Abstract
Acute application of topical capsaicin produces spontaneous burning and stinging pain similar to that seen in some neuropathic states, with local hyperalgesia. Use of capsaicin applied topically or injected intradermally has been described as a model for neuropathic pain, with patterns of activation in brain regions assessed using functional magnetic resonance imaging (fMRI) and positron emission tomography. The Contact Heat Evoked Potential Stimulator (CHEPS) is a noninvasive clinically practical method of stimulating cutaneous A-delta nociceptors. In this study, topical capsaicin (1%) was applied to the left volar forearm for 15 minutes of twelve adult healthy human volunteers. fMRI scans and a visual analog pain score were recorded during CHEPS stimulation precapsaicin and postcapsaicin application. Following capsaicin application there was a significant increase in visual analog scale (mean ± standard error of the mean; precapsaicin 26.4 ± 5.3; postcapsaicin 48.9 ± 6.0; P < 0.0001). fMRI demonstrated an overall increase in areas of activation, with a significant increase in the contralateral insular signal (mean ± standard error of the mean; precapsaicin 0.434 ± 0.03; postcapsaicin 0.561 ± 0.07; P = 0.047). The authors of this paper recently published a study in which CHEPS-evoked A-delta cerebral potential amplitudes were found to be decreased postcapsaicin application. In patients with neuropathic pain, evoked pain and fMRI brain responses are typically increased, while A-delta evoked potential amplitudes are decreased. The protocol of recording fMRI following CHEPS stimulation after topical application of capsaicin could be combined with recording of evoked potentials to provide a simple, rapid, and robust volunteer model to develop novel drugs for neuropathic pain.
Keywords: capsaicin, fMRI, contact heat evoked potentials, neuropathy, pain model
Introduction
Capsaicin is the pharmacologically active vanilloid compound found in chili peppers which, when applied topically, causes a burning or tingling sensation due to the activation of polymodal C and A-delta nociceptive fibers, and the release of substance P and calcitonin gene related peptide.1 Repeated application of 0.075% capsaicin to skin for 3 weeks results in the degeneration of protein gene product 9.5 immunoreactive intraepidermal nerve fibers in skin blisters and biopsies.2 Within 24 hours, 0.075% capsaicin applied four times daily causes a dramatic loss of intraepidermal nerve fibers immunoreactive for protein gene product 9.5, the pan neuronal marker.3 Short term application of a high dose (8%) capsaicin patch or intradermal injection of capsaicin also causes significant degeneration.4,5 Regeneration of epidermal nerve fibers after discontinuation of capsaicin treatment has been shown to occur, with a return of sensory function to baseline levels.2–4,6–8 Repeated application of capsaicin leads to functional desensitization to mechanical, thermal, or chemical noxious stimuli with clinical studies showing efficacy in reducing pain associated with postherpetic neuralgia, diabetic neuropathy, osteoarthritis, and musculoskeletal disorders.9–11
Acutely, capsaicin (either intradermally injected or applied topically) has been used in human models of neuropathic pain, to reproduce the symptoms commonly reported by neuropathic pain patients, including spontaneous burning, pricking, and stinging pain around the site of injection/application. Primary and secondary hyperalgesia to mechanical and thermal stimuli, and neurogenic inflammation – an area of flare spreading from the point of injection/site of application – can be measured.12–14 Several investigators report variability in aspects of the response to intradermal and topical capsaicin, and that some subjects are nonresponders. However, with carefully designed methods to reduce variability, the pain phenomena induced by capsaicin can be reproducible,12,15–17 and the model is acceptable for pharmacological profiling of novel analgesics.12
Recently, this research team reported that contact heat evoked potentials using a clinically practical stimulator (Contact Heat Evoked Potential Stimulator [CHEPS]) were suitable to assess nociceptive pathways in patients, particularly neuropathic pain patients.18 Further, a study was carried out to assess the feasibility of recording contact heat evoked potentials in the magnetic resonance imaging (MRI) environment, which revealed a positive correlation between the amplitude of contact heat evoked potentials and evoked pain scores in healthy volunteers, as reported by others.19–24 Previous investigations of capsaicin-induced changes using imaging techniques such as positron emission tomography and functional MRI (fMRI) have illustrated that application of capsaicin to the skin causes activation in the primary and secondary somatosensory cortices, supplementary motor area, cingulate gyrus, insular cortex, prefrontal cortex, thalamus, and brainstem.25–31 Coregistration of contact heat evoked potentials with functional MRI is highly desirable, particularly in the early studies of novel analgesics, for a number of documented reasons.19 There is a high unmet clinical need for patients with chronic neuropathic pain.
Using these techniques of assessment (CHEPS and fMRI), and the known effect of topical capsaicin, the aim was to develop a simple, rapid, and practical potential surrogate model of “neuropathic pain.” In a recently published study, it was shown that following topical application of capsaicin with CHEPS stimulation, there is an increase in the evoked pain score and a trend for a decrease in contact heat evoked potentials.32 In this study, changes in evoked pain scores and fMRI responses with CHEPS following topical application of capsaicin were assessed.
Methods
Subjects
Twelve healthy volunteers were recruited to take part in this study (six female, six male). The average age of participants was 26.08 years (range 20–34 years). Informed consent was taken from all subjects prior to commencement of the study. The study was approved by Riverside Research Ethics Committee (REC reference 07/H0706/78).
Capsaicin
Capsaicin cream was purchased from The Specials Laboratory, Northumberland, United Kingdom and contained Capsicum Oleoresin BPC 1973 12.5% in Unguentum M (equivalent to 1% capsaicin).
Experimental protocol
An event-related protocol was used to apply contact heat stimuli to a defined area of the left volar forearm of the subject during simultaneous fMRI acquisition, as reported previously.19 The thermode was moved slightly within the defined area of forearm skin to avoid habituation of the subject to the stimulus. The event-related protocol consisted of 32 stimuli at 51°C target temperature of 800 milliseconds duration, with an interstimulus interval that varied between 8 seconds and 32 seconds. Subjects were scanned twice using this protocol: (1) baseline (precapsaicin application) and (2) following topical application of 1% capsaicin which was removed after 15 minutes (postcapsaicin application).
During the precapsaicin and postcapsaicin protocols, subjects were asked to rate the pain of each contact heat stimulus on an electronic visual analog scale by moving a marker along a sliding scale that was graded from zero to 100, zero being not painful at all and 100 being the worst pain imaginable. Each pain score was recorded and an average taken.
fMRI acquisition
In each scanning session, 275 fMRI scans were acquired on a MAGNETOM Avanto 1.5T magnetic resonance scanner (Siemens AG, Erlangen, Germany) with a standard head array coil. Nineteen slices parallel to the anterior commissure and posterior commissure were acquired using a gradient echo EPI (echo-planar imaging) sequence (repetition time/echo time = 2.3 seconds/53 milliseconds, flip angle = 90°, field-of-view = 23 cm, matrix = 64 × 64, voxel size = 3.6 mm × 3.6 mm × 5 mm). A high resolution T1-weighted MP-RAGE anatomical scan was also obtained (repetition time/echo time = 11 milliseconds/5.2 milliseconds, flip angle = 15°, field-of-view = 23 cm, matrix = 256 × 256, voxel size = 0.9 mm × 0.9 mm × 1 mm).
To achieve synchronization, the trigger output of the scanner was used to initialize the fMRI paradigm and triggers from CHEPS stimulation and the scanner were recorded together.
fMRI processing
Processing of fMRI images was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). Due to T1 saturation effects, the first five volumes of each acquisition were discarded, leaving 270 volumes. Each dataset was realigned to correct for any motion during the acquisition, the time was corrected to account for differences in image acquisition time between slices, followed by normalization to transform the data to match the SPM template. The template images supplied with SPM conform to the space defined by the International Consortium for Brain Mapping project (National Institutes of Health P-20 grant). Finally, images were smoothed using an isotropic Gaussian kernel (8 mm full-width-at-half-maximum).
Statistical analysis was based on the general linear model. For intrasubject analysis, the scanning paradigm was specified in SPM and a first level model estimation performed. The event responses were modeled onto a design matrix by specifying their onset times and duration. For group analysis of twelve subjects, a canonical random effects model was used to make a population inference (P < 0.05 corrected for multiple comparisons by the family-wise error method). Spatial extent thresholding (voxel threshold = 135 mm3) was additionally carried out to exclude isolated voxels or small groups to show clusters of activation only. Significantly activated regions resulting from the group analysis were superimposed on a normalized structural volume scan. The location of the activated regions was assessed using SPM Anatomy toolbox (Wellcome Trust Center).
Statistical analysis
Graphs were created and statistical tests (Mann–Whitney) were performed using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, CA). Paired t-tests were used to compare variables precapsaicin and postcapsaicin application.
Results
Pain ratings
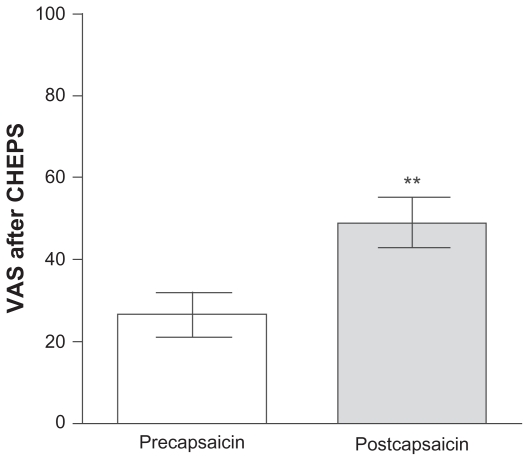
All subjects reported that 1% capsaicin evoked an unpleasant burning sensation. The mean pain rating during CHEPS stimulation at 51°C precapsaicin was 26.4 ± 5.3 and ratings increased significantly postcapsaicin to 48.9 ± 6.0 (P < 0.0001; Figure 1).
Figure 1.
Pain rating of 51°C stimulation with Contact Heat Evoked Potential Stimulator (CHEPS), reported on an electronic visual analog scale (VAS) precapsaicin and postcapsaicin application.
Note: **P < 0.0001.
fMRI
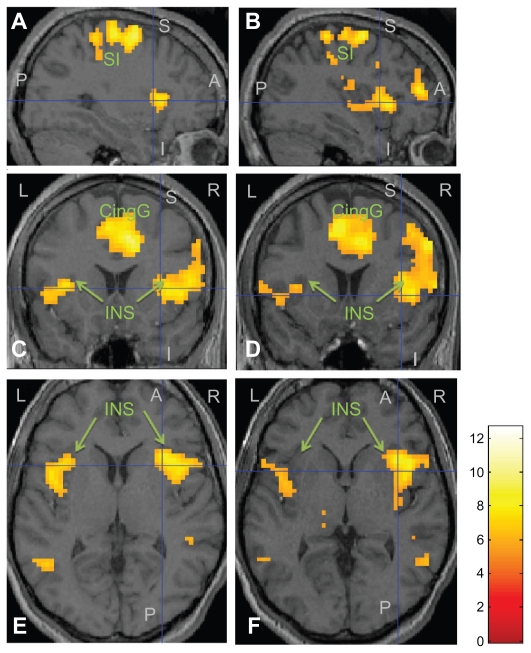
Group analysis of fMRI data acquired from all twelve volunteers following CHEPS stimulation precapsaicin revealed areas of blood oxygen level-dependent (BOLD) activation across the “pain matrix” in a pattern similar to that observed in a previous feasibility study (Figure 2).19
Figure 2.
Functional MRI group analysis results ((A) and (B): sagittal view; (C) and (D): coronal view; (E) and (F): axial view) in twelve healthy volunteers following Contact Heat Evoked Potential Stimulator stimulation at 51°C, precapsaicin (left panel), and 15 minutes postcapsaicin application (right panel).
Note: The intersection of the blue cross hairs represents the contralateral insular cortex with significant increase in activation postcapsaicin application.
Abbreviations: L, left side; R, right side; A, anterior; P, posterior; S, superior; I, inferior; INS, insular; CingG, cingulated gyrus; S1, somatosensory area.
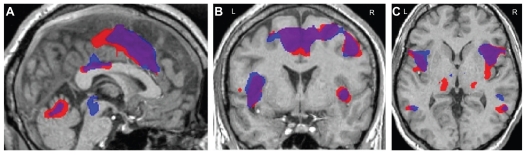
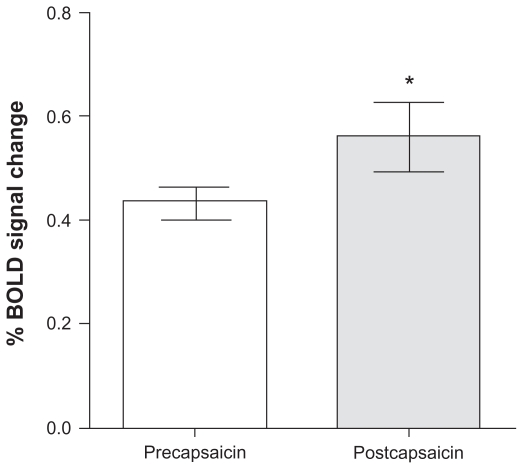
Analysis of fMRI data acquired postcapsaicin revealed increased levels of BOLD activation in the contralateral posterior cingulate gyrus, precentral and postcentral gyrus, and the superior frontal gyrus. Increased activation was also seen in the left anterior cingulate gyrus, middle frontal gyrus, and cuneus with bilateral activation in the caudate nucleus (Figure 2). Activations of precapsaicin and postcapsaicin application are summarized in Table 1. Overlaying the areas of contact heat induced BOLD activation both precapsaicin and postcapsaicin onto the normalized structural volume scan revealed that following the application of capsaicin there was an overall increase in the size of the area activation (Figure 3). Region of interest analysis was carried out in several areas in the “pain matrix” including primary somatosensory cortex, anterior cingulate cortex, and insular cortex, but only insular cortex showed significant change. There was a significant increase in BOLD signal over the contralateral insular region following capsaicin application (mean ± standard error of the mean; precapsaicin 0.434 ± 0.03; postcapsaicin 0.561 ± 0.07; P = 0.047) (Figure 4).
Table 1.
Summary of areas of blood oxygen level-dependent functional magnetic resonance imaging activation (SPM8 [Wellcome Trust Centre for Neuroimaging, London, United Kingdom] group analysis) following stimulation with Contact Heat Evoked Potential Stimulator precapsaicin and postcapsaicin application
| Precapsaicin | Postcapsaicin | |||
|---|---|---|---|---|
| Contralateral x, y, z (T) | Ipsilateral x, y, z (T) | Contralateral x, y, z (T) | Ipsilateral x, y, z (T) | |
| Postcentral gyrus | 54, −29, 48 (9.99) | −24, −47, 63 (7.23) | 27, −38, 60 (8.67) | −24, −50, 63 (7.27) |
| Insular cortex | 36, 13, 6 (8.38) | −45, 4, 6 (8.08) | 39, 13, 6 (8.31) | −33, 10, 12 (6.78) |
| Middle cingulate gyrus | 9, 13, 45 (11.09) | −9, −23, 39 (6.61) | 9, 13, 42 (10.05) | |
| Middle frontal gyrus | 45, −5, 60 (7.49) | −38, 37, 30 (5.78) | 60, 7, 12 (7.52) | −30, −8, 57 (8.47) |
| Inferior frontal gyrus | 51, 10, 15 (7.77) | −57, 10, 9 (5.7) | ||
| Anterior cingulate gyrus | 6, 28, 27, (6.1) | −6, 13, 30 (5.89) | ||
| Cerebellum | 27, −59, −21 (5.98) | −27, −62, −18 (7.86) | 27, −59, −18 (6.27) | −27, −62, −18 (9.08) |
| Thalamus | −15, −20, 12 (5.72) | −18, −32, 12 (6.15) | ||
Note: Within each area, the maximum T value (T) and its Talairach coordinates (x,y,z) are provided (SPM Anatomy Toolbox).
Figure 3.
Areas of blood oxygen level-dependent activation following contact heat stimulation overlaid on a high resolution structural scan ((A): sagittal view; (B): coronal view; (C): axial view).
Note: Blue indicates precapsaicin activation, red indicates postcapsaicin activation, and purple indicates overlapping areas.
Abbreviations: L, left; R, right.
Figure 4.
Functional magnetic resonance imaging signal intensity in the contralateral insular cortex precapsaicin and postcapsaicin application.
Note: *P = 0.047.
Abbreviation: BOLD, blood oxygen level-dependent.
Discussion
In this study, topical application of 1% capsaicin (for 15 minutes) evoked a spontaneous, unpleasant burning sensation, and resulted in an increase in pain scores following heat stimulation (with CHEPS). The authors were able to obtain fMRI images which showed areas of activation consistent with the “pain matrix.” The areas of activity which follow stimulation with CHEPS have been shown to increase in size following acute application of capsaicin, with a significant increase in the signal intensity over the contralateral insular cortex. Correlation between perceived pain intensity and magnitude of signals over various areas such as the somatosensory cortex and the insular cortex have been shown using fMRI and positron emission tomography scans.33,34 These findings would support the use of this topical capsaicin model for the study of neuropathic pain.
The use of fMRI in the study of capsaicin induced thermal hyperalgesia has been reported previously.35 In Brooks et al’s study,35 the thermode temperature which evoked pain on a visual analog scale between five and seven was noted (designated as TPAIN). fMRI was performed with thermode at baseline temperature of 35°C and repeated with the thermode at a temperature of TPAIN for 9 seconds. This protocol was then repeated following application of capsaicin, with the new thermode temperature evoking pain on the visual analog scale between five and seven (designated as TCAPS). The temperature eliciting the pain after capsaicin application was significantly lower than that before application of capsaicin, suggesting capsaicin induced thermal hyperalgesia. Subjects did not report any spontaneous pain following capsaicin application. Following capsaicin application, with fMRI, there was a significantly increased activation of ipsilateral prefrontal and bilateral parietal regions. The stimulation protocol of the present study was different from Brooks et al35 study, due to interest in studying the effects of capsaicin application alone on pain and pattern of fMRI activation following stimulation of the nociceptive fibers. The same thermode destination temperature was used in both precapsaicin and postcapsaicin application. All subjects described a burning sensation over the skin following capsaicin application.
Recently, the authors of this study reported on a new volunteer pain model with recording of CHEPS-evoked cerebral A-delta potentials following topical application of transient receptor potential (TRP) channel agonists including capsaicin.32 In this previous study fMRI responses were not measured, unlike the present study. There was an increase in pain score during stimulation with CHEPS following capsaicin and a trend for reduction in evoked A-delta amplitudes, whereas there was a positive correlation between evoked pain scores and A-delta potential amplitudes precapsaicin;19 pain scores were correlated negatively with CHEPS evoked A-delta potentials after capsaicin application.32 In the present study, topical application of capsaicin resulted in an increase in pain scores following heat stimulation with CHEPS, and fMRI images were able to be obtained which showed areas of activation consistent with the “pain matrix.”
Unpleasant sensations, heat hyperalgesia, and negative correlations between amplitude and pain scores caused by acute capsaicin application are similar to what is observed in some neuropathic pain patients, ie, a reduction in amplitude of contact heat evoked potentials, but a preserved or increased sensation of pain. Topical capsaicin acts in the periphery by eliciting spontaneous discharges from TRP channel, subfamily V, member 1-expressing A-delta and C-fiber nociceptors, but after capsaicin treatment afferent volleys to the brain are less synchronous, leading to decreased amplitudes of pain evoked cerebral potentials. In healthy volunteers, microneurographic recordings show that spontaneous burning sensations elicited by capsaicin are accompanied by firing of mechanical and heat sensitive polymodal C-fiber nociceptors.36 Spontaneous activity similar to this has also been shown in recordings from patients with peripheral neuropathy and positive sensory symptoms.37 Topical capsaicin application may provide a correlate of clinical changes in peripheral neuropathy.
One of the limitations of this study is the defined order of performing fMRI scans. As it is necessary to perform a baseline scan prior to application of topical capsaicin as in other studies,35 there is a potential bias due to the effects of the order of the scan. It is difficult to avoid this, given the need to stimulate the same region of skin, and the prolonged duration of effect of topical capsaicin.
Cerebral potentials can provide a useful biomarker, but their limitation is the lack of spatial resolution. fMRI overcomes this limitation. Simultaneous recording of electroencephalogram with CHEPS and fMRI offers the advantages of high temporal resolution of electroencephalogram and high spatial resolution provided by fMRI. The authors have previously reported on the feasibility of this combination in healthy volunteers.19 The capsaicin application protocol in the present study, when used with simultaneous recording of CHEPS, evoked potentials, and fMRI could be useful to test novel analgesics for neuropathic pain, in particular TRP channel, subfamily V, member 1 antagonists.
Conclusion
Topical capsaicin application produces a burning pain with a corresponding increase in pain scores following CHEPS, an increase in areas of activation with fMRI, and increased signal change in the insular cortex. This offers the potential to develop a simple, rapid model to study pain in human volunteers and the effect of treatments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75(2):157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- 2.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81(1–2):135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 3.Khalili N, Wendelschafer-Crabb G, Kennedy WR, Simone DA. Influence of thermode size for detecting heat pain dysfunction in a capsaicin model of epidermal nerve fiber loss. Pain. 2001;91(3):241–250. doi: 10.1016/S0304-3959(00)00444-9. [DOI] [PubMed] [Google Scholar]
- 4.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18(21):8947–8959. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111(3):360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127(Pt 7):1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 7.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47(3):285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter SE, Lynn B. Vascular and sensory responses of human skin to mild injury after topical treatment with capsaicin. Br J Pharmacol. 1981;73(3):755–758. doi: 10.1111/j.1476-5381.1981.tb16812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 10.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328(7446):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes A, Macleod A, Growcott J, Thomas I. Assessment of the reproducibility of intradermal administration of capsaicin as a model for inducing human pain. Pain. 2002;99(1–2):323–331. doi: 10.1016/s0304-3959(02)00161-6. [DOI] [PubMed] [Google Scholar]
- 13.Kilo S, Schmelz M, Koltzenburg M, Handwerker HO. Different patterns of hyperalgesia induced by experimental inflammation in human skin. Brain. 1994;117(Pt 2):385–396. doi: 10.1093/brain/117.2.385. [DOI] [PubMed] [Google Scholar]
- 14.Culp WJ, Ochoa J, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Cross modality threshold modulation in human C nociceptors. Brain. 1989;112(Pt 5):1317–1331. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- 15.Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003;4(3):122–128. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Max MB, Robinovitz E, Gracely RH, Bennett GJ. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction. J Pain Symptom Manage. 1998;16(1):10–20. doi: 10.1016/s0885-3924(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 17.Harding LM, Murphy A, Kinnman E, Baranowski AP. Characterization of secondary hyperalgesia produced by topical capsaicin jelly – a new experimental tool for pain research. Eur J Pain. 2001;5(4):363–371. doi: 10.1053/eujp.2001.0253. [DOI] [PubMed] [Google Scholar]
- 18.Atherton DD, Facer P, Roberts KM, et al. Use of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007;7:21. doi: 10.1186/1471-2377-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts K, Papadaki A, Goncalves C, et al. Contact heat evoked potentials using simultaneous EEG and fMRI and their correlation with evoked pain. BMC Anesthesiol. 2008;8:8. doi: 10.1186/1471-2253-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granovsky Y, Granot M, Nir RR, Yarnitsky D. Objective correlate of subjective pain perception by contact heat-evoked potentials. J Pain. 2008;9(1):53–63. doi: 10.1016/j.jpain.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Ohara S, Crone NE, Weiss N, Treede RD, Lenz FA. Amplitudes of laser evoked potential recorded from primary somatosensory, parasylvian and medial frontal cortex are graded with stimulus intensity. Pain. 2004;110(1–2):318–328. doi: 10.1016/j.pain.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Chen AC, Niddam DM, Arendt-Nielsen L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci Lett. 2001;316(2):79–82. doi: 10.1016/s0304-3940(01)02374-6. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Y, Noguchi Y, Honda M, et al. Brain processing of the signals ascending through unmyelinated C fibers in humans: an event-related functional magnetic resonance imaging study. Cereb Cortex. 2006;16(9):1289–1295. doi: 10.1093/cercor/bhj071. [DOI] [PubMed] [Google Scholar]
- 24.Chen IA, Hung SW, Chen YH, et al. Contact heat evoked potentials in normal subjects. Acta Neurol Taiwan. 2006;15(3):184–191. [PubMed] [Google Scholar]
- 25.Baron R. Capsaicin and nociception: from basic mechanisms to novel drugs. Lancet. 2000;356(9232):785–787. doi: 10.1016/S0140-6736(00)02649-0. [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Baron Y, Disbrow E, Roberts TP. Brain processing of capsaicin-induced secondary hyperalgesia: a functional MRI study. Neurology. 1999;53(3):548–557. doi: 10.1212/wnl.53.3.548. [DOI] [PubMed] [Google Scholar]
- 27.Iadarola MJ, Berman KF, Zeffiro TA, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121(Pt 5):931–947. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- 28.Iannetti GD, Zambreanu L, Wise RG, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A. 2005;102(50):18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron. 2002;35(2):383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 30.Maihofner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. Neuroimage. 2005;28(4):996–1006. doi: 10.1016/j.neuroimage.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 31.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Roberts K, Shenoy R, Anand P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J Clin Neurosci. 2011;18(7):926–932. doi: 10.1016/j.jocn.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125(Pt 6):1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 34.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 35.Brooks JCW, Bimson WE, Roberts N, Nurmikko TJ. Functional magnetic resonance imaging of capsaicin-induced thermal hyperalgesia. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain. Seattle: IASP Press; 2003. pp. 295–304. [Google Scholar]
- 36.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campero M, Serra J, Marchettini P, Ochoa JL. Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve. 1998;21(12):1661–1667. doi: 10.1002/(sici)1097-4598(199812)21:12<1661::aid-mus6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]