.2003.22119.x. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick TB. The validity and practicality of sun reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 14.Moiniche S, Dahl JB, Kehlet H. Time course of primary and secondary hyperalgesia after heat injury to the skin. Br J Anaesth. 1993;71(2):201–205. doi: 10.1093/bja/71.2.201. [DOI] [PubMed] [Google Scholar]