Abstract
Polycystic ovary syndrome (PCOS) is a common lifestyle-related endocrinopathy in women of reproductive age and is associated with several mental health problems. We examined the genotypic distributions of IRS-1 Gly972Arg and CYP11B2 -344T/C, which were previously described as influencing PCOS, and assayed the serum levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), in a set of female patients with borderline personality disorder (BPD) with comorbid major depressive disorder (MDD) (n = 50) and age-matched control subjects (n = 100), to investigate the predisposition for BPD with MDD. The results showed that the patients were more frequently IRS-1 972Arg variant allele carriers (P = 0.013; OR 6.68; 95% CI = 1.30–34.43) and homozygous for the CYP11B2 −344C variant allele (P = 0.022; OR = 3.32; 95% CI = 1.18–9.35) than the control subjects. The IL-6 level was significantly higher in the patients than in the controls (P < 0.0001). There was no significant difference in the serum TNF-α level between patients with BPD with MDD and the healthy comparison group (P = 0.5273). In conclusion, the predisposition for BPD with MDD is associated with that for PCOS, in the female Japanese population. An elevated serum IL-6 level is considered to be a possible biomarker of BPD with MDD.
Keywords: borderline personality disorder, depression, polycystic ovary syndrome, genetic polymorphism, insulin resistance
Introduction
Borderline personality disorder (BPD) is a serious public health problem predominant in women, and is characterized by interpersonal stress, affective instability, impulsivity, stress-related dissociation, repeated self-mutational behavior, and chronic suicidal tendencies.1 BPD affects 1%–2% of the general population, and 41%–83% of patients with BPD report a history of major depressive disorder (MDD).1 The etiology of BPD is not yet clear, and relatively little data exist regarding biological causes compared with psychosocial ones, such as traumatic life events and the family environment in their childhood.2,3 Biological causes underlying BPD have been investigated mainly from the viewpoint of monoamine neurotransmission,4–6 although some metabolic and endocrinal disturbances also have been assessed to investigate the biological etiology involved. Dysregulation of the hypothalamic-pituitary axis, elevations in levels of pro-inflammatory cytokines (interleukin-6 [IL-6] and tumor necrosis factor-alpha [TNF-α]), cortisol, and cortisol-dehydroepiandrosterone ratio, low bone mineral density, reduced insulin sensitivity, and increased visceral fat deposition have been suggested as possible contributing etiological factors among female patients with BPD with MDD.7–10 There is also some support for a psychosocial cause of BPD with MDD; specifically, chronic family stress in childhood has been reported to contribute to adult metabolic function and atypical activity of the hypothalamicpituitary axis.11 However, previous reports also suggest that some forms of BPD with MDD are life-style related immuneendocrine disorders.7–10
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting up to 10% of women of reproductive age. PCOS is characterized by polycystic ovaries demonstrable by ultrasound, chronic anovulation, and reduced fertility. Several metabolic and endocrinal abnormalities such as a rapid luteinizing hormone pulse frequency, insulin resistance, hyperandrogenemia, and elevated levels of serum cytokines maintain the PCOS status.12,13 Above all, insulin resistance has been considered to be the most important etiology of the reproductive and metabolic abnormalities in PCOS.14 Accordingly, PCOS is a lifestyle-related endocrine disorder. PCOS has also been reported to be associated with several mental health problems such as depressive disorder, bipolar disorder, anxiety disorder, posttraumatic stress disorder, eating disorder, and female-to-male transsexuality.15–18 However, there is no consensus on the criteria for the diagnosis of PCOS. Most clinicians and researchers from the USA and from Southern Europe use the criteria derived from the conference held at the National Institute of Child Health and Human Development (NICHD) in 1990 such as clinical and/or biochemical hyperandrogenism, menstrual dysfunction, and exclusion of specific etiologies.19 According to these criteria, the presence of polycystic ovaries on ultrasound examination is not needed for the diagnosis. To diagnose as PCOS strictly is not easy even for specialists in the field of gynecology.
Our main a priori hypothesis was that the predisposition for BPD with MDD is associated with that for PCOS. The genetic correlates of BPD have been tested with regard to central nervous system monoamine transporters or receptors.20–23 However, the results are not yet definitive. Among the large number of genetic variants found in association with PCOS,24 insulin receptor substrate-1 (IRS-1) Gly972Arg and aldosterone synthetase (CYP11B2) -344T/C have been reported to influence PCOS in East-Asian populations.25,26 In order to investigate the predisposition to BPD with MDD, we evaluated its possible association with these two genomic variants, and assessed the influence of this predisposition on the serum concentrations of IL-6 and TNF-α.
Material and methods
Subjects
A total of 50 Japanese female patients suffering from BPD with MDD (age, 27.4 ± 4.8 years [mean ± SD; range, 19–40 years]) participated. All met the DSM-IV diagnostic criteria for BPD with current or lifetime major depressive episode, and had been consecutively treated at Tokyo Jikei University Hospital, Iwate Medical University Hospital, and Mizusawa General Hospital. Nineteen (age, 27.2 ± 5.0 years [mean ± SD; range, 20–35 years]) of these 50 patients had a current major depressive episode, and the remaining 31 (age, 27.5 ± 4.7 years [mean ± SD; range, 19–40 years]) had a lifetime history of major depressive disorder but no current major depressive episode. One hundred age-matched mentally healthy Japanese women (age, 27.6 ± 4.8 years [mean ± SD; range, 20–40 years]) were recruited as the comparison group.
Diagnoses in the patient group and exclusion of psychiatric disorders in the comparison group were determined by using the Japanese versions of the Structured Clinical Interview for DSM-IV (SCID) and the SCID for Personality Disorders. Patients with current or lifetime anorexia nervosa and schizophrenia, current oligophrenia, pregnancy, estrogen deficiency, amenorrhea, infectious or auto-inflammatory disease, and aged 17 years or younger were excluded. All interviews were carried out by six trained and experienced clinicians.
The Ethics Committee on Genetics at each participating institution approved the present study, and the investigation was performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants before personal data and blood samples were collected.
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the NucleoMag 96 Blood (Macherey–Nagel, Düren, Germany) with the Biomek 3000 Laboratory Automation System (Beckman Coulter, Fullerton, CA) in accordance with the manufacturers’ instructions. Two single- nucleotide polymorphisms (SNPs), IRS-1 Gly972Arg (rs1801278) and CYP11B2 −344T/C (rs1799998), which had been reported to influence PCOS in East-Asian populations,23,24 were selected.
Each SNP was genotyped by TaqMan® SNP Genotyping Assays (Applied Biosystems, Foster City, CA) using Real- Time allelic discrimination on an ABI 7300 Real-Time polymerase chain reaction (PCR) System (Applied Biosystems, Foster City, CA). Briefly, a fluorogenic probe consisting of an oligonucleotide labeled with both a fluorescent reporter dye and a quencher dye was included in a typical PCR. Amplification of the probe-specific product caused cleavage of the probe, generating an increase in reporter fluorescence. By using two different reporter dyes (5-carboxyfluorescein [FAM] or VIC), cleavage of allele-specific probes were detectable in a single PCR. The reaction mixture contained 1 μL of genomic DNA, 0.625 μL of 40× SNP Genotyping Assay Mix (Applied Biosystems, Foster City, CA), 12.5 μL of TaqMan® Universal PCR Master Mix, No AmpErase® UNG (Applied Biosystems, Foster City, CA), and 10.875 μL of distilled water in a total volume of 25 μL. A negative control (no-template control) was included in every assay. The amplification protocol was provided by the manufacturer and entailed an initial step at 50°C for 2 minutes and then 95°C for 10 minutes, followed by 40 cycles of denaturation at 92°C for 15 seconds and one annealing/extension step at 58°C for 1 minute.
Cytokine assays
Blood was drawn into a serum-separator collection tube and centrifuged for 10 minutes at 2500 rpm: the serum was then removed and stored at −80°C until assay. The Bio-Plex Human Cytokine 2-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile the expression of IL-6 and TNF-α. The assay was performed in accordance with the manufacturer’s instructions. Briefly, serum samples were thawed and then centrifuged at 4500 rpm for 3 minutes at 4°C. Serum samples were then incubated with micro-beads labeled with specific antibodies against one of the aforementioned cytokines for 30 minutes. After a washing step, the beads were incubated with the detection antibody cocktail, each antibody specific to a single cytokine. After another washing step, the beads were incubated with streptavidin-phycoerythrin for 10 minutes and washed, and the concentration of each cytokine was determined using the array reader. All samples were assayed in duplicate.
Statistical analysis
Fisher’s exact probability test was used to examine the association between BPD with MDD and the genotypes of the two SNPs. Hardy–Weinberg equilibrium analyses were used to compare the observed and expected genotype frequencies using χ2-test. We also performed logistic regression analysis to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) associated with the genotypes. Bartlett’s test revealed that the measured serum concentrations of IL-6 and TNF-α in the present study population did not have a normal distribution. Therefore, statistical analyses of differences in serum cytokine levels between participants in the BPD with MDD group and the healthy comparison group were performed by Mann–Whitney U test, and among the two patient groups (BPD plus current major depressive episode and BPD plus lifetime major depressive episode) and the healthy comparison group by the Kruskal–Wallis test followed by the Scheffé F-test as a post hoc test.
Differences were considered statistically significant at P < 0.05. All analyses were conducted using SPSS 11.0J for Windows (SPSS Inc, Chicago, IL).
Results
Concentrations of serum cytokines in BPD with MDD
Concentrations of IL-6 and TNF-α were compared between 50 Japanese women suffering from BPD with MDD and 100 healthy controls (Table 1). Bartlett’s test revealed that both the serum IL-6 and TNF-α concentration in the present study population did not have a normal distribution.
Table 1.
Sociodemographic data and serum cytokine levels in 50 female patients with borderline personality disorder with comorbid major depressive disorder and 100 comparison subjects
| Group | Age (years) | IL-6 (pg/mL) | TNF-α (pg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | SEM | Mean | SD | SEM | |
| Comparison group (n = 100) | 27.6 | 4.8 | 0.893 | 1.132 | 0.113 | 1.332 | 2.184 | 0.218 |
| Patients with borderline personality disorder with comorbid major depressive disorder | ||||||||
| BPD plus lifetime major depressive disorder (n = 31) | 27.5 | 4.7 | 7.769* | 18.10 | 3.251 | 5.184 | 22.06 | 3.961 |
| BPD plus current major depressive episode (n = 19) | 27.2 | 5.0 | 2.432 | 2.688 | 0.617 | 0.989 | 0.858 | 0.197 |
Note: P = 0.0004 versus the comparison group.
Abbreviations: BPD, borderline personality disorder; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; SD, standard deviation; SEM, standard error of the mean.
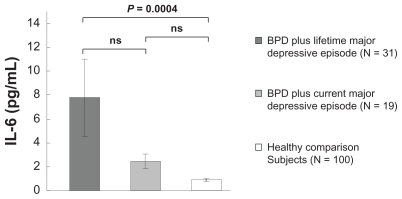
A Mann–Whitney U test revealed that the serum IL-6 level was significantly higher within the sample of patients with BPD with MDD than within the healthy comparison group (P < 0.0001). Kruskal–Wallis tests demonstrated significant differences in serum IL-6 levels among the two patient groups and the healthy comparison group (P < 0.0001). The Scheffé F-test used as a post hoc test showed that the serum IL-6 level was significantly higher in patients with BPD plus lifetime major depressive episode than in the healthy comparison group (P = 0.0004), but there was no significant difference in the concentration of IL-6 between patients with BPD plus current major depressive episode and the healthy comparison group (P = 0.7595). There was also no significant difference in the serum concentration of IL-6 between patients with BPD plus current major depressive episode and those with BPD plus lifetime major depressive episode (P = 0.0902) (Figure 1).
Figure 1.
IL-6 was significantly higher in patients with BPD plus lifetime major depressive disorder than in the healthy comparison group (P = 0.0004), but there was no significant difference in the concentration of IL-6 between patients with BPD plus current major depressive disorder and the healthy comparison group.
Note: Error bar: ±standard error of the mean.
Abbreviations: BPD, borderline personality disorder; IL-6, interleukin-6; ns, no significant difference.
There was no significant difference in the serum TNF-α level either between patients with BPD with MDD and the healthy comparison group (P = 0.5273, Mann–Whitney U test), or among the two patient groups and the healthy comparison group (P = 0.7515, Kruskal–Wallis test).
Distributions of genomic variants associated with PCOS
The frequencies of the IRS-1 and CYP11B2 genotypes were compared between 50 Japanese patients suffering from BPD with MDD and 100 healthy controls (Table 2). The distributions of genotypes in all groups were consistent with the Hardy–Weinberg equilibrium. Six (12%) BPD with MDD patients were heterozygous for the 972Arg variant of IRS-1, as compared with two (2%) heterozygous variant controls. Eighteen (36%) BPD with MDD patients were heterozygous for the −344C variant of CYP11B2 and ten (20%) were homozygous, as compared with 44 (44%) heterozygous and seven (7%) homozygous variant controls. The frequency of the IRS-1 972Arg variant allele was significantly higher among women with BPD with MDD than among the controls (P = 0.018), and the frequency of the CYP11B2-344C variant allele showed a P value close to 0.1 (P = 0.118). A logistic regression model retained women carrying the IRS-1 972Arg variant allele (OR = 6.68; 95% CI = 1.30–34.43; P = 0.013) and homozygous for the CYP11B2 −344C variant allele (OR = 3.32; 95% CI = 1.18–9.35; P = 0.022) for prediction of BPD with MDD (Table 2).
Table 2.
Distribution of IRS-1 and SYP11B2 genotypes among 50 patients with borderline personality disorder with comorbid major depressive disorder and 100 normal comparison subjects
| Genotype | BPD with MDD | Comparison group | ORa (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| IRS-1 | ||||||
| G ly/Gly | 44 | 88 | 98 | 98 | – | – |
| G ly/Arg | 6 | 12 | 2 | 2 | 6.68 (1.30–34.43) | 0.013 |
| CYP11B2 | ||||||
| −344T/T | 22 | 44 | 49 | 49 | – | – |
| −344T/C | 18 | 36 | 44 | 44 | – | – |
| −344C/C | 10 | 20 | 7 | 7 | 3.32 (1.18–9.35) | 0.022 |
Note: Logistic regression analysis.
Abbreviations: OR, odds ratio; CI, confidence interval; BPD, borderline personality disorder; MDD, major depressive disorder.
The genotypes did not affect the serum concentration of IL-6 or TNF-α. (data not shown).
Discussion
The present study demonstrated that serum IL-6 levels were significantly higher in women with BPD with MDD than that in healthy controls (P < 0.0001). The level of IL-6 increases with increasing glucose intolerance,27 and an elevated serum level of IL-6 in patients suffering from BPD with MDD is reportedly correlated with reduced insulin sensitivity.9 Although indices of insulin resistance were not investigated in the present study, we wondered whether there could be an association between insulin resistance and BPD with MDD because a previous study had observed a correlation between reduced insulin sensitivity and serum concentrations of IL-6 in patients with BPD with MDD.9 In this and other previous studies, it was also suggested that increased pro-inflammatory cytokines in BPD with MDD were related to the presence of MDD.8–10 However, our results suggested a different interpretation. We observed that as compared with healthy controls, IL-6 levels were higher in the case of BPD with lifetime MDD (Figure 1) and not in BPD with current MDD as the above studies had found. These results suggest a possible association between elevation of the IL-6 level and personality disorder in some forms of BPD with MDD, rather than major depressive episode. Although elevation of the serum IL-6 level is a non-specific feature, it is nevertheless considered to be a possible biomarker of BPD with MDD.
Here, we found no significant difference in the concentration of TNF-α between women suffering from BPD with MDD and the healthy comparison group. However, other researchers found that TNF-α levels were increased in patients with BPD with MDD.8–10 Although its levels are reportedly increased in individuals with adiposity and/or insulin resistance,28,29 a considerable number of investigators have failed to observe an increased TNF-α level in obese, compared with lean, individuals.30–32 Also, it has been reported that the activity of the TNF-α system is reflected by the levels of soluble TNF-α receptors rather than by TNF-α expression.32 Although it is unclear why there should be a dissociation between IL-6 and TNF-α in our study, soluble TNF receptors could possibly account for the normal TNF-α expression and elevated IL-6 level in BPD with MDD in the present study.
In the present study population, BPD with MDD patients were more frequently two genotypes of IRS-1 and CYP11B2, which are reportedly associated with PCOS in East-Asian populations,25,26 compared to the healthy controls (Table 2). Although the limitations of this study are largely attributable to the nature of association studies, including a small patient sample size, and the indices of insulin resistance and endocrinal disturbance such as luteinizing hormone/follicle stimulating hormone ratio and serum androgen were not investigated, BPD with MDD was considered to overlap with PCOS in the genetic backgrounds of this Japanese population. The most important “upstream” driver for reproductive and metabolic abnormalities in PCOS is reportedly insulin resistance, and thus insulin sensitizing agents, such as thiazolidinediones and metformin, are useful for treatment.14 Moreover, an elevated serum aldosterone level is reportedly related to insulin resistance in women with PCOS.33 Both IRS-1 and CYP11B2 were previously described to be associated with insulin resistance,34,35 and have been identified in the central nervous system (CNS).
Insulin receptor substrate-1 (IRS-1) occupies a key position in the insulin signaling pathway. After insulin binding to the α-subunit of the insulin receptor, the β-subunit of the receptor undergoes autophosphorylation, and in turn, phosphorylates other endogenous protein substrates in the insulin cascade. IRS-1 is the first direct substrate for the insulin receptor kinase.36,37 IRS-1 Gly972Arg is a genetic variant of glycine (Gly) to arginine (Arg) change at codon 972 in IRS-1. IRS-1 972Arg protein has been reported to act as a competitive inhibitor of insulin receptor autophosphorylation and to allow IRS-1 to act as an inhibitor of the insulin receptor kinase, leading to insulin resistance.38 Actually, carriers of this variant have a 25% increased risk for developing Type 2 diabetes.39 IRS-1 is widely distributed in the CNS, and is particularly abundant in the cerebral cortex, the hippocampus, many hypothalamic and thalamic nuclei, the basal ganglia, the cerebellar cortex, and the brainstem nuclei.40
CYP11B2 -344T/C is a genetic variant in a putative binding site for the steroidogenic factor-1 in the 5′ transcriptional regulatory region (−344 thymidine/cytosine). Homozygosity for the −344C variant of CYP11B2 has been reported to increase the aldosterone-to-renin ratio.41 Aldosterone decreases IRS-1 expression and suppresses insulin signaling,42 and clinical reports have indicated that patients with primary aldosteronism commonly have impaired glucose tolerance.43 It is known that the CNS produces aldosterone. CYP11B2 mRNA has been identified in whole rat brain homogenates and in the cerebellum, hippocampus, hypothalamus, cerebral cortex and amygdala.44 It has also been detected in the human brain, although its expression is much lower than in the adrenal.45
Accordingly, both the IRS-1 972Arg and CYP11B2 −344C alleles are considered to reduce insulin sensitivity in the CNS. The brain is an insulin-responsive organ and reduced CNS insulin signaling leads to disturbances of fat and glucose metabolism.46 Although it remains unclear whether insulin plays a specific neurotransmitter or metabolic role in the brain, insulin receptors are widespread throughout the brain with dendritic fields receiving rich synaptic input to regulate at least energy disposal, fuel metabolism, appetite, autonomic function, cognition, and reproduction.47–49 Insulin can down-regulate the function and synthesis of the re-uptake transporter for norepinephrine, and can up-regulate those for dopamine.50 Therefore, insulin resistance can interfere with catecholamine clearance in the CNS, and theoretically this could lead to emotional instability. Possible evidence for this is given in the next paragraph.
Although little is known about its etiology, many reports have demonstrated the hypothalamic origin of PCOS.51–53 Both IRS-1 and CYP11B2 are distributed in the hypothalamic area and can cause insulin resistance, as described previously. Disturbances or functional alterations in the hypothalamus and/or its interactions can cause personality changes, lack of restraint and inhibitions, and endocrine dysfunction.54 Several reports have suggested an association between insulin resistance and behavioral-pathological problems. Amelioration of insulin resistance by arm-cutting behavior in depressed adolescents,55 reduced glucose metabolism in the temporo-parietal cortices of women with BPD,56 and the effectiveness of omega-3 fatty acid treatment for BPD57 have been reported. We have also reported a case of conduct disorder in an adolescent girl who was treated with an insulin sensitizing agent, pioglitazone.58 From our present findings and previous reports, we suppose that some forms of BPD with MDD are a diencephalic disorder54 originating from insulin resistance in the CNS.
Insulin sensitizing agents and aldosterone blockers are effective for attenuating insulin resistance associated with the roles of IRS-1 or CYP11B2. The IRS-1 972Arg variant impairs insulin signaling, and treatment with insulin sensitizing agents ameliorates this condition. Pioglitazone, a thiazolidinedione, restores normal differentiation of adipocytes and insulin signaling defect induced by IRS-1 Gly972Arg variant, and the variant modulates the response to metformin therapy in women with PCOS.59–61 Moreover, treatment with the selective mineralocorticoid receptor antagonist eplerenone has been reported to suppress aldosterone and attenuate aldosterone-induced degeneration of IRS-1.42 Therefore, insulin sensitizing agents (eg, metformin, thiazolidinediones) and selective mineralocorticoid receptor antagonists (eg, eplerenone) might have therapeutic potential for individuals with a predisposition to some forms of BPD with MDD.
Our study had several limitations. Despite the results of genetic analysis seeming to support the involvement of IRS-1 and SYP11B2 in BPD with MDD, the possibility of false-positive findings cannot be excluded because of the small sample size. In addition, metabolic and endocrinal disturbances and indices of obesity in the participants were not investigated in the present study. Thus, our findings need to be confirmed in larger Japanese case control populations where metabolic and endocrinal disturbances associated with PCOS are investigated. Nevertheless, this is the first study to examine the relationship between PCOS associated polymorphisms and BPD with MDD.
Conclusion
In conclusion, the predisposition for BPD with MDD is associated with that for PCOS, in the female Japanese population. An elevated serum IL-6 level is considered to be a possible biomarker of BPD with MDD, and to be associated with personality disorder rather than major depression. The remaining challenge is to investigate the hypothalamus-pituitary-ovarian axis dysregulation in female patients with BPD with MDD and its symptomatic relevance to the menstrual cycle.
Acknowledgments
This research was supported by the “Open Research Center Project” for Private Universities; matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology, Japan), 2004–2008; and research funding from Iwate Medical University.
Footnotes
Disclosure
The authors declare that they have no conflicts of interest in relation to the subject of this manuscript.
References
- 1.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364(9432):453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 2.Katerndahl D, Burge S, Kellogg N. Predictors of development of adult psychopathology in female victims of childhood sexual abuse. J Nerv Ment Dis. 2005;193(4):258–264. doi: 10.1097/01.nmd.0000158362.16452.2e. [DOI] [PubMed] [Google Scholar]
- 3.Bandelow B, Krause J, Wedekind D, Broocks A, Hajak G, Ruther E. Early traumatic life events, parental attitudes, family history, and birth risk factors in patients with borderline personality disorder and healthy controls. Psychiatry Res. 2005;134(2):169–179. doi: 10.1016/j.psychres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Coccaro EF, Lee R, McCloskey M. Norepinephrine function in personality disorder: plasma free MHPG correlates inversely with life history of aggression. CNS Spectr. 2003;8(10):731–736. doi: 10.1017/s1092852900019106. [DOI] [PubMed] [Google Scholar]
- 5.Hansenne M, Pitchot W, Pinto E, et al. 5-HT1A dysfunction in borderline personality disorder. Psychol Med. 2002;32(5):935–941. doi: 10.1017/s0033291702005445. [DOI] [PubMed] [Google Scholar]
- 6.Koch W, Schaaff N, Popperl G, et al. [I-123] ADAM and SPECT in patients with borderline personality disorder and healthy control subjects. J Psychiatry Neurosci. 2007;32(4):234–240. [PMC free article] [PubMed] [Google Scholar]
- 7.Schweitzer I, Tuckwell V, Maguire K, Tiller J. Personality pathology, depression and HPA axis functioning. Hum Psychopharmacol. 2001;16(4):303–308. doi: 10.1002/hup.297. [DOI] [PubMed] [Google Scholar]
- 8.Kahl KG, Rudolf S, Stoeckelhuber BM, et al. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am J Psychiatry. 2005;162(1):168–174. doi: 10.1176/appi.ajp.162.1.168. [DOI] [PubMed] [Google Scholar]
- 9.Kahl KG, Bester M, Greggersen W, et al. Visceral fat deposition and insulin sensitivity in depressed women with and without comorbid borderline personality disorder. Psychosom Med. 2005;67(3):407–412. doi: 10.1097/01.psy.0000160458.95955.f4. [DOI] [PubMed] [Google Scholar]
- 10.Kahl KG, Bens S, Ziegler K, et al. Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry. 2006;59(7):667–671. doi: 10.1016/j.biopsych.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosom Med. 2005;67(6):846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- 12.Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- 13.Tarkun I, Cetinarslan B, Turemen E, Canturk Z, Biyikli M. Association between circulating tumor necrosis factor-alpha, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metab Syndr Relat Disord. 2006;4(2):122–128. doi: 10.1089/met.2006.4.122. [DOI] [PubMed] [Google Scholar]
- 14.Palomba S, Falbo A, Orio F, Zullo F. Insulin-sensitizing agents and reproductive function in polycystic ovary syndrome patients. Curr Opin Obstet Gynecol. 2008;20(4):364–373. doi: 10.1097/GCO.0b013e328307ebc5. [DOI] [PubMed] [Google Scholar]
- 15.Baba T, Endo T, Honnma H, et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007;22(4):1011–1016. doi: 10.1093/humrep/del474. [DOI] [PubMed] [Google Scholar]
- 16.Dobie DJ, Kivlahan DR, Maynard C, et al. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- 17.Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv. 2006;61(11):723–732. doi: 10.1097/01.ogx.0000243772.33357.84. [DOI] [PubMed] [Google Scholar]
- 18.Klipstein KG, Goldberg JF. Screening for bipolar disorder in women with polycystic ovary syndrome: a pilot study. J Affect Disord. 2006;91(2–3):205–209. doi: 10.1016/j.jad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 20.Joyce PR, McHugh PC, McKenzie JM, et al. A dopamine transporter polymorphism is a risk factor for borderline personality disorder in depressed patients. Psychol Med. 2006;36(6):807–813. doi: 10.1017/S0033291706007288. [DOI] [PubMed] [Google Scholar]
- 21.Ni X, Sicard T, Bulgin N, et al. Monoamine oxidase a gene is associated with borderline personality disorder. Psychiatr Genet. 2007;17(3):153–157. doi: 10.1097/YPG.0b013e328016831c. [DOI] [PubMed] [Google Scholar]
- 22.Tadic A, Victor A, Baskaya O, et al. Interaction between gene variants of the serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT) in borderline personality disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(4):489–495. doi: 10.1002/ajmg.b.30843. [DOI] [PubMed] [Google Scholar]
- 23.Zaboli G, Gizatullin R, Nilsonne A, et al. Tryptophan hydroxylase- 1 gene variants associate with a group of suicidal borderline women. Neuropsychopharmacology. 2006;31(9):1982–1990. doi: 10.1038/sj.npp.1301046. [DOI] [PubMed] [Google Scholar]
- 24.Escobar-Morreale HF, Luque-Ramirez M, San Millán JL. The molecular- genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26(2):251–282. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- 25.Baba T, Endo T, Sata F, et al. Polycystic ovary syndrome is associated with genetic polymorphism in the insulin signaling gene IRS-1 but not ENPP1 in a Japanese population. Life Sci. 2007;81(10):850–854. doi: 10.1016/j.lfs.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhao SP, Tang XM, Shao DH, Dai HY, Dai SZ. Association study between a polymorphism of aldosterone synthetase gene and the pathogenesis of polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2003;38(2):94–97. [PubMed] [Google Scholar]
- 27.Deepa R, Velmurugan K, Arvind K, et al. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance – the Chennai Urban Rural Epidemiology Study (CURES) Metabolism. 2006;55(9):1232–1238. doi: 10.1016/j.metabol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Corica F, Allegra A, Corsonello A, et al. Relationship between plasma leptin levels and the tumor necrosis factor-alpha system in obese subjects. Int J Obes Relat Metab Disord. 1999;23(4):355–360. doi: 10.1038/sj.ijo.0800826. [DOI] [PubMed] [Google Scholar]
- 29.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauner H, Bender M, Haastert B, Hube F. Plasma concentrations of soluble TNF-alpha receptors in obese subjects. Int J Obes Relat Metab Disord. 1998;22(12):1239–1243. doi: 10.1038/sj.ijo.0800773. [DOI] [PubMed] [Google Scholar]
- 31.Hube F, Birgel M, Lee YM, Hauner H. Expression pattern of tumor necrosis factor receptors in subcutaneous and omental human adipose tissue: role of obesity and non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1999;29(8):672–678. doi: 10.1046/j.1365-2362.1999.00520.x. [DOI] [PubMed] [Google Scholar]
- 32.Bullo M, Garcia-Lorda P, Salas-Salvado J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol. 2002;146(3):325–331. doi: 10.1530/eje.0.1460325. [DOI] [PubMed] [Google Scholar]
- 33.Cascella T, Palomba S, Tauchmanova L, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91(11):4395–4400. doi: 10.1210/jc.2006-0399. [DOI] [PubMed] [Google Scholar]
- 34.Almind K, Inoue G, Pedersen O, Kahn CR. A common amino acid polymorphism in insulin receptor substrate-1 causes impaired insulin signaling. Evidence from transfection studies. J Clin Invest. 1996;97(11):2569–2575. doi: 10.1172/JCI118705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo P, Lauria F, Loguercio M, et al. −344C/T Variant in the promoter of the aldosterone synthase gene (CYP11B2) is associated with meta-bolic syndrome in men. Am J Hypertens. 2007;20(2):218–222. doi: 10.1016/j.amjhyper.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Sun XJ, Rothenberg P, Kahn CR, et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 37.White MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- 38.McGettrick AJ, Feener EP, Kahn CR. Human insulin receptor substrate- 1 (IRS-1) polymorphism G972R causes IRS-1 to associate with the insulin receptor and inhibit receptor autophosphorylation. J Biol Chem. 2005;280(8):6441–6446. doi: 10.1074/jbc.M412300200. [DOI] [PubMed] [Google Scholar]
- 39.Jellema A, Zeegers MP, Feskens EJ, Dagnelie PC, Mensink RP. Gly972Arg variant in the insulin receptor substrate-1 gene and association with Type 2 diabetes: a meta-analysis of 27 studies. Diabetologia. 2003;46(7):990–995. doi: 10.1007/s00125-003-1126-4. [DOI] [PubMed] [Google Scholar]
- 40.Folli F, Bonfanti L, Renard E, Kahn CR, Merighi A. Insulin receptor substrate-1 (IRS-1) distribution in the rat central nervous system. J Neurosci. 1994;14(11 Pt 1):6412–6422. doi: 10.1523/JNEUROSCI.14-11-06412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim PO, Macdonald TM, Holloway C, et al. Variation at the aldosterone synthase (CYP11B2) locus contributes to hypertension in subjects with a raised aldosterone-to-renin ratio. J Clin Endocrinol Metab. 2002;87(9):4398–4402. doi: 10.1210/jc.2001-012070. [DOI] [PubMed] [Google Scholar]
- 42.Hitomi H, Kiyomoto H, Nishiyama A, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50(4):750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 43.Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965;273(21):1135–1143. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Sanchez CE, Zhou MY, Cozza EN, et al. Aldosterone biosynthesis in the rat brain. Endocrinology. 1997;138(8):3369–3373. doi: 10.1210/endo.138.8.5326. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Romero DG, Gomez-Sanchez CE, Gomez-Sanchez EP. Steroidogenic enzyme gene expression in the human brain. Mol Cell Endocrinol. 2002;190(1–2):9–17. doi: 10.1016/s0303-7207(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 46.Koch L, Wunderlich FT, Seibler J, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118(6):2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 48.Gerozissis K. Brain insulin: regulation, mechanisms of action and functions. Cell Mol Neurobiol. 2003;23(1):1–25. doi: 10.1023/A:1022598900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werther GA, Hogg A, Oldfield BJ, et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121(4):1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 50.Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994;644(2):331–334. doi: 10.1016/0006-8993(94)91698-5. [DOI] [PubMed] [Google Scholar]
- 51.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. doi: 10.1210/jc.2004-2359. [DOI] [PubMed] [Google Scholar]
- 52.Hayden BJ, Balen AH. The role of the central nervous system in the pathogenesis of polycystic ovary syndrome. Minerva Ginecol. 2006;58(1):41–54. [PubMed] [Google Scholar]
- 53.Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(4):1394–1397. doi: 10.1210/jc.2007-1716. [DOI] [PubMed] [Google Scholar]
- 54.Campbell RJ. Campbell’s Psychiatric Dictionary. 7th ed. New York, Oxford; Oxford University Press; 1996. [Google Scholar]
- 55.Allen AD, Pitts FN, Jr, Allen RE. Arm cutting and glucose level in depression. Am J Psychiatry. 1989;146(5):680–681. doi: 10.1176/ajp.146.5.680a. [DOI] [PubMed] [Google Scholar]
- 56.Lange C, Kracht L, Herholz K, Sachsse U, Irle E. Reduced glucose metabolism in temporo-parietal cortices of women with borderline personality disorder. Psychiatry Res. 2005;139(2):115–126. doi: 10.1016/j.pscychresns.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Zanarini MC, Frankenburg FR. Omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160(1):167–169. doi: 10.1176/appi.ajp.160.1.167. [DOI] [PubMed] [Google Scholar]
- 58.Kawamura S, Sakai A, Endo T, Maruta M. Conduct disorder in an adolescent girl treated with an insulin-sensitizing agent. Psychiatry Clin Neurosci. 2008;62(6):750. doi: 10.1111/j.1440-1819.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 59.Ertunc D, Tok EC, Aktas A, Erdal EM, Dilek S. The importance of IRS-1 Gly972Arg polymorphism in evaluating the response to metformin treatment in polycystic ovary syndrome. Hum Reprod. 2005;20(5):1207–1212. doi: 10.1093/humrep/deh747. [DOI] [PubMed] [Google Scholar]
- 60.Sentinelli F, Filippi E, Cavallo MG, Romeo S, Fanelli M, Baroni MG. The G972R variant of the insulin receptor substrate-1 gene impairs insulin signaling and cell differentiation in 3T3L1 adipocytes; treatment with a PPARgamma agonist restores normal cell signaling and differentiation. J Endocrinol. 2006;188(2):271–285. doi: 10.1677/joe.1.06290. [DOI] [PubMed] [Google Scholar]
- 61.Yamada K, Yuan X, Ishiyama S, et al. Codon 972 polymorphism of the insulin receptor substrate-1 gene in impaired glucose tolerance and late-onset NIDDM. Diabetes Care. 1998;21(5):753–756. doi: 10.2337/diacare.21.5.753. [DOI] [PubMed] [Google Scholar]