Abstract
Serratia marcescens S-95, which displayed an unusually high degree of resistance to aminoglycosides, including kanamycins and gentamicins, was isolated in 2002 from a patient in Japan. The resistance was mediated by a large plasmid which was nonconjugative but transferable to an Escherichia coli recipient by transformation. The gene responsible for the aminoglycoside resistance was cloned and sequenced. The deduced amino acid sequence of the resistance gene shared 82% identity with RmtA, which was recently identified as 16S rRNA methylase conferring high-level aminoglycoside resistance in Pseudomonas aeruginosa. Histidine-tagged recombinant protein showed methylation activity against E. coli 16S rRNA. The novel aminoglycoside resistance gene was therefore designated rmtB. The genetic environment of rmtB was further investigated. The sequence immediately upstream of rmtB contained the right end of transposon Tn3, including blaTEM, while an open reading frame possibly encoding a transposase was identified downstream of the gene. This is the first report describing 16S rRNA methylase production in S. marcescens. The aminoglycoside resistance mechanism mediated by production of 16S rRNA methylase and subsequent ribosomal protection used to be confined to aminoglycoside-producing actinomycetes. However, it is now identified among pathogenic bacteria, including Enterobacteriaceae and P. aeruginosa in Japan. This is a cause for concern since other treatment options are often limited in patients requiring highly potent aminoglycosides such as amikacin and tobramycin.
Aminoglycoside antibiotics are widely used in clinical settings, especially for treatment of life-threatening infections caused by gram-negative bacteria. They bind to the highly conserved A-site of the 16S rRNA of the prokaryotic 30S ribosomal subunits, interfering with the protein synthesis with subsequent bacterial death (16). The most frequently encountered mechanism of resistance to aminoglycosides is their structural modification by specific enzymes produced by resistant organisms. The three classes of such enzymes are aminoglycoside acetyltransferases (AAC), aminoglycoside nucleotidyltransferases (ANT or AAD), and aminoglycoside phosphotransferases (APH) (20). Other mechanisms of resistance include ribosomal alterations, efflux of the agents by extrusion pump, or altered permeability leading to reduced uptake (3). While ribosomal protection by methylation of 16S rRNA in aminoglycoside-producing actinomycetes gives high-level resistance to intrinsic aminoglycosides, no clinical isolate with this mechanism has been found among nosocomial bacteria (5, 6).
However, a novel plasmid-mediated 16S rRNA methylase which conferred an extraordinarily high level of resistance to aminoglycosides was identified quite recently in a Pseudomonas aeruginosa clinical strain in Japan (23) and submitted to the DNA Data Bank of Japan in April 2002 (DDBJ accession no. AB083212). It was the first report of aminoglycoside resistance mediated by 16S rRNA methylase in gram-negative bacteria. Acquisition of such an efficacious resistance mechanism by P. aeruginosa was alarming, and there was concern about possible further dissemination of 16S rRNA methylase genes among P. aeruginosa and also into other gram-negative bacterial species (23).
Serratia marcescens S-95 isolated from an inpatient in Japan displayed a very high degree of resistance to many aminoglycosides, including arbekacin. This was an unusual observation, because arbekacin is generally stable under enzymatic modification, and only the bifunctional enzyme AAC(6′)-APH(2′′) is known to confer low-level resistance to arbekacin (15). Quite recently, another putative 16S rRNA methylase, ArmA, conferring high-level resistance to aminoglycosides, was found in a Klebsiella pneumoniae clinical isolate in France (10). The ArmA showed a moderate degree of similarity in amino acid sequence with some 16S rRNA methylases previously reported from actinomycetes. In the present study, we aimed to elucidate the mechanism of the high-level resistance to various aminoglycosides, including arbekacin, found in a clinically isolated S. marcescens strain.
MATERIALS AND METHODS
Clinical background.
S. marcescens S-95 was isolated in March 2002 from sputum of a 76-year-old male patient at a 500-bed general hospital in Japan. He was originally admitted due to cerebral hemorrhage, but the course had been complicated with subdural hematoma, bronchial asthma, pneumonia, and urinary tract infection. The patient had received panipenem, minocycline, vancomycin, and levofloxacin in the month before isolation of the strain.
Bacterial strains and plasmids.
The strains and plasmids used in the study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth or agar plates (BD Diagnostic Systems, Sparks, Md.) supplemented with appropriate antibiotics.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain | Plasmid | Characteristic(s) |
|---|---|---|
| S. marcescens S-95 | pKRC | Clinical isolate from sputum, Kochi, Japan |
| E. coli CSH2 | Resistant to rifampin and nalidixic acid | |
| E. coli XL1-Blue | supE44 recA1 endA1 gyrA96 thi hsdR17 (rK− mK+) relA1 lac [F′ proAB+lacIqZΔM15::Tn10(Tetr)] | |
| E. coli XL1-Blue | pKRC | Transformant |
| E. coli XL1-Blue | pBCSK+ | Chloramphenicol-resistant cloning vector |
| E. coli XL1-Blue | pS95B2 | Transformant containing a 4.6-kb BamHI fragment with rmtB ligated to pBCSK+ |
| E. coli XL1-Blue | pS95S8 | Transformant containing a 1.2-kb Sau3AI fragment with rmtB ligated to pBCSK+ |
| E. coli XL1-Blue | pET29a(+) | Kanamycin-resistant cloning-expression vector |
| E. coli BL21(DE3)pLysS | F−ompT hsdSB (rB− mB−) dcm gal, λ(DE3) pLysS Cmr | |
| E. coli BL21(DE3)pLysS | pS95H5 | Transformant containing a PCR-amplified rmtB ligated to pET29a(+) |
Antibiotics and susceptibility testing.
The following antibiotics were obtained from the indicated sources: amikacin, Bristol Pharmaceuticals Y. K., Tokyo, Japan; arbekacin, kanamycin, and streptomycin, Meiji Seika Kaisha Ltd., Tokyo, Japan; chloramphenicol, Sankyo Co., Ltd., Tokyo, Japan; gentamicin and sisomicin, Schering-Plough K. K., Osaka, Japan; hygromycin B, Sigma Aldrich Japan K. K., Tokyo, Japan; isepamicin, Asahi Kasei Corporation, Tokyo, Japan; neomycin, Nippon Kayaku Co., Ltd., Tokyo, Japan; rifampin, Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan; and tobramycin, Shionogi Pharmaceutical Co., Osaka, Japan.
MICs were determined by the agar dilution method using Mueller-Hinton agar (BD Diagnostic Systems) and according to the protocol recommended by the National Committee for Clinical Laboratory Standards (17).
Transfer of aminoglycoside resistance genes.
Conjugation experiments were conducted using Escherichia coli CSH2 as the recipient by broth mating and filter mating methods (7, 9). Transconjugants were selected on LB agar supplemented with rifampin (50 μg/ml), nalidixic acid (50 μg/ml), and kanamycin (25 μg/ml). Plasmid DNA of S. marcescens S-95 was purified by the method of Kado et al. (13). Transformation of E. coli XL1-Blue with the plasmid DNA of S. marcescens S-95 was performed using standard electroporation techniques. Transformants were selected on LB agar containing kanamycin (25 μg/ml).
Cloning and sequencing of aminoglycoside resistance genes.
The basic recombinant DNA techniques were carried out as described by Sambrook et al. (19). The plasmid DNA of S. marcescens S-95 was digested with BamHI, and the resultant fragments were ligated in plasmid vector pBCSK+ (Stratagene, La Jolla, Calif.). Electrocompetent E. coli XL1-Blue was then transformed with these recombinant plasmids. Transformants were selected by resistance to chloramphenicol (30 μg/ml) and kanamycin (25 μg/ml). The enzymes used for gene manipulation were purchased from New England Biolabs, Inc. (Beverly, Mass.) or Takara Bio Inc. (Otsu, Japan). The DNA sequences were determined on both strands using BigDye Terminator Cycle Sequencing Ready Reaction kits and an ABI 3100 DNA sequencer (Applied Biosystems, Foster City, Calif.). The alignments of nucleotide and amino acid sequences were performed with GENETYX-MAC (version 10.1.1; Software Development Co., Ltd., Tokyo, Japan).
Preparation of 30S ribosomal subunits.
30S ribosomal subunits of E. coli XL1-Blue were prepared as described by Skeggs et al. (21). After ultracentrifugation with sucrose density gradients, fractions corresponding to 30S ribosomal subunits were collected and concentrated by centrifugation with an Ultrafree-15 centrifugal filter device (Millipore Corporation, Bedford, Mass.). The purity of the 30S ribosomal subunits was checked by denatured agarose gel electrophoresis of 16S rRNA derived from the material, and the 30S ribosomal subunits were stored at −80°C in aliquots until use.
Expression and purification of histidine-tagged RmtB.
For use in methylation assays, RmtB was purified using a histidine-tag purification system. The entire coding region of rmtB was amplified by PCR with primers MBH-F (5′-GGAATTCCATATGAACATCAACGATGCCCT-3′) and MBH-R (5′-CCGCTCGAGTCCATTCTTTTTTATCAAGTA-3′). The product was partially double digested with NdeI and XhoI, and ligated to pET29a(+) (Novagen, Madison, Wis.) double digested with the same enzymes. Electrocompetent E. coli XL1-Blue was transformed with the recombinant plasmids, and transformants were selected on LB agar containing arbekacin (20 μg/ml). Several colonies obtained were found to harbor plasmids with inserts encoding RmtB tagged with six histidine residues at the C-terminal end. E. coli BL21(DE3)pLysS (Novagen) was transformed with one such plasmid, pS95H5. The transformants were cultured in 1 liter of LB broth supplemented with kanamycin (25 μg/ml) to an optical density (A620) of approximately 0.7. IPTG (isopropyl-d-thiogalactopyranoside) (0.5 mM) was then added to the culture, and a further 2-h incubation was conducted before harvesting. The pellet was washed once with 50 mM phosphate buffer (pH 7.0) and suspended in 20 mM phosphate buffer (pH 7.4) containing 10 mM imidazole. The suspension was passed through a French pressure cell (Ohtake Works Co., Ltd., Tokyo, Japan) at 120 MPa twice and then centrifuged at 30,000 × g for 30 min. Histidine-tagged RmtB contained in the supernatant was purified using HiTrap Chelating HP included in the HisTrap kit (Amersham Biosciences, K. K., Tokyo, Japan) according to the manufacturer's instructions. It was eluted at an imidazole concentration of 300 mM, and found to be over 95% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Finally, the enzyme was dialyzed at 4°C against 2 × 200 volumes of HRS buffer (10 mM HEPES-KOH, pH 7.5; 10 mM MgCl2; 50 mM NH4Cl; 3 mM 2-mercaptoethanol) and stored at −80°C in aliquots until use.
Methylation assay of 16S rRNA.
The reaction mixtures for methylation experiments contained 10 pmol of 30S ribosomal subunits from E. coli XL1-Blue, 10 pmol of histidine-tagged RmtB, and 2.5 μCi of [methyl-3H]S-adenosyl methionine (SAM) and were adjusted to 100 μl with methylation buffer (50 mM HEPES-KOH, pH 7.5; 7.5 mM MgCl2; 37.5 mM NH4Cl; 3 mM 2-mercaptoethanol). In control experiments, histidine-tagged RmtB was replaced by an equal volume of HRS buffer. The reactions were carried out at 35°C, and 30-μl aliquots of reaction mixtures were sampled at 0, 10, and 30 min, respectively. Each sample was purified immediately using an RNeasy Mini kit (QIAGEN K. K., Tokyo, Japan) according to the instructions provided by the manufacturer. The eluate (50 μl) containing purified 16S rRNA was spotted on DEAE Filtermat for MicroBeta (Perkin-Elmer Life Sciences Japan Co., Ltd., Tokyo, Japan). The filter mat was then covered with MeltiLex for MicroBeta filters (Perkin-Elmer) on a hot plate. Finally, it was applied to 1450 MicroBeta TRILUX (Perkin-Elmer), and the radioactivity of each spot was determined.
Nucleotide sequence accession number.
The entire nucleotide sequence containing rmtB and determined in this study appears in the EMBL/GenBank/DDBJ databases under accession number AB103506.
RESULTS
Aminoglycoside resistance of S. marcescens S-95.
The MICs of aminoglycosides for S. marcescens S-95 are listed in Table 2. S-95 showed a high level of resistance to kanamycin, tobramycin, amikacin, arbekacin, gentamicin, sisomicin, isepamicin, streptomycin (MIC, >1,024 μg/ml), and hygromycin B (MIC, 128 μg/ml), but not neomycin.
TABLE 2.
Results of antibiotic susceptibility testing
| Aminoglycoside | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| S. marcescens S-95 | E. coli XL1-Blue(pKRC) | E. coli XL1-Blue(pS95B2) | E. coli XL1-Blue(pS95S8) | E. coli XL1-Blue(pBCSK+) | |
| Kanamycin | >1,024 | >1,024 | >1,024 | >1,024 | 0.5 |
| Tobramycin | >1,024 | 1,024 | 64 | 128 | 0.25 |
| Amikacin | >1,024 | 1,024 | 1,024 | >1,024 | 0.5 |
| Arbekacin | >1,024 | 256 | 256 | 1,024 | 0.13 |
| Gentamicin | >1,024 | >1,024 | 1,024 | 1,024 | 0.13 |
| Sisomicin | >1,024 | >1,024 | 128 | 512 | 0.13 |
| Isepamicin | >1,024 | >1,024 | 1,024 | 1,024 | 0.25 |
| Neomycin | 2 | 0.5 | 0.5 | 0.5 | 0.5 |
| Hygromycin B | 128 | 16 | 8 | 16 | 16 |
| Streptomycin | 1,024 | 128 | 0.5 | 0.5 | 2 |
Transfer of aminoglycoside resistance.
The aminoglycoside resistance of S. marcescens S-95 could not be transferred to the recipient E. coli strain CSH2 by conjugation despite repeated attempts. However, the aminoglycoside resistance could be transferred to E. coli XL1-Blue by electroporation, and the resultant transformants harbored a large nonconjugative plasmid of the parental strain, which was designated pKRC.
Cloning of aminoglycoside resistance gene.
Competent cells of E. coli XL1-Blue were electrotransformed with recombinant plasmids of pBCSK+ carrying a BamHI-digested fragment of total DNA from S. marcescens S-95. Transformants obtained with selection by kanamycin and chloramphenicol were found to possess recombinant plasmids with a 4.6-kb BamHI insert. One such plasmid (pS95B2) was selected for further study. The 1.2-kb Sau3AI fragment was recloned with BamHI-cleaved pBCSK+, and the resultant recombinant plasmid was assigned pS95S8. The MICs of aminoglycosides for E. coli XL1-Blue(pKRC), XL1-Blue(pS95B2), and XL1-Blue(pS95S8) are listed in Table 2. The spectrum of resistance of XL1-Blue(pS95B2) included aminoglycosides belonging to the kanamycin and gentamicin groups, while the degree of resistance was generally lower than that of the parental strain. MICs of aminoglycosides for XL1-Blue(pS95S8) were generally higher than those for XL1-Blue(pS95B2), and this might due to probable multicopy effect of small plasmid. Both transformants were susceptible to streptomycin and neomycin. XL1-Blue(pKRC) carrying the large plasmid from S-95 was resistant to streptomycin as well. This streptomycin resistance was attributed to the presence of the integron-borne streptomycin resistance gene aadA2 on pKRC (data not shown).
DNA sequencing of pS95B2.
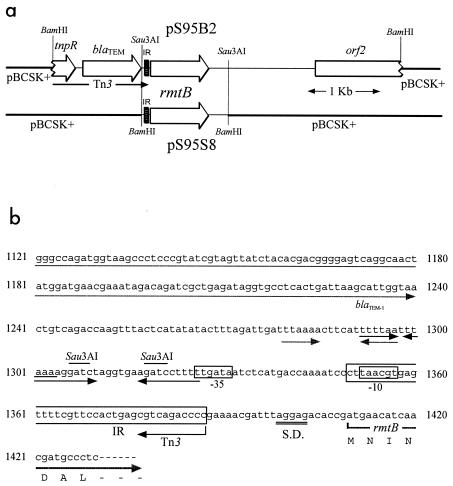
The entire 4.6-kb insert of pS95B2 was sequenced in the search for a kanamycin-gentamicin resistance determinant. The overall structure of the sequenced region is depicted in Fig. 1. The first 1.4 kb comprised the right end of Tn3 and included part of tnpR and blaTEM, ending with the right-hand inverted repeat (11). An open reading frame encoding 251 amino acids was located immediately downstream of the inverted repeat. It showed 82% amino acid identity with rmtA, which was recently reported as an aminoglycoside resistance gene encoding 16S rRNA methylase in a P. aeruginosa clinical isolate (23), and therefore was designated rmtB. A comparison of deduced amino acid sequences of RmtA and RmtB is shown in Fig. 2. The identity of amino acid residues between RmtB and ArmA was 29%. Identities with other 16S rRNA methylases produced by Streptomyces and Micromonospora species were generally lower. Amino acid identities of RmtB were 33 and 32% with GrmB and Sgm methylases of sisomicin-producing Micromonospora rosea and Micromonospora zionensis, respectively (14); 32% with GrmA methylase of gentamicin-producing Micromonospora purpurea (14); 31% with Kmr methylase of kanamycin-producing Streptomyces kanamyceticus (8); 30% with FmrO methylase of fortimicin-producing Micromonospora olivasterospora (18); and 27% with KgmB of nebramycin-producing Streptomyces tenebrarius (12). The putative promoter region of rmtB appeared to be located within the right-hand end of Tn3, just upstream of the inverted repeat (Fig. 1b). The nucleotide sequence upstream of rmtB shared no significant similarity with that of rmtA. On the other hand, the sequences downstream of the two genes showed 78% identity for approximately 0.8 kb and then diverged. The only other open reading frame identified was truncated at the end of the cloned insert. The available sequence indicated that it encoded at least 358 amino acids, which shared 99% identity with Orf2, a transposase-like protein of Salmonella enterica serovar Typhimurium (2), and 56% identity with Orf513, a putative transposase known to be associated with sul1-type complex integrons (9).
FIG. 1.
(a) Schematic presentation of the 4.6-kb BamHI fragment of pS95B2 and 1.2-kb Sau3AI fragment of pS95S8. Shaded boxes indicate terminal inverted repeats (IR) of Tn3. The 1.2-kb insert of pS95S8 carries only IR and the rmtB gene as well as its possible promoter. (b) Region upstream of the rmtB gene. The nucleotide sequence containing the inverted repeat (IR) of the transposon 3 (Tn3) and region upstream of the rmtB gene are shown. The open reading frame of blaTEM is terminated at 1,238TAA. Several dyad symmetries are indicated with horizontal arrows. Possible −35 and −10 regions are boxed. IR sequence of Tn3 is enclosed with an open oblong box. A Shine-Dalgarno-like sequence (S.D.) (1,399AGGAG) is located just upstream of the initiation codon (1410ATG) of the rmtB gene.
FIG. 2.
Alignment of the deduced amino acid sequence of RmtB with those of RmtA and ArmA. Asterisks indicate amino acid identities, and dots denote conserved replacements. Residues conserved among representative 16S rRNA methylases (GenBank accession no. AB083212, AB103506, AY220558, M55520, M55521, M87057, S60108, and Y15838) are underlined.
Methylation activity of RmtB.
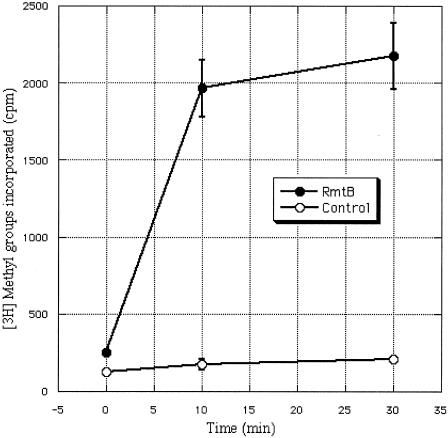
Histidine-tagged RmtB-producing E. coli XL1-Blue demonstrated a high-level resistance to arbekacin (MIC, >128 μg/ml), as well as to the other aminoglycosides (data not shown). Therefore, this recombinant protein was purified and used as the enzyme in the methylation assay. The result is depicted in Fig. 3. The vigorous incorporation of radiolabeled methyl groups into 16S rRNA of 30S ribosomal subunits from E. coli XL1-Blue in the presence of purified RmtB confirmed that RmtB was in fact a functional 16S rRNA methylase.
FIG. 3.
Methylation of 16S rRNA. The 16S rRNA from E. coli XL1-Blue was incubated with purified RmtB using [methyl-3H]SAM as a cofactor. The value of each point was calculated with three data points. Error bars, standard deviations.
DISCUSSION
Ribosomal protection by methylation of 16S rRNA has been known as a principal mechanism of aminoglycoside resistance among some aminoglycoside-producing organisms such as Streptomyces spp. and Micromonospora spp. Although production of such 16S rRNA methylases confers a very high level of aminoglycoside resistance to the producers, it had been thought that this mechanism was confined to environmental bacterial species without clinical relevance (5, 6).
This picture changed when a P. aeruginosa clinical strain AR-2 was found to produce 16S rRNA methylase, which conferred an extremely high level of resistance (MIC, >1,024 μg/ml) to a wide spectrum of aminoglycosides (23). The responsible gene, rmtA, was located on a self-transmissible plasmid, and therefore further dissemination of the gene among P. aeruginosa and other gram-negative bacteria was anticipated (23).
In fact, the present study identified the emergence of an S. marcescens clinical strain producing 16S rRNA methylase. This novel enzyme RmtB conferred high-level resistance to various aminoglycosides. The spectrum included 4,6-disubstituted deoxytreptamine aminoglycosides such as kanamycin, tobramycin, amikacin, arbekacin, gentamicin, sisomicin, and isepamicin. However, RmtB did not confer resistance to neomycin, streptomycin, and hygromycin B, all of which have different aminocyclitol components. This resistance pattern is consistent with that conferred by RmtA and includes most of the parenteral bactericidal aminoglycosides administered for serious infections caused by gram-negative bacteria (23).
RmtB shared 82% identity with RmtA of P. aeruginosa, while its similarity with the 16S rRNA methylases of the genera Streptomyces and Micromonospora was relatively low (up to 33%). As to the origin of the cluster of enzymes including RmtB and RmtA, at this stage we assume that the responsible genes have been mobilized to S. marcescens and P. aeruginosa independently from some yet unidentified aminoglycoside-producing bacterial species which are likely related to one another.
In previous studies, crude extracts of the 16S rRNA methylase-producing organisms were used as the enzyme for methylation assays (21, 22, 23). The incorporation rate of SAM was approximately twofold compared with controls in these reports. For improved specificity, we constructed histidine-tagged RmtB, which rendered its producer resistant to kanamycins and gentamicins. The protein was readily purified and subsequently used for methylation assay in place of crude enzyme. As a result, vigorous methylation of 16S rRNA could be observed, resulting in more than a 20-fold difference in the rate of incorporation of SAM between the RmtB-containing reaction mixtures and controls (Fig. 3).
16S rRNA methylases produced by aminoglycoside-producing actinomycetes are known to confer either a kanamycin-gentamicin resistance pattern or a kanamycin-apramycin resistance pattern (5). The MICs shown in Table 2 indicate that RmtB belongs to the former group of enzymes. GrmB produced by M. purpurea, which belongs to the kanamycin-gentamicin group, was previously shown to methylate G1405 within the A-site of 16S rRNA, resulting in resistance of the producer to kanamycin and gentamicin but not neomycin or apramycin (1). This methylation is known to prevent the formation of hydrogen bonds with ring III of gentamicin C1a, a major component of gentamicin which usually interacts with G1405 · C1496 and U1406 · U1495 base pairs (24). We may therefore speculate that RmtB methylates a nucleotide within the A-site in a similar fashion.
rmtB was carried on a large plasmid, pKRC, which was nonconjugative but transferable to E. coli by electroporation. The neighboring sequence of rmtB was interrupted only 22 bp upstream of the initiation codon in the presence of Tn3. However, the 0.8-kb region downstream of rmtB shared significant identity with the corresponding region of rmtA, thus reenforcing the idea that the two genes may have come from similar bacterial species. The mode of acquisition of rmtB by pKRC was not clear from the sequence information obtained in this study alone. This large plasmid also possessed an integron-mediated streptomycin resistance gene, aadA2, so the aminoglycoside-resistant phenotype seen in S-95 could be accounted for solely by the presence of pKRC. The integron is a well-recognized DNA recombination system that mediates the integration of antibiotic resistance genes through site-specific recombination (4). Future acquisition of additional antibiotic resistance genes by pKRC may well be possible.
Nosocomial bacteria producing RmtB or RmtA have been uniformly pan-resistant to 4,6-disubstituted deoxytreptamines, which cannot be accounted for by production of a single aminoglycoside-modifying enzyme except for the bifunctional enzyme AAC(6′)-APH(2′′) produced by some methicillin-resistant Staphylococcus aureus strains. When a gram-negative pathogen with high-level resistance (MIC, >128 μl/ml) to both gentamicin and amikacin or tobramycin is detected in the clinical laboratory, additional susceptibility testing using arbekacin may prove useful. If the MIC of arbekacin exceeds 128 μl/ml, it strongly suggests that the strain produces 16S rRNA methylase.
In conclusion, we have described the emergence of high-level aminoglycoside resistance mediated by production of a novel 16S rRNA methylase in an S. marcescens clinical isolate. Dissemination of rmtB to other enterobacterial species as well as among S. marcescens is of concern.
Acknowledgments
We thank Kumiko Kai for expert technical assistance. We also thank Yoshiichi Yamaji for providing S. marcescens S-95 and Hiroya Tada for providing relevant clinical information.
This work was supported by grants H12-Shinkou-19 and H12-Shinkou-20 from the Ministry of Health, Labor, and Welfare of Japan. Part of the genetic analyses was supported by Grant-in-Aid for Young Scientists (B) 14771358 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Beauclerk, A. A., and E. Cundliffe. 1987. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J. Mol. Biol. 193:661-671. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, D., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryan, L. E. 1988. General mechanisms of resistance to antibiotics. J. Antimicrob. Chemother. 22(Suppl. A):1-15. [DOI] [PubMed] [Google Scholar]
- 4.Collis, C. M., and R. M. Hall. 1992. Site-specific deletion and rearrangement of integron insert genes catalyzed by the integron DNA integrase. J. Bacteriol. 174:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cundliffe, E. 1989. How antibiotic-producing organisms avoid suicide. Annu. Rev. Microbiol. 43:207-233. [DOI] [PubMed] [Google Scholar]
- 6.Davies, J., and G. D. Wright. 1997. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5:234-240. [DOI] [PubMed] [Google Scholar]
- 7.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 8.Demydchuk, J., Z. Oliynyk, and V. Fedorenko. 1998. Analysis of a kanamycin resistance gene (kmr) from Streptomyces kanamyceticus and a mutant with increased aminoglycoside resistance. J. Basic Microbiol. 38:231-239. [DOI] [PubMed] [Google Scholar]
- 9.Doi, Y., N. Shibata, K. Shibayama, K. Kamachi, H. Kurokawa, K. Yokoyama, T. Yagi, and Y. Arakawa. 2002. Characterization of a novel plasmid-mediated cephalosporinase (CMY-9) and its genetic environment in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 46:2427-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galimand, M., P. Courvalin, and T. Lambert. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffron, F., B. J. McCarthy, H. Ohtsubo, and E. Ohtsubo. 1979. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell 18:1153-1163. [DOI] [PubMed] [Google Scholar]
- 12.Holmes, D. J., and E. Cundliffe. 1991. Analysis of a ribosomal RNA methylase gene from Streptomyces tenebrarius which confers resistance to gentamicin. Mol. Gen. Genet. 229:229-237. [DOI] [PubMed] [Google Scholar]
- 13.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelemen, G. H., E. Cundliffe, and I. Financsek. 1991. Cloning and characterization of gentamicin-resistance genes from Micromonospora purpurea and Micromonospora rosea. Gene 98:53-60. [DOI] [PubMed] [Google Scholar]
- 15.Kondo, S., and K. Hotta. 1999. Semisynthetic aminoglycoside antibiotics: development and enzymatic modifications. J. Infect. Chemother. 5:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Kotra, L. P., J. Haddad, and S. Mobashery. 2000. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 44:3249-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; 12th informational supplement. M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Ohta, T., and M. Hasegawa. 1993. Analysis of the self-defense gene (fmrO) of a fortimicin A (astromicin) producer, Micromonospora olivasterospora: comparison with other aminoglycoside-resistance-encoding genes. Gene 127:63-69. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skeggs, P. A., J. Thompson, and E. Cundliffe. 1985. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol. Gen. Genet. 200:415-421. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J., P. A. Skeggs, and E. Cundliffe. 1985. Methylation of 16S ribosomal RNA and resistance to the aminoglycoside antibiotics gentamicin and kanamycin determined by DNA from the gentamicin-producer, Micromonospora purpurea. Mol. Gen. Genet. 201:168-173. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama, K., Y. Doi, K. Yamane, H. Kurokawa, N. Shibata, K. Shibayama, T. Yagi, H. Kato, and Y. Arakawa. 2003. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 362:1888-1893. [DOI] [PubMed]
- 24.Yoshizawa, S., D. Fourmy, and J. D. Puglisi. 1998. Structural origins of gentamicin antibiotic action. EMBO J. 17:6437-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]