Abstract
Multidrug-resistant strains of Shigella dysenteriae type 1 were implicated in three outbreaks and sporadic cases of dysentery in eastern India in 2002 and 2003. After a hiatus of 14 years, this pathogen reemerged with an altered antibiotic resistance pattern. In addition to ampicillin, co-trimoxazole, tetracycline, chloramphenicol, and nalidixic acid, all the recent strains were resistant to norfloxacin, lomefloxacin, pefloxacin, and ofloxacin and showed reduced susceptibility to ciprofloxacin. Pulsed-field gel electrophoresis identified a new clone of S. dysenteriae type 1 that was associated with the recent outbreaks and sporadic cases. Based on the spatial and temporal spread of multidrug-resistant S. dysenteriae type 1, we predict that this clonal type may spread further in this region.
Shigellosis caused by multiply antibiotic-resistant Shigella strains constitutes an increasing problem in developing countries. The most virulent serotype of Shigella dysenteriae, S. dysenteriae type 1, has been responsible for large dysentery epidemics in India (S. C. Pal, Letter, Lancet i:1462, 1984), Guatemala and other parts of Central America (9), Zaire (2), Kenya (Y. Iijima, J. O. Oundo, K. Taga, S. M. Saidi, and T. Honda, Letter, Lancet 345:69-70, 1995), Bangladesh (M. Bennish, A. Eusof, B. Kay, and T. Weirzba, Letter, Lancet ii:441, 1985), and recently West Africa (3). To our knowledge, the last cases of dysentery caused by S. dysenteriae type 1 were reported from eastern India in 1988 (D. Sen, P. Dutta, B. C. Deb, and S. C. Pal, Letter, Lancet ii:911, 1988), and except for one strain in 1995, this serotype was not found again till 2001 (14, 15). We report here a comparative study of S. dysenteriae type 1 strains isolated from an early dysentery outbreak in 1988 and in recent outbreaks and sporadic cases in eastern India.
From March to June 2002, a total of 1,124 patients reported with acute bacillary dysentery to the subdivisional hospital, Diamond Harbor, West Bengal. During almost the same time (April to May 2002), a similar outbreak occurred in a northern part of West Bengal (Alipurduar, Siliguri) among tea garden laborers, affecting 1,728 persons with an attack rate and death rate of 25.6 and 6%, respectively. The details of these two outbreaks have been published elsewhere (K. Sarkar, S. Ghosh, S. K. Niyogi, and S. K. Bhattacharya, Letter, Lancet 361:785, 2003; D. Sur, S. K. Niyogi, S. Sur, K. K. Datta, Y. Takeda, G. B. Nair, and S. K. Bhattacharya, Letter, Emerg. Infect. Dis. 9:404-405, 2003). A year later (2003), another outbreak of dysentery from the first week of April until the first week of June was reported from a remote village of Aizal in Mizoram State. The total number of reported cases was 169 with an attack rate of 17%. For bacteriological examination, 51 stool specimens were collected from dysentery patients from these three affected areas.
Strains of S. dysenteriae type 1 isolated from 904 and 950 acute diarrheal patients with sporadic cases who sought treatment at the Infectious Diseases Hospital (IDH) and B. C. Roy Children's Hospital (BCRCH), Calcutta, respectively, between January and December 2002 were also analyzed in this study. Stool specimens were collected from patients with dysentery, by the use of sterile catheters, into MacCartney bottles or with rectal swabs and tested for common enteric pathogens by standard microbiological techniques (18). The identity of these isolates was confirmed by biochemical tests (18). Shigella strains were serotyped with an antiserum kit (Denka Seiken Co., Ltd., Tokyo, Japan).
The antimicrobial susceptibility test was done by the standard disk diffusion method, according to the National Committee for Clinical Laboratory Standards guidelines (12). The Escherichia coli strain ATCC 25922 was used for quality control. Strains of S. dysenteriae type 1 were tested for susceptibility by using commercially available disks (HiMedia, Mumbai, India, and Difco) of ampicillin (10 μg), co-trimoxazole (25 μg), chloramphenicol (30 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), norfloxacin (10 μg), lomefloxacin (10 μg), pefloxacin (5 μg), ofloxacin (5 μg), and tetracycline (30 μg). MICs of nalidixic acid, norfloxacin, ciprofloxacin, and ofloxacin were determined by the E-test (AB Biodisk, Solna, Sweden).
DNA fingerprinting was done by pulsed-field gel electrophoresis (PFGE) with the restriction enzyme XbaI (Takara Shuzo, Otsu, Japan) according to a standard procedure (4). New and old strains of S. dysenteriae type 1, representing outbreak and sporadic cases, were included for comparison. Run conditions were generated by the autoalgorithm mode of the CHEF Mapper PFGE system with a size range of 30 to 600 kb (Bio-Rad Laboratories, Hercules, Calif.). After electrophoresis, the ethidium bromide (Sigma, St. Louis, Mo.)-stained agarose gel was visualized and the gel images were digitized for computer-aided analysis (Gel Doc 2000; Bio-Rad) and stored in a personal computer. The images were retrieved and analyzed with the Diversity Database fingerprinting software (Bio-Rad). The differences in the banding patterns of the PFGE profiles were compared to ascertain the clonal relationship between the strains. An unrooted dendrogram was made with the unweighted pair group with arithmetic mean (UPGMA) method.
Seventeen strains of Shigella spp. were isolated from the outbreak-affected regions, and sporadic case strains were serologically confirmed as S. dysenteriae type 1. The incidence rate of S. dysenteriae type 1 among sporadic cases was 0.8 and 0.95% in IDH and BCRCH, respectively. No other enteric pathogens were detected from the S. dysenteriae type 1-positive stool specimens. Cyclic epidemic patterns with major shifts in serogroups, with S. dysenteriae type 1 strains initially predominating and subsequently being replaced by S. flexneri, which is then replaced by S. sonnei, have been observed since the turn of the 20th century (7). This trend seems to be true in Calcutta (1, 14). Even though other Shigella serotypes prevailed (1, 14), S. dysenteriae type 1 appeared in this region after a hiatus of 14 years. The incidence of S. dysenteriae type 1 appears to be a recurrent phenomenon, associated with large-scale morbidity and mortality in eastern India (Pal, letter; Sarkar et al., letter; Sen et al., letter; Sur et al., letter).
Antibiotic susceptibility testing showed that S. dysenteriae type 1 strains were resistant to ampicillin, co-trimoxazole, nalidixic acid, chloramphenicol, and tetracycline (Table 1). In addition, the strains isolated in epidemic (2002 to 2003) and sporadic (2002) cases were resistant to norfloxacin, lomefloxacin, ofloxacin, and pefloxacin and showed reduced susceptibility to ciprofloxacin. The MIC ranges of ciprofloxacin, norfloxacin, and ofloxacin for recent S. dysenteriae type 1 strains were found to be 2 to 6, 6 to 24, and 8 to 16 μg/ml, respectively (Table 1). Antibiotic therapy is essential in the treatment of shigellosis, especially when the patients are infected with S. dysenteriae type 1. In this study, we recorded the isolation of S. dysenteriae strains with unique resistance profiles compared to those isolated previously in this region. The emergence of multidrug-resistant strains of Shigella was previously reported in Bangladesh (16), Indonesia (8), Tanzania (13), and India (15). An interesting observation in this study was the emergence of S. dysenteriae type 1 strains with altered antibiotic resistance profiles during each epidemic in eastern India. Nalidixic acid-resistant strains caused an epidemic in 1988 (Sen et al., letter), followed by the 2002 epidemic in which all the S. dysenteriae type 1 strains were resistant to fluoroquinolones (Sarkar et al., letter; Sur et al., letter).
TABLE 1.
Antimicrobial resistance and PFGE profiles of S. dysenteriae type 1 strains isolated from patients with sporadic and outbreak cases of dysentery
| Strain | Place (location)/yr of isolation | Case type | MIC (μg/ml)
|
Resistance profilea | Pulsotype | |||
|---|---|---|---|---|---|---|---|---|
| Nalidixic acid | Ciprofloxacin | Norfloxacin | Ofloxacin | |||||
| HU8 | Tripura (northeastern state)/1988 | Outbreak | 1 | 0.08 | 0.027 | 0.047 | ACoTCNa | A |
| HU10 | Tripura (northeastern state)/1988 | Outbreak | 1.25 | 0.012 | 0.047 | 0.047 | ACoTCNa | A |
| HU29 | Tripura (northeastern state)/1988 | Outbreak | 1 | 0.006 | 0.023 | 0.047 | ACoTCNa | A |
| BCH518 | Calcutta (West Bengal)/1995 | Sporadic | >256 | 0.094 | 0.19 | 0.038 | ACoTCNa | B1 |
| D1 | Diamond Harbor (West Bengal)/2002 | Outbreak | >256 | 6 | 16 | 8 | ACoTCNaNxLoOfPe | B2 |
| D2 | Diamond Harbor (West Bengal)/2002 | Outbreak | >256 | 4 | 12 | 8 | ACoTCNaNxLoOfPe | B3 |
| D44 | Diamond Harbor (West Bengal)/2002 | Outbreak | >256 | 4 | 16 | 12 | ACoTCNaNxLoOfPe | B |
| 18 | Siliguri (North Bengal)/2002 | Outbreak | >256 | 2 | 12 | 12 | ACoTCNaNxLoOfPe | B |
| 21 | Siliguri (North Bengal)/2002 | Outbreak | >256 | 6 | 16 | 12 | ACoTCNaNxLoOfPe | B |
| 25 | Siliguri (North Bengal)/2002 | Outbreak | >256 | 2 | 12 | 12 | ACoTCNaNxLoOfPe | B4 |
| NK2217 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 6 | 24 | 12 | ACoTCNaNxLoOfPe | B5 |
| NK2490 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 4 | 12 | 12 | ACoTCNaNxLoOfPe | B6 |
| NK2678 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 4 | 12 | 12 | ACoTCNaNxLoOfPe | B7 |
| NT4359 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 4 | 12 | 12 | ACoTCNaNxLoOfPe | C |
| H14174 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 6 | 16 | 10 | ACoTCNaNxLoOfPe | B8 |
| H16576 | Calcutta (West Bengal)/2002 | Sporadic | >256 | 6 | 16 | 8 | ACoTCNaNxLoOfPe | B9 |
| Az11 | Aizal (Mizoram State)/2003 | Outbreak | >256 | 3 | 6.0 | 16 | ACoTCNaNxLoOfPe | B |
Abbreviations: A, ampicillin; Co, co-trimoxazole; T, tetracycline; C, chloramphenicol; Na, nalidixic acid; Nx, norfloxacin; Lo, lomefloxacin; Of, ofloxacin; Pe, pefloxacin.
S. dysenteriae type 1 strains resistant to nalidixic acid, a narrow-spectrum quinolone, were reported as early as 1981 from Rwanda (Iijima et al., letter). The subsequent appearance of nalidixic acid-resistant strains was reported in the Indian subcontinent (11; Sen et al., letter), and since then the proportion of resistance is increasing in other species of Shigella (14). Treatment of patients with nalidixic acid was successful in 1984 when an S. dysenteriae type 1 epidemic occurred in northeastern India (R. Bose, J. N. Nashipuri, P. K. Sen, P. Dutta, S. K. Bhattacharya, D. Datta, D. Sen, and M. K. Bhattacharya, Letter, Lancet ii:1160, 1984). In many areas nalidixic acid still remains the drug of choice for shigellosis (5; A. Mahmood, Letter, J. Pak. Med. Assoc. 51:101, 2001) including West Africa (3).
Norfloxacin and ciprofloxacin, expanded-spectrum quinolone derivatives, are effective in the treatment of shigellosis (6). These drugs have helped to control and manage shigellosis in areas of endemicity (10). The current epidemic is particularly disturbing due to the emergence of S. dysenteriae type 1 strains resistant to norfloxacin and ciprofloxacin, which are the drugs of choice for dysentery. Ampicillin and co-trimoxazole were formerly the drugs of choice for treating shigellosis in developing countries due to their low cost and broad spectrum of activity (13). These drugs are no longer useful because of the emergence of widespread resistance (16).
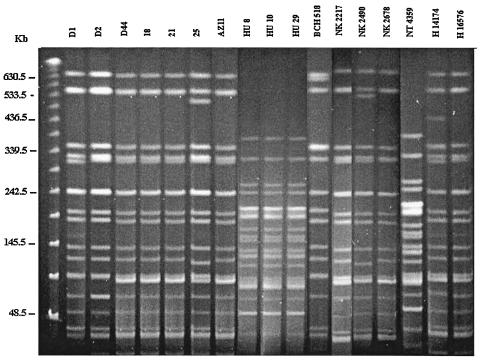
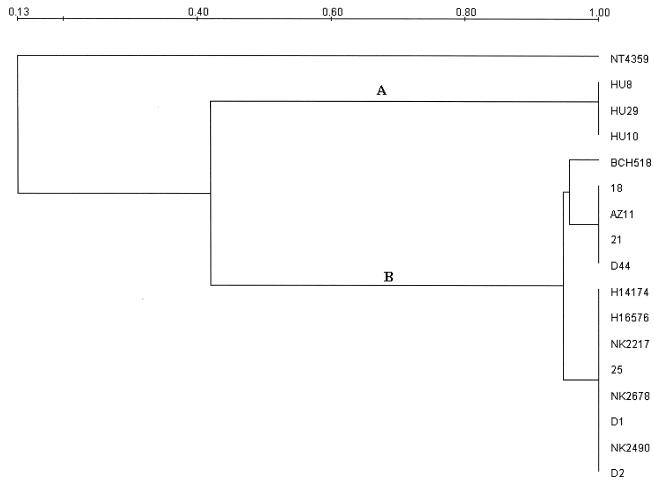
The PFGE patterns of the S. dysenteriae type 1 strains isolated in the 1988 epidemic and a strain isolated in 1995 in a sporadic case in Calcutta differed from those isolated during 2002 and 2003. All the recent epidemic strains were found to be closely related (Fig. 1), and the strains isolated from patients with sporadic cases admitted to the BCRCH during almost the same time frame exhibited strong similarity to the epidemic strains. However, discrete clones were also detected among patients with sporadic cases from Calcutta (strains BCH518 and NT4359) (Fig. 1). Dendrogram analysis indicated that the 1988 epidemic strains are identical (Fig. 2, cluster A) but different from seven recent epidemic strains (Fig. 2, cluster B).
FIG. 1.
PFGE profiles of S. dysenteriae type 1 strains after digestion with XbaI. Strains D1, D2, D44, 18, 21, and 25 represent the 2002 dysentery epidemic. Strain AZ11 was isolated from Aizal in 2003. Strains HU8, HU10, and HU29 were isolated during a 1988 outbreak in Tripura. BCH518 was isolated from a patient with a sporadic case in 1995 in Calcutta. Strains NK2217, NK2490, and NK2678 and strains NT4359, H14174, and H16576 were isolated from patients with sporadic cases of dysentery at BCRCH and IDH, respectively, in 2002. The molecular size marker (leftmost lane) used here is the PFG lambda ladder.
FIG. 2.
Dendrogram analysis of the S. dysenteriae type 1 strains based on the PFGE profiles with Diversity Database software (Bio-Rad) employing the UPGMA method, which grouped clusters A and B.
Considering the PFGE clonal criteria (17), we observed that many strains from patients with sporadic cases were clonally related to epidemic strains isolated during the same period (Table 1). Chronologically, we first detected a new clone of S. dysenteriae type 1 among 2002 epidemic case patients, followed by sporadic diarrheal case patients admitted to the BCRCH, Calcutta, and in outbreak cases in Mizoram in 2003. This result indicates that the recent multiply drug-resistant strains of S. dysenteriae type 1 are spreading from the outbreak-affected areas to other regions. The reemergence of S. dysenteriae type 1 with epidemic potential is cause for concern. This trend should be carefully monitored in India and neighboring countries.
Acknowledgments
This work was supported in part by the Indian Council of Medical Research (project no. 5/3/3/5/99-ECD-I) and the Japan International Cooperation Agency (JICA/NICED project 054-1061-E-0).
REFERENCES
- 1.Dutta, P., D. Dutta, S. K. Bhattacharya, D. Sen, U. Mitra, A. R. Ghose, M. Lahiri, and B. C. Deb. 1989. Clinical and bacteriological profiles of shigellosis in Calcutta before and after an epidemic (1984-87). Indian J. Med. Res. 89:132-137. [PubMed] [Google Scholar]
- 2.Goma Epidemiology Group. 1995. Public health impact of Rwandan refugee crisis: what happened in Goma, Zaire, in July 1994? Lancet 345:339-344. [PubMed] [Google Scholar]
- 3.Guerin, P. J., C. Brasher, E. Baron, D. Mic, F. Grimont, M. Rayan, P. Aavitsland, and D. Legros. 2003. Shigella dysenteriae serotype 1 in West Africa: intervention strategy for an outbreak in Sierra Leone. Lancet 362:705-706. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson, K., and G. Chu. 1991. Pulsed-field electrophoresis of megabase-sized DNA. Mol. Cell. Biol. 11:3348-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwalokun, B. A., G. O. Gbenle, S. I. Smith, A. Ogunledun, K. A. Akinsinde, and E. A. Omonigbehin. 2001. Epidemiology of shigellosis in Lagos, Nigeria: trends in antimicrobial resistance. J. Health Popul. Nutr. 19:183-190. [PubMed] [Google Scholar]
- 6.Khan, W. A., C. Seas, U. Dhar, M. A. Salam, and M. L. Bennish. 1997. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind randomized, control trial. Ann. Intern. Med. 126:697-703. [DOI] [PubMed] [Google Scholar]
- 7.Kostrzewski, J., and H. S. Misiurewicz. 1968. Changes in the epidemiology of dysentery in Poland and the situation in Europe. Arch. Immunol. Ther. Exp. 16:429-451. [PubMed] [Google Scholar]
- 8.Malakooti, M. A., J. Alaii, G. D. Shanks, and P. A. Phillips-Howard. 1997. Epidemic dysentery in western Kenya. Trans. R. Soc. Trop. Med. Hyg. 91:541-543. [DOI] [PubMed] [Google Scholar]
- 9.Mendizabal-Morris, C. A., I. Malta, E. J. Gangarisa, and G. Guzman. 1971. Epidemic Shiga-bacillus dysentery in Central America. Derivation of the epidemic and its progression in Guatemala, 1968-69. Am. J. Trop. Med. Hyg. 20:927-933. [DOI] [PubMed] [Google Scholar]
- 10.Moolasart, M. A., B. Eampokalap, and M. Ratanasrithong. 1999. Comparison of the efficacy of ceftibuten and norfloxacin in the treatment of acute gastrointestinal infection in children. Southeast Asian J. Trop. Med. Public Health 30:764-769. [PubMed] [Google Scholar]
- 11.Munshi, M. H., K. Haider, M. M. Rahaman, D. A. Sack, Z. U. Ahmed, and M. G. Morshed. 1987. Plasmid-mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet ii:419-421. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk susceptibility tests. 12th informational supplement. Document M100-512. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Navia, M. M., L. Capitano, J. Ruiz, M. Vargas, H. Urassa, D. Schellemberg, J. Gascon, and J. Vila. 1999. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J. Clin. Microbiol. 37:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyogi, S. K., U. Mitra, and P. Dutta. 2001. Changing patterns of serotypes and antimicrobial susceptibilities of Shigella species isolated from children in Calcutta, India. Jpn. J. Infect. Dis. 54:121-122. [PubMed] [Google Scholar]
- 15.Niyogi, S. K., and G. P. Pazhani. 2003. Multiresistant Shigella species isolated from childhood diarrhoea cases in Kolkata, India. Jpn. J. Infect. Dis. 56:33-34. [PubMed] [Google Scholar]
- 16.Sack, R. B., M. Rahamn, M. Yunus, and E. H. Khan. 1997. Antimicrobial resistance in organisms causing diarrhoeal disease. Clin. Infect. Dis. 24:S102-S105. [DOI] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. 1987. Manual for laboratory investigation of acute enteric infections. CDD/83.3. World Health Organization, Geneva, Switzerland.