Abstract
A class of betulinic acid derivatives was synthesized to target two critical steps in the human immunodeficiency virus type 1 (HIV-1) replication cycle, entry and maturation. Each mechanism of HIV-1 inhibition is distinct from clinically available anti-HIV therapeutics. The viral determinants of the antientry and antimaturation activities are the bridging sheet of HIV-1 gp120 and the P24/p2 cleavage site, respectively.
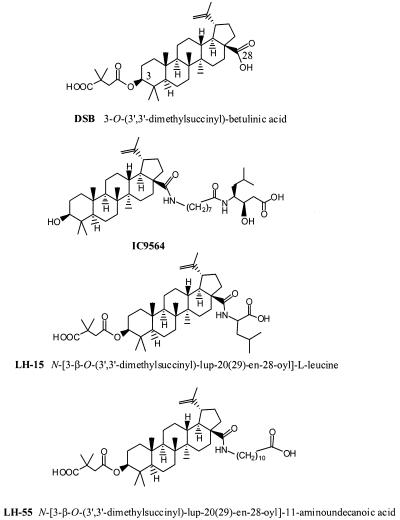
Betulinic acid derivatives are a class of small molecules that exhibit anti-human immunodeficiency virus type 1 (anti-HIV-1) activity (6, 7, 11). The targets of betulinic acid derivatives are varied, depending primarily on the side chain structures of the compounds (6) (Fig. 1). For example, betuliniyl aminooctanoylamino-3R-hydroxy-6-methylheptanoic acid (IC9564), which has aside chain at position 28 of betulinic acid, is a potent HIV-1 entry inhibitor (5). In contrast, dimethyl-succinyl-betulinic acid (DSB), which has a side chain at position 3, exhibits potent anti-HIV activity by inhibiting the maturation of the viral progeny (4, 7). IC9564 and DSB share a five-membered betulinic acid ring structure. Based on the molecular targets and chemical structures of IC9564 and DSB, we synthesized a class of compounds possessing both of these structural features, which resulted in an anti-HIV-1 profile superior to those of IC9564 and DSB. N-[3-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oyl]leucine (LH15) and N-[3-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oyl]-11-aminoundecanoic acid (LH55) are two of these compounds with potent anti-HIV activity.
FIG. 1.
Chemical structures of anti-HIV-1 betulinic acid derivatives.
Synthesis and anti-HIV-1 activity of bifunctional small molecules LH15 and LH55.
The protocol used to synthesize LH15 and LH55 was modified from previously described procedures (4, 12). The antientry functional groups, leucine and aminoundecanoic acid methyl esters, were introduced into the backbone of betulinic acid at position 28. The resulting intermediates were refluxed with 2,2-dimethylsuccinic anhydride in the presence of pyridine and dimethylaminopyridine to introduce the antimaturation side chains at position 3. The final products were purified with high-performance liquid chromatography to yield LH15 and LH55. 1H nuclear magnetic resonance with signal assignment and mass spectrometry were performed to verify the structures.
The anti-HIV activity of these compounds was evaluated with an HIV-1 infectivity assay described previously (5). A diluted HIV-1 stock at a multiplicity of infection of 0.001 50% tissue culture infective dose per cell was used to infect MT4 cells in the presence of various concentrations of the compounds. The compounds IC9564 and DSB (4, 5) inhibit various HIV-1 isolates at submicromolar concentrations but not the protease inhibitor-resistant strain PI-R, which is less sensitive to DSB (Table 1). LH15 and LH55, in general, are at least a log more potent than either IC9564 or DSB. The results shown in Table 1 also demonstrate that LH15 and LH55 are potent inhibitors of the multiple-protease-inhibitor-resistant strain PI-R (2) and the multiple-reverse transcriptase (RT)-inhibitor-resistant strain RTI-R (10).
TABLE 1.
Anti-HIV activity of betulinic acid derivatives
| Virusa | IC50 of indicated compound (μM)b
|
|||
|---|---|---|---|---|
| DSB | IC9564 | LH55 | LH15 | |
| NL4-3 | 0.096 ± 0.012 | 0.11 ± 0.02 | 0.0065 ± 0.0008 | 0.016 ± 0.003 |
| PI-R | 1.71 ± 0.15 | 0.12 ± 0.03 | 0.032 ± 0.004 | 0.049 ± 0.005 |
| RTI-R | 0.085 ± 0.008 | 0.093 ± 0.016 | 0.01 ± 0.001 | 0.019 ± 0.003 |
PI-R is an HIV-1 strain, HIV-1M46I/L63P/V82P/I84V, resistant to multiple protease inhibitors (2). RTI-R is an HIV-1 strain, HIV-1RTMDR1/MT2 (RT 74V, 41L, 106A, 215Y), resistant to multiple HIV-1 reverse transcriptase inhibitors (10). PI-R and RTI-R were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program.
IC50 is the drug concentration required to inhibit 50% of HIV-1 replication. The highest concentration tested for these compounds was 5 μM; there was no observable cytotoxicity at this concentration. The numbers in the table represent the means ± the standard deviations of three independent experiments done in duplicate.
Inhibition of HIV-1 entry.
The side chain at position 28 is critical for the antifusion activity of the betulinic acid derivatives. Betulinic acid derivatives with the same side chains as LH15 and LH55 at position 28 but without a functional side chain at position 3 exhibit antifusion activity (12). Figure 2a shows that the potency of LH55 against NL4-3 envelope-induced membrane fusion is similar to that of IC9564 and T20. DSB, lacking a side chain at position 28, does not significantly affect the envelope-mediated membrane fusion. It has previously been demonstrated that the key determinant for IC9564 sensitivity is gp120 (5). However, the detailed mechanism of fusion inhibition remains unclear. IC9564 does not affect CD4-gp120 interaction. The binding of gp120 to CD4 and the subsequent interaction with chemokine receptors are two critical fusion events for HIV-1 entry. Thus, it is likely that the chemokine receptor interactive site on gp120 is a key determinant for the antifusion activity of IC9564.
FIG. 2.
(a) LH15 and LH55 inhibit HIV-1 entry. A fusion assay modified from a previously reported protocol (5) was used in this study. The fusions between COS cells expressing NL4-3 envelope glycoproteins and HeLa cells expressing CD4 and CXCR4 (1) were performed in the presence of the anti-HIV-1 betulinic acid derivatives and T20, as indicated in the figure. T20 is a peptide derived from gp41 of HIV-1. The fused syncytia were stained blue and enumerated as previously described (5, 15). Each data point in the figure represents the mean of three independent experiments. (b) An amino acid in the bridging sheet of gp120 determines the sensitivity of HIV-1 to IC9564. The same fusion assay described for Fig. 2a was used for the experiments. Each data point in the figure represents the mean of three independent experiments.
To test this hypothesis, we chose a pair of HIV-1 strains, NLDH120 and M2-NLDH, that exhibit significant differences in the accessibility of their chemokine receptor interactive sites (15). The two viruses differ in one amino acid at position 198 in the bridging sheet of gp120 (15). The envelope sequences of these two viruses were cloned into an expression vector, pSRHS, and used in an envelope-mediated membrane fusion assay (5). The fusion mediated by the M2-NLDH envelope, M2-pSW120, is approximately 10-fold more sensitive to IC9564 than that of the NLDH120 envelope, pSW120 (Fig. 2b). Since the bridging sheet is a critical structural motif involved in HIV-1 entry (9), the results strongly support the notion that the bridging sheet is involved in IC9564 sensitivity.
Interference with the processing of p25 resulted in an inhibition of HIV-1 maturation.
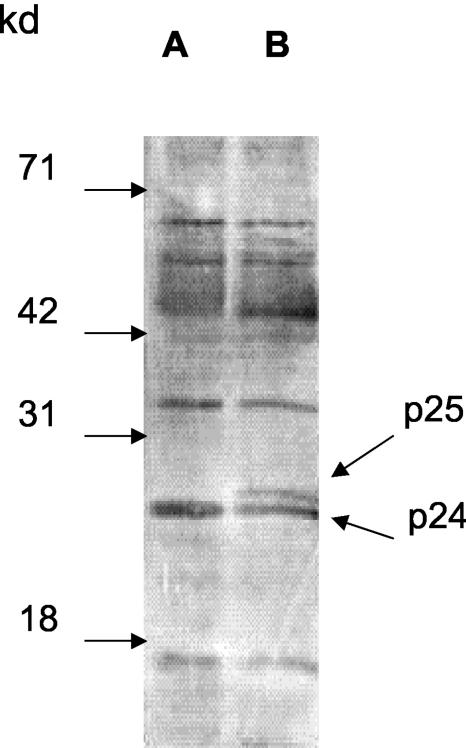
Based on our previous observation that DSB does not affect the production of HIV-1 viral particles (4), we speculated that DSB treatment might lead to the production of immature viral particles that have lost infectivity. In order to test this hypothesis, HIV-1 particles produced in the presence of LH55 were lysed and analyzed using Western blots. There was an accumulation of p25 in the virus produced in the presence of LH55 (Fig. 3). Accumulation of p25 was also observed in the virus particles produced in the presence of DSB (data not shown). The processing of p25 to p24 and p2 is the last step in sequential protease cleavage of Gag precursor into mature Gag proteins, which is critical for viral infectivity (3, 8, 13). The unique mode of action of DSB or LH55 is that these compounds affect the processing of only p25. The processing of other Gag proteins, such as p17, is not affected by these compounds (Fig. 3).
FIG. 3.
LH55 inhibits HIV-1 maturation. HIV-1 viral particles were produced from ACH-2 cells in the absence (A) or presence (B) of LH55. The virus particles were purified by ultracentrifugation using a sucrose gradient (14). The collected viral samples were lysed and analyzed using a sodium dodecyl sulfate-12.5% polyacrylamide gel followed by a Western blot using an HIV-1-positive human serum. kd, molecular mass standards in kilodaltons.
The key structural feature that enables LH55 and its analogs to possess the dual mode of action is the presence of both side chains at position 3 and position 28. The unique biological activity of these compounds is that they not only inhibit the viruses resistant to HIV-1 RT and protease inhibitors but also inhibit viruses that are resistant to compounds which bear the side chains only at position 3 or position 28 (data not shown). The bridging sheet of gp120 and the maturation of p24 are the critical determinants for drug sensitivity to this class of compounds. The antifusion activity of LH55 or LH15 allows the compound to block HIV-1 before it enters the cell. The virus that survives this blockage will have to face the antimaturation activity of these compounds. Since the molecular targets of these bifunctional anti-HIV betulinic acid derivatives are different from those of clinically available anti-HIV-1 drugs, it is possible that LH15, LH55, or their derivatives might have the potential to become useful additions to current anti-HIV therapy.
Acknowledgments
This work was supported by National Institutes of Health grant R01AI49096.
REFERENCES
- 1.Chackerian, B., E. M. Long, P. A. Luciw, and J. Overbaugh. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, D. Titus, T. Yang, H. Teppler, K. E. Squires, P. J. Deutsch, and E. A. Emini. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. [DOI] [PubMed] [Google Scholar]
- 3.Gottlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto, F., Y. Kashiwada, L. M. Cosentino, C. H. Chen, P. E. Garrett, and K. H. Lee. 1997. Anti-AIDS agents XXVII. Synthesis and anti-HIV activity of betulinic acid and dihydrobetulinic acid derivatives. Bioorg. Med. Chem. 5:2133-2143. [DOI] [PubMed] [Google Scholar]
- 5.Holz-Smith, S. L., I.-C. Sun, L. Jin, T. J. Matthews, K.-H. Lee, and C. H. Chen. 2001. Role of human immunodeficiency virus (HIV) type 1 envelope in the anti-HIV activity of the betulinic acid derivative IC9564. Antimicrob. Agents Chemother. 45:60-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, L., and C. H. Chen. 2002. Molecular target of anti-HIV-1 triterpene derivatives. Curr. Drug Targets Infect. Disord. 2:33-36. [DOI] [PubMed] [Google Scholar]
- 7.Kanamoto, T., Y. Kashiwada, K. Kanbara, K. Gotoh, M. Yoshimori, T. Goto, K. Sano, and H. Nakashima. 2001. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob. Agents Chemother. 45:1225-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krausslich, H.-G., M. Facke, A. M. Heuser, J. Konvalinka, and H. Zentgraf. 1995. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J. Virol. 69:3407-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larder, B. A., P. Kellam, and S. D. Kemp. 1993. Convergent combination therapy can select viable multidrug-resistant HIV-1 in vitro. Nature 365:451-453. [DOI] [PubMed] [Google Scholar]
- 11.Mayaux, J. F., A. Bousseau, R. Pauwels, T. Huet, Y. Henin, N. Dereu, M. Evers, F. Soler, C. Poujade, E. De Clercq, and J. P. Le Pecq. 1994. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 91:3564-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun, I. C., C. H. Chen, Y. Kashiwada, J. H. Wu, W. K. Wang, and K. H. Lee. 2002. Anti-AIDS agents 49. Synthesis, anti-HIV, and anti-fusion activities of IC9564 analogues based on betulinic acid. J. Med. Chem. 45:4271-4275. [DOI] [PubMed] [Google Scholar]
- 13.Wiegers, K., G. Rutter, H. Kottler, U. Tessmer, H. Hohenberg, and H. G. Krausslich. 1998. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J. Virol. 72:2846-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyma, D. J., A. Kotov, and C. Aiken. 2000. Evidence for a stable interaction of gp41 with Pr55Gag in immature human immunodeficiency virus type 1 particles. J. Virol. 74:9381-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, C. B., L. Zhu, S. Holz-Smith, T. J. Matthews, and C. H. Chen. 2001. The role of the third β-strand in gp120 conformation and neutralization sensitivity of the HIV-1 primary isolate DH012. Proc. Natl. Acad. Sci. USA 98:15227-15232. [DOI] [PMC free article] [PubMed] [Google Scholar]