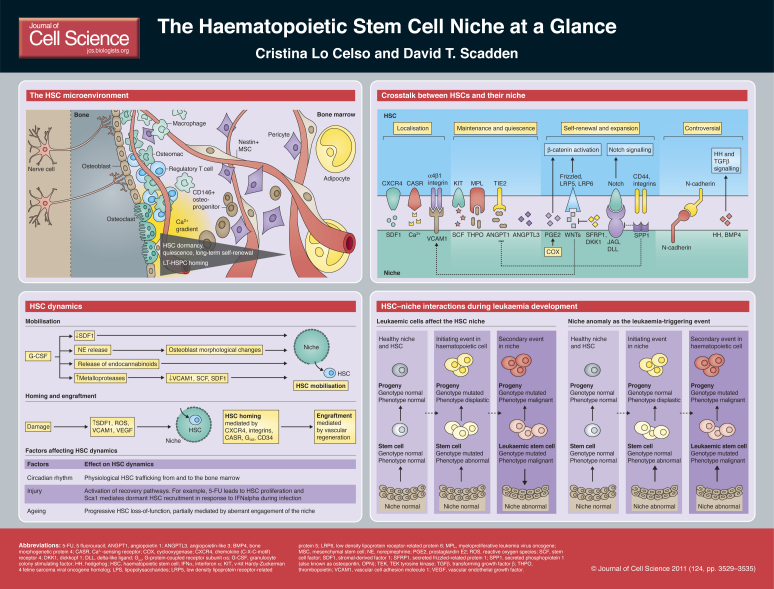
Haematopoietic stem cells (HSCs) regulate the balanced turnover of erythrocytes, platelets and all immune cells by switching between self-renewal, differentiation, quiescence and dormancy (Trumpp et al., 2010) and, thereby, maintain homeostasis both in the steady state as well as in response to injury. HSCs are among the longest studied and utilised somatic stem cells. However, they cannot yet be maintained and expanded in vitro because a complex and dynamic molecular crosstalk between HSC and their endogenous microenvironment (or ‘niche’) directs their fate. The importance of the stem cell niche in regulating HSC function was first postulated in 1978 by Ray Schofield, when observing that the spleen is unable to support HSCs in the same way that bone marrow can (Schofield, 1978). Since then, it has become clear that not only HSCs but all somatic stem cells maintain homeostasis because they sense and respond to the need of an organism for their differentiated progeny as well as to stem cells themselves. The stem cell niche is the functional and anatomical ‘node’ that allows integration of signals from the periphery into the appropriate stem cell behaviour. The constantly evolving technological and experimental approaches have provided insights into the nature of the HSC niche, the specific function of different niche components and how perturbations in this highly integrated system are involved in the development of haematopoietic disease.
In this Cell Science at a Glance article, we provide an update of the cellular and molecular components of the HSC niche, describe how they adapt in response to specific stimuli, and discuss their potential role in the development of haematopoietic disease. As several controversial observations have led to the most recently proposed niche models and because future studies are likely to further change our views again, we do not present a definitive, detailed portrait of the HSC niche but, instead, integrate currently accepted niche elements and dynamic models to provide an up-to-date overview. Box 1 contains details on the specific nomenclature used in this article. Box 2 contains a brief summary of recent
experimental models and technological advances that were used to study the HSC niche.
Box 1. Notes on nomenclature
An increasing number of haematopoietic stem and progenitor cell (HSPC) subpopulations continues to be identified. Furthermore, the steady introduction of new markers has demonstrated the heterogeneity of cell populations used in previous studies. In this Cell Science at a Glance article, we use the term HSC when referring to the experimental demonstration of HSC function and the term HSPC to indicate phenotypically defined cell populations that have been shown to contain HSCs.
The term HSC niche often refers strictly to the microenvironment of the most primitive HSCs. However, as a result of the continuous experimental refinement of this cell population and the increasing – and often controversial – number of bone marrow components shown to affect HSC function, this term is best used to refer to the entire network of extrinsic factors that regulates HSC fate.
Finally, various osteoblastic cell subpopulations have been proposed to differentially interact with and affect HSCs, but a widespread consensus on markers that make their distinction possible is missing. We, therefore, use the terms osteoblast and osteoblastic cell to refer to the whole lineage and not to cells at a specific stage of differentiation or with a particular metabolic activity.
Box 2. Recently developed experimental protocols to study the HSC niche
Functional studies in mice
Analysis of the effects of perturbation of cell adhesion molecules, signalling pathways or specific components of the bone marrow microenvironment on HSC number and functional ability to reconstitute irradiated recipient mice provides clues about the mechanisms that regulate the HSC niche. Haematopoietic defects that are observed following transplantation of wild-type haematopoietic cells into mutant recipients and can be rescued by transplantation of mutant marrow into wild-type recipients have provided the first indication of niche-mediated molecular mechanisms (Walkley et al., 2007a; Walkley et al., 2007b; Fleming et al., 2008; Renstrom et al., 2009). Immunodeficient, KIT-deficient mice were generated as universal recipients that allow the study of human HSPCs (Waskow et al., 2009). The use of W/Wv KIT-mutant recipient mice is becoming increasingly popular because these mice uniquely enable the study of HSC engraftment in near-physiological, unconditioned bone marrow (Lo Celso et al., 2009; Migliaccio et al., 1999).
Observing the HSC niche using imaging techniques
The direct observation of HSPCs within the bone marrow microenvironment allows direct assessment of HSC niche anatomy and dynamics. Confocal–multiphoton hybrid microscopy can be used to follow homing and initial proliferation of haematopoietic cells. We have shown that minimal surgery is necessary to observe the marrow space contained within the mouse frontal bones, where bone, osteoblasts, ex-vivo-labelled HSPCs, endogenous autofluorescent cells and the vasculature can be observed simultaneously (Lo Celso et al., 2011). Alternatively, careful thinning of the tibeal bone allows imaging of the peri-endoseal diaphyseal marrow (Kohler et al., 2009), whereas insertion of fibre optics inside the femural marrow makes it possible to carry out fluorescence microscopy of central marrow space (Lewandowski et al., 2010). Furthermore, real-time analysis of HSPC behaviour within the femur bone marrow was also carried out, by positioning femur fragments on a coverslip and by monitoring the exposed marrow for a few hours ex vivo (Xie et al., 2009). In vivo immunostaining protocols have been used to highlight expression of endothelial and peri-vascular antigens (Lewandowski et al., 2010; Sipkins et al., 2005).
Cellular components of the HSC niche
HSCs reside within the bone marrow, which presents a complex microenvironment that is made up of different cell types and extracellular elements. An increasing number of bone marrow lineages, structures and molecular components have been demonstrated to affect HSC fate and function.
The endosteal surface of the bone and cells of the osteoblastic lineage were shown first to be components of the HSC niche (Calvi et al., 2003; Grassinger et al., 2010; Lord et al., 1975; Visnjic et al., 2004; Zhang et al., 2003). Whether a specific subpopulation of osteoblastic cells is interacting with HSCs is currently under investigation. Recently, secreted phosphoprotein 1 [SPP1; also known as osteopontin (OPN) and one of the main extracellular proteins secreted by osteoblasts] and ALCAM (an adhesion molecule widely expressed across different lineages) were proposed as markers of a subpopulation of osteoblastic cells that affect HSC function. (Mayack and Wagers, 2008; Nakamura et al., 2010). Interestingly, haematopoiesis is dramatically affected by the conditional deletion of the ribonuclease Dicer in osteoprogenitors. However, the same deletion in fully mature, osteocalcin-positive osteoblasts does not lead to haematopoietic defects (Raaijmakers et al., 2010), and the selective ablation of terminally differentiated osteoblasts does not affect haematopoiesis either (Corral et al., 1998; Raaijmakers et al., 2010). The association between HSCs and osteoprogenitors might be rooted even earlier within the osteoblastic lineage, as was suggested by the fact that nestin-positive MSC-like cells are marrow stroma cells that can interact with HSCs (Mendez-Ferrer et al., 2010). Furthermore, human CD146+ osteoprogenitor cells are able to direct ectopic bone formation accompanied by haematopoietic seeding (Sacchetti et al., 2007). PDGFRα+, Sca1+ mesenchymal progenitors have been successfully transplanted and have been shown to localise to areas of the bone marrow that are generally recognised to contain HSC niches (Morikawa et al., 2009). Taken together, these studies show that osteoblastic cells at various stages of differentiation can support different HSC functions and states.
The endosteal HSC niche model was challenged, however, by immunofluorescence studies, which showed that the only bone marrow structure that is consistently located adjacent to HSCs is the sinusoidal vasculature (Kiel et al., 2005). Integrity and regeneration of bone marrow vasculature are, indeed, fundamental for HSC recovery from myeloablative injuries and following bone marrow transplantation (Hooper et al., 2009; Kobayashi et al., 2010). Several reports agree on the location of functional, engrafting haematopoietic stem and progenitor cells (HSPCs, Box 1) being near the endosteal surface but not exclusively adjacent to osteoblastic cells (Jiang et al., 2009; Lo Celso et al., 2009; Xie et al., 2009). Whereas endosteal surfaces are highly vascularised, the question remains whether HSCs that are located at varying distances from osteoblastic cells are functionally distinct from those located near osteoblastic cells.
Other HSC regulators include perivascular, non-endothelial supportive cells (Sugiyama et al., 2006), adipocytes, which have been shown to inhibit HSC engraftment (Naveiras et al., 2009), and the autonomous nervous system, which influences HSC mobilisation (Katayama et al., 2006). In addition, several cells of haematopoietic origin have a role in the HSC niche. For example, the activation of osteoclasts, a specialised subpopulation of endosteal macrophages that are responsible for bone resorption, leads to HSC egress from the bone marrow (Kollet et al., 2006), whereas their pharmacological inhibition leads to a reduction of HSPC numbers (Lymperi et al., 2011). A different subpopulation of bone marrow macrophages, the ‘osteomacs’, form a canopy of cells near active osteoblasts and carry out the opposite role: their depletion leads to the loss of osteoblast activity and increased HSC mobilisation (Winkler et al., 2010). Very recently regulatory T cells have been demonstrated to make the HSC niche a site of immune privilege (Fujisaki et al., 2011).
The question remains whether we should think about one or several HSC niches. The observation that different HSPC localisations exist within the marrow and that a growing number of cell types are involved in HSC regulation (as illustrated above), together with the increasing number of reports that describe the heterogeneity of even highly purified HSPC populations (Dykstra et al., 2007; Lo Celso et al., 2009; Wilson et al., 2008), could be an indication of the complex microenvironments through which HSCs navigate. One proposed model suggests that osteoblastic cells provide a context for HSC dormancy, whereas a perivascular, quiescent niche provides an intermediate niche for activated HSCs that are ready to either generate differentiating progeny or revert to dormancy, depending on the needs of the organism (Malhotra and Kincade, 2009a; Trumpp et al., 2010). It is possible that the number of functional niches increases further if additional HSC subpopulations and states are identified. Real-time long-term cell tracking coupled with a greater number of reporter strategies to simultaneously highlight multiple stroma components and HSPC subpopulations will be instrumental to fully understand the interactions between HSCs and their niche.
Molecular regulators of HSC fate
Independent of the identity of the niche cell that generates a signal for the HSC, a multitude of molecular regulators of HSC fate have been described. Some are known products of osteoblastic cells; however, it is possible that an increasing number of these factors are produced by multiple cell types.
Secreted ligands and their receptors
The stroma-derived factor 1 (SDF1; also known as CXCL12)–C-X-C chemokine receptor type 4 (CXCR4) axis is the best-defined regulator of HSC localisation in the bone marrow. Osteoblastic cells produce SDF1 and upregulate its expression in response to irradiation (Xie et al., 2009). However, analysis of SDF1-reporter mice has highlighted numerous perivascular cells throughout the marrow that are SDF1-positive (Sugiyama et al., 2006), which indicates that SDF1 directs HSC localisation not only near osteoblastic cells but probably also throughout perivascular areas.
If SDF1 and CXCR4 are responsible for HSC localisation, other cytokines, signalling pathways and adhesion molecules known to have a role in the HSC niche might, instead, regulate HSC fate. Indeed, the ligand–receptor pairs stem cell factor (SCF) and KIT, thrombopoietin (TPO) and myeloproliferative leukemia virus oncogene (MPL), as well as angiopoietin 1 (ANGPT1) and TIE2 tyrosine kinase (officially known as TEK) have pivotal roles in regulating the interaction between osteoblasts and HSCs and, specifically, HSC maintenance and in vivo quiescence (Arai et al., 2004; Barker, 1997; Yoshihara et al., 2007). Recently, angiopoietin-like 3 (ANGPTL3), which is primarily expressed by endothelial cells, has been shown to control HSC quiescence as well as the number of HSCs both in the steady state and following transplantation (Zheng et al., 2011).
WNT signalling
The WNT signalling cascade has been implicated with a role in HSC regulation; but this is still highly controversial, mostly because of the complexity of the signalling pathway itself (Malhotra and Kincade, 2009b). Following initial reports that the obliteration of the canonical WNT pathway does not affect HSCs, later studies that focussed on interactions between HSCs and their niche provided further detailed information on the involvement of this pathway (Cobas et al., 2004; Koch et al., 2008). It was shown that osteoblast-specific overexpression of the WNT inhibitor dickkopf homolog 1 (DKK1) leads to impairment of HSC self-renewal (Fleming et al., 2008). Similarly, the knockout of secreted frizzled-related protein 1 (SFRP1) – another negative modulator of WNT signalling – leads to an initial increase in long-term reconstituting HSPCs (LT-HSPCs), followed by their premature exhaustion (Renstrom et al., 2009). Interestingly, early B-cell factor 2 (EBF2, a transcription factor known to synergise with WNT signalling in certain cells and under certain conditions) knockout mice are affected by environment-dependent loss of HSCs, and their osteoblastic cells have altered expression of – among others – SFRP1 and SFRP2 (Kieslinger et al., 2010). Another modulator of WNT signalling is the secreted eicosanoid prostaglandin E2 (PGE2), which stabilises WNT signalling in HSCs and is, therefore, a new player in the complexity of WNT-mediated crosstalk between HSCs and the niche (Goessling et al., 2009; North et al., 2007). Further interactions between WNT and other signalling pathways affecting the HSC–osteoblastic-cell crosstalk were uncovered very recently by studying transgenic mice that express WNT inhibitor factor 1 (WIF) in osteoblasts, which display a similar phenotype to that observed following overexpression of Dkk1 (Shaniel et al., 2011).
Notch and hedgehog signalling
A controversy similar to that surrounding WNT signalling accompanies the role of Notch signalling in the HSC niche. Initial gain-of-function studies indicated its ability to expand HSCs (Varnum-Finney et al., 1998). Furthermore, studies in which transgenic reporters have been used revealed Notch activity in transplanted HSPCs (Duncan et al., 2005), but in vivo knockout studies could not confirm these results (Maillard et al., 2008; Mancini et al., 2005). However, the Notch ligand JAG1 (jagged1), was first found to be upregulated at the endosteal surface of parathyroid hormone receptor transgenic mice – which are characterised by increased HSC number and activity (Calvi et al., 2003) – and is present on human CD146+ HSC supportive bone marrow stroma cells (Sacchetti et al 2007). Moreover, expression of Notch1 and Notch2 in HSCs was recently shown to be important for their response to endothelial-derived maintenance factors (Butler et al., 2010).
Studies that investigate the role of hedgehog (HH) signalling in the HSC niche seem to follow a similar paradigm compared with those focusing Notch signalling (Bhardwaj et al., 2001; Hofmann et al., 2009). The initial indication that HH signalling can cause HSC expansion through activation of bone morphogenetic protein (BMP) signalling pathways (Bhardwaj et al., 2001) was followed by the finding that HH signalling is dispensable for adult haematopoiesis (Bhardwaj et al., 2001; Hofmann et al., 2009). However, stromal BMP4 was shown to contribute to HSC maintenance (Goldman et al., 2009).
The extracellular matrix
As a result of their ability to influence stem cell fate, components of the extracellular matrix (ECM) have gained increasing attention with regards to the HSC niche (Connelly et al., 2010). Although it is still impossible to test how contact area, shape and matrix stiffness impact HSC fate in vivo, it could be shown that HSCs seeded on microwells actively produce their own ECM and undergo quiescence or proliferation depending on the size of the well (Kurth et al., 2009). In addition, SPP1 was the first osteoblast-derived ECM protein that was shown to influence HSC number and function (Nilsson et al., 2005; Stier et al., 2005). Lack of SPP1 leads to a stroma-dependent increase of LT-HSPCs and increased JAG1 and ANGPT1 expression in stroma cells, which perhaps explains how, in Spp1-deficient mice, HSC expansion is not accompanied by their exhaustion (Nilsson et al., 2005; Staal and Clevers, 2005).
Glycans are non-protein components of the bone marrow stroma that have a role in the HSC niche. They are likely to mediate the formation of chemokine and growth factor gradients (Haylock and Nilsson, 2006). Moreover, eicosanoids (including PGE2, as mentioned above) affect the strength of signalling cascades, and neurotransmitters regulate the response to HSC mobilizing agents (see below).
Adhesion molecules
For a number of years, the role of β1 integrins in the crosstalk between HSCs and their niche has been of interest. Integrin α4β1 (ITGA4; also known as VLA4) mediates HSC retention within the bone marrow microenvironment (Priestley et al., 2006), whereas integrins α1β1 and α5β1 (ITGA3 and ITGA5; also known as VLA1 and VLA5, respectively) mediate adhesion of HSCs to – among others – SPP1 (Nilsson et al., 2005). Furthermore, these three integrins mediate SDF1 function (Peled et al., 2000). Interestingly, there is a link between WNT signalling and integrin expression in HSCs: the expression of constitutively active β-catenin leads to the loss of HSCs and rapid exhaustion of their progeny, but also to higher expression of integrins α2, β1 and β7 in HSPCs (Kirstetter et al., 2006).
Integrins interact with ECM proteins, but integrin α4β1 is also the main binding partner of vascular cell adhesion molecule 1 (VCAM1), which is expressed on the surface of endothelial cells (Ulyanova et al., 2007) as well as cells of the osteoblastic lineage (Jiang et al., 2009). Interestingly, VCAM1 expression correlates with HSC homing (Lewandowski et al., 2010) and is upregulated in response to WNT signalling (Malhotra and Kincade, 2009a). Cadherins, which make up another class of adhesion molecules, might also contribute to HSC fate but their role in HSC homeostasis remains controversial (Levesque et al., 2010). Future studies will indicate whether the HSC niche is similar to other niches in that the direct interaction of HSCs with osteoblastic, endothelial and other cell types is fundamental to ensure their correct function.
Chemical gradients
Calcium and oxygen are the main chemical elements that have been studied in relation to the HSC niche. Ca2+ is released by osteoclasts during bone resorption, which leads to the formation of a concentration gradient that spreads out from the endosteal surface (Levesque et al., 2010). HSCs express the G-protein coupled Ca2+-sensing receptor (CASR) and depend on it for their peri-endosteal localisation and function (Adams et al., 2006).
In contrast to the acknowledged role of Ca2+ in the HSC nice, the relevance of oxygen tension is a controversial topic. Adaptations of the protocol for the isolation of side populations (Goodell, 2005), which use Hoechst dye in vivo staining to label efficiently perfused cells, suggested that HSCs reside in hypoxic areas of the bone marrow (Parmar et al., 2007; Winkler et al., 2010). However, both endosteal and non-endosteal LT-HSPCs are localised near vasculature (Kiel et al., 2005; Lo Celso et al., 2009). Therefore, in vivo measurements of bone marrow O2 concentration are necessary to definitively assess whether O2 gradients are present within the marrow space and what their defining features and function are. This question has recently gained further attention, as O2 levels were shown to affect transcription and WNT signalling through the activity of HIF1α, the hypoxia-responsive transcription factor that is also expressed in HSPCs (Levesque et al., 2007).
The HSC niche is a paramount example of the complex molecular interactions that take place in living tissues, and the evolutionary robustness of vertebrate haematopoietic systems undoubtedly relies on the intricacy of its molecular regulation. Increasingly detailed spatio-temporal analysis of the molecular composition of the HSC niche will allow us to fit current and new data into a functional HSC niche model.
HSC niche dynamics
All somatic stem cell niches are characterised by intrinsic dynamics, due to the oscillation of stem cells between quiescence, self-renewal and differentiation. The HSC niche, however, has to accommodate a unique trait: HSCs periodically leave the niche and enter circulation (‘mobilisation’) to later re-enter the bone marrow space (‘homing’) (Bhattacharya et al., 2009; Wright et al., 2001). This physiological phenomenon is exploited therapeutically during bone marrow transplantation. Because bone marrow engraftment requires conditioning of the recipient by means of irradiation or chemotherapy, bone marrow transplantation provides a prime example of the remarkable regenerative potential of the HSC niche. The dramatic perturbations caused by pharmacological HSC mobilisation and transplantation have been studied for a long time in order to improve clinical practice, and recent technological advances have allowed to unravel the more subtle changes that occur in the HSC niche during physiological responses and throughout the development of disease.
Induced HSC mobilisation
Granulocyte colony-stimulating factor [G-CSF, officially known as colony stimulating factor 3 (CSF3)] is the most widely used mobilising agent in clinical practice. It has a highly multifaceted mode of action: G-CSF administration results in the transient reduction of SDF1 expression, the activation of metalloproteases that cleave SCF, VCAM1 and SDF1, as well as the release of norepinephrine from the sympathetic nervous system, which affects the morphology of osteoblasts and their ability to retain HSCs within the niche (Brouard et al., 2010; Katayama et al., 2006). Endocannabinoids and reactive oxygen species (ROS) have recently been identified as non-protein mediators of the effects of G-CSF action (Jiang et al., 2011; Jiang et al., 2010; Tesio et al., 2011). Interestingly, whereas HSC mobilisation is normally transient, the continuous administration of the SDF1 antagonist AMD3100 can lengthen this process (Bonig et al., 2009).
HSC transplantation
HSC homing is the first step of a successful transplantation and crucially depends on the SDF1–CXCR4 signalling axis and β1 integrins. Subsequently, the CASR, in response to a Ca2+ gradient, mediates the lodging of HSC near the endosteum (see above) (Adams et al., 2006; Jung et al., 2006). Although HSCs exit and re-enter the niche normally, some molecules are dispensable during steady-state haematopoiesis but are crucial for transplanted HSC homing. For example, the stimulatory class of the Gα subunit of G-protein-coupled receptors (Gαs) is necessary for transplanted HSPCs to extravasate and reach the bone marrow space (Adams et al., 2009). Furthermore, CD34 might have a role in HSC movement and transmigration to the niche but, so far, CD34-dependent defects have only been observed in very specific transplantation settings (Nielsen and McNagny, 2007).
Bone marrow conditioning with cytotoxic agents or irradiation prior to transplantation triggers several molecular signals and is crucial for HSC homing in the endosteal region (Jiang et al., 2009; Lo Celso et al., 2009). The consequences of conditioning on cells of the osteoblastic and other stroma lineages are only partially known, but upregulation of SDF1, ROS-mediated induction of VCAM1 and vascular endothelial growth factor (VEGF)-mediated endothelial recovery have been highlighted as part of the regenerative process triggered by lethal irradiation (Hooper et al., 2009; Lewandowski et al., 2010; Xie et al., 2009).
Physiological HSC trafficking
Recent studies uncovered the ability of the HSC niche to orchestrate dynamic responses to sub-lethal injuries through the recruitment of dormant HSCs. An analysis of the response to interferon α (IFNα) treatment (which mimics activation of the immune response) was the first study that highlighted a clear function for Sca1 (stem cell antigen 1) (Essers et al., 2009), which has long been recognised as a marker of mouse HSPCs.
The physiological egress of HSCs from, and their return to, the bone marrow niche is probably regulated by mechanisms that are similar to those linked to successful mobilisation, engraftment and recovery from injury, albeit on a smaller scale. Circadian rhythms affect niche anatomy and the number of circulating HSCs (Mendez-Ferrer et al., 2008), and bone remodelling itself might have a crucial role in HSC homeostasis (Levesque et al., 2010). Ageing is another physiological process that is likely to affect HSC–niche interactions. Although long-term follow up of endogenous HSCs is currently unachievable, differences in cell volume oscillation and position relative to the endosteal surface were observed in young and old transplanted HSPCs (Kohler et al., 2009).
The HSC niche in disease
There is evidence for the HSC niche both supporting the development of and being affected by haematopoietic disease. Aberrant bone marrow stroma can lead not only to loss of HSC function but also to myelofibrosis and myelodysplastic syndromes (Lane et al., 2009; Raaijmakers et al., 2010; Walkley et al., 2007a). For example, mice with defective microRNA processing in the osteoblastic lineage develop profound bone defects as well as severe myelodysplasia, which can occasionally develop into leukaemia (Raaijmakers et al., 2010). Whereas it is clear that aggressive leukaemia depends on the accumulation of genetic abnormalities in cells of the haematopoietic lineage, this model indicates that the bone marrow stroma has a role in disease development, presumably by exerting different selection criteria that support leukaemia cells or confer a disadvantage to normal haematopoietic cells. Niche aberrations might, therefore, favour the development of disease. In addition, leukaemia cells might acquire the ability to respond differently to physiological niche signals than regular HSCs. For example, transforming growth factor β (TGFβ) is thought to contribute to the dormancy of normal HSCs (Yamazaki et al., 2009) and, by contrast, contributes to the survival and proliferation of leukaemia-initiating cells in a model of chronic myelogenous leukaemia (Naka et al., 2010).
Whether normal HSCs and leukaemia stem cells compete for the same niche space is still an open question, but it appears to be that normal haematopoiesis is impaired in the setting of leukaemia. Indeed, the homing pattern of human HSPCs is dramatically altered when they are transplanted into recipient leukaemic mice (Colmone et al., 2008). Whether leukaemia impairs HSC niche function is unclear (Podar et al., 2009), but human chronic myeloid leukaemia stem (or leukaemia-initiating) cells have been shown to reside near the endosteal surface when injected into newborn irradiated recipient mice (Saito et al., 2010). HSCs, whether normal or leukaemic, might have a role in shaping their niche and it remains an interesting question, whether that interaction can be pharmacologically manipulated to selectively affect the support of normal or malignant cells (Colmone et al., 2008).
Perspectives
The number of known cellular components and molecular mechanisms that regulate HSC–niche interactions has dramatically increased during the past few years. An interesting trend in the HSC niche field has been the progression from several controversies to the clarification that HSC niches present a scenario that is more complex than previously thought. Whereas initial studies highlighted the role of osteoblastic cells in regulating HSC number and function, it is now widely accepted that several other stromal cell types, ranging from mesenchymal stem cells to various differentiated lineages, interact with HSC and influence their fate through an array of secreted and cell–cell adhesion molecules as well as by affecting the protein and chemical composition of bone marrow extracellular space. The current picture of the components involved in the HSC niche is likely to grow in complexity, and the same is true for the intricacy of signalling pathways and subtleness of effects based on intensity and localisation of each signal.
Further technological advances and increasingly refined experimental models will allow us to explore new dimensions of the HSC niche itself and to unravel several points that are currently under discussion. For example, an open question is how many functionally distinct HSC niches exist and whether they are spatially segregated or, rather, represent dynamic oscillations in the bone marrow microenvironment. Studies on invertebrate niches have already indicated their ability to tightly regulate symmetry during stem cell division and to instruct the reversion of differentiating cells to a stem cell stage. Further development of imaging-based and functional assays will allow the investigation of whether this is also the case for the HSC niche. Finally, understanding the relationship between normal and diseased HSC–niche interactions will allow us to harness these mechanisms for therapeutic purposes through effective ex vivo expansion of HSCs and the development of new treatments for haematopoietic disease.
Supplementary Material
Footnotes
Funding
The work of the authors is supported by Cancer Research UK [grant number CEA C36195/A11831], the Kay Kendall Leukaemia Fund [grant number KKL460], the UK Biotechnology and Biological Sciences Research Council [grant number BB/I004033/1], the Human Frontiers Science Program [grant number RGP0051/2011] to C.L.C., and the National Institute of Health [grant numbers NHLBI HL097794, HL097748, HL100402 and HL044851] and the Ellison Foundation and the Harvard Stem Cell Institute to D.T.S. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.074112/-/DC1
This article is part of an Article Series on Stem Cells, available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.074112/-/DC2
References
- Adams G. B., Chabner K. T., Alley I. R., Olson D. P., Szczepiorkowski Z. M., Poznansky M. C., Kos C. H., Pollak M. R., Brown E. M., Scadden D. T. (2006). Stem cell engraftment at the‘ endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599-603 [DOI] [PubMed] [Google Scholar]
- Adams G. B., Alley I. R., Chung U. I., Chabner K. T., Jeanson N. T., Lo Celso C., Marsters E. S., Chen M., Weinstein L. S., Lin C. P., et al. (2009). Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature 459, 103-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F., Hirao A., Ohmura M., Sato H., Matsuoka S., Takubo K., Ito K., Koh G. Y., Suda T. (2004). Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149-161 [DOI] [PubMed] [Google Scholar]
- Barker J. E. (1997). Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp. Hematol. 25, 542-547 [PubMed] [Google Scholar]
- Bhardwaj G., Murdoch B., Wu D., Baker D. P., Williams K. P., Chadwick K., Ling L. E., Karanu F. N., Bhatia M. (2001). Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol 2, 172-180 [DOI] [PubMed] [Google Scholar]
- Bhattacharya D., Czechowicz A., Ooi A. G., Rossi D. J., Bryder D., Weissman I. L. (2009). Niche recycling through division-independent egress of hematopoietic stem cells. J. Exp. Med. 206, 2837-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonig H., Chudziak D., Priestley G., Papayannopoulou T. (2009). Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp. Hematol. 37, 402-415e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard N., Driessen R., Short B., Simmons P. J. (2010). G-CSF increases mesenchymal precursor cell numbers in the bone marrow via an indirect mechanism involving osteoclast-mediated bone resorption. Stem Cell Res. 5, 65-75 [DOI] [PubMed] [Google Scholar]
- Butler J. M., Nolan D. J., Vertes E. L., Varnum-Finney B., Kobayashi H., Hooper A. T., Seandel M., Shido K., White I. A., Kobayashi M., et al. (2010). Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi L. M., Adams G. B., Weibrecht K. W., Weber J. M., Olson D. P., Knight M. C., Martin R. P., Schipani E., Divieti P., Bringhurst F. R., et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841-846 [DOI] [PubMed] [Google Scholar]
- Cobas M., Wilson A., Ernst B., Mancini S. J., MacDonald H. R., Kemler R., Radtke F. (2004). Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmone A., Amorim M., Pontier A. L., Wang S., Jablonski E., Sipkins D. A. (2008). Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322, 1861-1865 [DOI] [PubMed] [Google Scholar]
- Connelly J. T., Gautrot J. E., Trappmann B., Tan D. W., Donati G., Huck W. T., Watt F. M. (2010). Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 12, 711-718 [DOI] [PubMed] [Google Scholar]
- Corral D. A., Amling M., Priemel M., Loyer E., Fuchs S., Ducy P., Baron R., Karsenty G. (1998). Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc. Natl. Acad. Sci. USA 95, 13835-13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A. W., Rattis F. M., DiMascio L. N., Congdon K. L., Pazianos G., Zhao C., Yoon K., Cook J. M., Willert K., Gaiano N., et al. (2005). Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314-322 [DOI] [PubMed] [Google Scholar]
- Dykstra B., Kent D., Bowie M., McCaffrey L., Hamilton M., Lyons K., Lee S., Brinkman R., Eaves C. (2007). Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218-229 [DOI] [PubMed] [Google Scholar]
- Essers M. A., Offner S., Blanco-Bose W. E., Waibler Z., Kalinke U., Duchosal M. A., Trumpp A. (2009). IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458, 904-908 [DOI] [PubMed] [Google Scholar]
- Fleming H. E., Janzen V., Guo J., Celso C. L., Leahy K. M., Kronenberg H. M., Scadden D. T. (2008). Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A. L., Silberstein L., Putheti P., Gao W., Saito T., Lo Celso C., Tsuyuzaki H., Sato T., et al. (2011). In vivo imaging of Tregs providing immune privilege to the hematopoietic stem cell niche. Nature 474, 216-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., North T. E., Loewer S., Lord A. M., Lee S., Stoick-Cooper C. L., Weidinger G., Puder M., Daley G. Q., Moon R. T., et al. (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. C., Bailey A. S., Pfaffle D. L., Al Masri A., Christian J. L., Fleming W. H. (2009). BMP4 regulates the hematopoietic stem cell niche. Blood 114, 4393-4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell M. A. (2005). Stem cell identification and sorting using the Hoechst 33342 side population (SP). Curr. Protoc. Cytom. Chapter 9, Unit9 18 [DOI] [PubMed] [Google Scholar]
- Grassinger J., Haylock D. N., Williams B., Olsen G. H., Nilsson S. K. (2010). Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood 116, 3185-3196 [DOI] [PubMed] [Google Scholar]
- Haylock D. N., Nilsson S. K. (2006). The role of hyaluronic acid in hemopoietic stem cell biology. Regen. Med. 1, 437-445 [DOI] [PubMed] [Google Scholar]
- Hofmann I., Stover E. H., Cullen D. E., Mao J., Morgan K. J., Lee B. H., Kharas M. G., Miller P. G., Cornejo M. G., Okabe R., et al. (2009). Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell 4, 559-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. T., Butler J. M., Nolan D. J., Kranz A., Iida K., Kobayashi M., Kopp H. G., Shido K., Petit I., Yanger K., et al. (2009). Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Zagozdzon R., Jorda M. A., Parmar K., Fu Y., Williams J. S., Wood J. A., Makriyannis A., Banu N., Avraham S., et al. (2010). Endocannabinoids are expressed in bone marrow stromal niches and play a role in interactions of hematopoietic stem and progenitor cells with the bone marrow microenvironment. J. Biol. Chem. 285, 35471-35478 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jiang S., Alberich-Jorda M., Zagozdzon R., Parmar K., Fu Y., Mauch P., Banu N., Makriyannis A., Tenen D. G., Avraham S., et al. (2011). Cannabinoid receptor 2 and its agonists mediate hematopoiesis and hematopoietic stem and progenitor cell mobilization. Blood 117, 827-838 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jiang Y., Bonig H., Ulyanova T., Chang K., Papayannopoulou T. (2009). On the adaptation of endosteal stem cell niche function in response to stress. Blood. 114, 3773-3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wang J., Schneider A., Sun Y. X., Koh-Paige A. J., Osman N. I., McCauley L. K., Taichman R. S. (2006). Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 38, 497-508 [DOI] [PubMed] [Google Scholar]
- Katayama Y., Battista M., Kao W. M., Hidalgo A., Peired A. J., Thomas S. A., Frenette P. S. (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407-421 [DOI] [PubMed] [Google Scholar]
- Kiel M. J., Yilmaz O. H., Iwashita T., Yilmaz O. H., Terhorst C., Morrison S. J. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109-1121 [DOI] [PubMed] [Google Scholar]
- Kieslinger M., Hiechinger S., Dobreva G., Consalez G. G., Grosschedl R. (2010). Early B cell factor 2 regulates hematopoietic stem cell homeostasis in a cell-nonautonomous manner. Cell Stem Cell 7, 496-507 [DOI] [PubMed] [Google Scholar]
- Kirstetter P., Anderson K., Porse B. T., Jacobsen S. E., Nerlov C. (2006). Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, 1048-1056 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J. M., O'Donnell R., Kobayashi M., Ding B. S., Bonner B., Chiu V. K., Nolan D. J., Shido K., Benjamin L., et al. (2010). Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U., Wilson A., Cobas M., Kemler R., Macdonald H. R., Radtke F. (2008). Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood 111, 160-164 [DOI] [PubMed] [Google Scholar]
- Kohler A., Schmithorst V., Filippi M. D., Ryan M. A., Daria D., Gunzer M., Geiger H. (2009). Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 114, 290-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O., Dar A., Shivtiel S., Kalinkovich A., Lapid K., Sztainberg Y., Tesio M., Samstein R. M., Goichberg P., Spiegel A., et al. (2006). Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12, 657-664 [DOI] [PubMed] [Google Scholar]
- Kurth I., Franke K., Pompe T., Bornhauser M., Werner C. (2009). Hematopoietic stem and progenitor cells in adhesive microcavities. Integr. Biol. (Camb) 1, 427-434 [DOI] [PubMed] [Google Scholar]
- Lane S. W., Scadden D. T., Gilliland D. G. (2009). The leukemic stem cell niche–current concepts and therapeutic opportunities. Blood 114, 1150-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J. P., Winkler I. G., Hendy J., Williams B., Helwani F., Barbier V., Nowlan B., Nilsson S. K. (2007). Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells 25, 1954-1965 [DOI] [PubMed] [Google Scholar]
- Levesque J. P., Helwani F. M., Winkler I. G. (2010). The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 24, 1979-1992 [DOI] [PubMed] [Google Scholar]
- Lewandowski D., Barroca V., Duconge F., Bayer J., Van Nhieu J. T., Pestourie C., Fouchet P., Tavitian B., Romeo P. H. (2010). In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 115, 443-452 [DOI] [PubMed] [Google Scholar]
- Lo Celso C., Fleming H. E., Wu J. W., Zhao C. X., Miake-Lye S., Fujisaki J., Cote D., Rowe D. W., Lin C. P., Scadden D. T. (2009). Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C., Lin C. P., Scadden D. T. (2011). In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat. Protoc. 6, 1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord B. I., Testa N. G., Hendry J. H. (1975). The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood 46, 65-72 [PubMed] [Google Scholar]
- Lymperi S., Ersek A., Ferraro F., Dazzi F., Horwood N. J. (2011). Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 117, 1540-1549 [DOI] [PubMed] [Google Scholar]
- Maillard I., Koch U., Dumortier A., Shestova O., Xu L., Sai H., Pross S. E., Aster J. C., Bhandoola A., Radtke F., et al. (2008). Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2, 356-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S., Kincade P. W. (2009a). Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp. Hematol. 37, 19-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S., Kincade P. W. (2009b). Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell 4, 27-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini S. J., Mantei N., Dumortier A., Suter U., MacDonald H. R., Radtke F. (2005). Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 105, 2340-2342 [DOI] [PubMed] [Google Scholar]
- Mayack S. R., Wagers A. J. (2008). Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood 112, 519-531 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mendez-Ferrer S., Lucas D., Battista M., Frenette P. S. (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442-447 [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., Macarthur B. D., Lira S. A., Scadden D. T., Ma'ayan A., Enikolopov G. N., Frenette P. S. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829-834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A. R., Carta C., Migliaccio G. (1999). In vivo expansion of purified hematopoietic stem cells transplanted in nonablated W/Wv mice. Exp. Hematol. 27, 1655-1666 [DOI] [PubMed] [Google Scholar]
- Morikawa S., Mabuchi Y., Kubota Y., Nagai Y., Niibe K., Hiratsu E., Suzuki S., Miyauchi-Hara C., Nagoshi N., Sunabori T., et al. (2009). Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 206, 2483-2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K., Hoshii T., Muraguchi T., Tadokoro Y., Ooshio T., Kondo Y., Nakao S., Motoyama N., Hirao A. (2010). TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463, 676-680 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Arai F., Iwasaki H., Hosokawa K., Kobayashi I., Gomei Y., Matsumoto Y., Yoshihara H., Suda T. (2010). Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood 116, 1422-1432 [DOI] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P. L., Hauschka P. V., Fahey F., Daley G. Q. (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. S., McNagny K. M. (2007). Influence of host irradiation on long-term engraftment by CD34-deficient hematopoietic stem cells. Blood 110, 1076-1077 [DOI] [PubMed] [Google Scholar]
- Nilsson S. K., Johnston H. M., Whitty G. A., Williams B., Webb R. J., Denhardt D. T., Bertoncello I., Bendall L. J., Simmons P. J., Haylock D. N. (2005). Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232-1239 [DOI] [PubMed] [Google Scholar]
- North T. E., Goessling W., Walkley C. R., Lengerke C., Kopani K. R., Lord A. M., Weber G. J., Bowman T. V., Jang I. H., Grosser T., et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K., Mauch P., Vergilio J. A., Sackstein R., Down J. D. (2007). Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA 104, 5431-5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A., Kollet O., Ponomaryov T., Petit I., Franitza S., Grabovsky V., Slav M. M., Nagler A., Lider O., Alon R., et al. (2000). The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95, 3289-3296 [PubMed] [Google Scholar]
- Podar K., Chauhan D., Anderson K. C. (2009). Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23, 10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley G. V., Scott L. M., Ulyanova T., Papayannopoulou T. (2006). Lack of alpha4 integrin expression in stem cells restricts competitive function and self-renewal activity. Blood 107, 2959-2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers M. H., Mukherjee S., Guo S., Zhang S., Kobayashi T., Schoonmaker J. A., Ebert B. L., Al-Shahrour F., Hasserjian R. P., Scadden E. O., et al. (2010). Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom J., Istvanffy R., Gauthier K., Shimono A., Mages J., Jardon-Alvarez A., Kroger M., Schiemann M., Busch D. H., Esposito I., et al. (2009). Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell 5, 157-167 [DOI] [PubMed] [Google Scholar]
- Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324-336 [DOI] [PubMed] [Google Scholar]
- Saito Y., Uchida N., Tanaka S., Suzuki N., Tomizawa-Murasawa M., Sone A., Najima Y., Takagi S., Aoki Y., Wake A., et al. (2010). Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat. Biotechnol. 28, 275-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaniel C., Sirabella D., Qiu J., Niu X, Lemischka I. R., Moore K. A. (2011). Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood 118, 2420-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7-25 [PubMed] [Google Scholar]
- Sipkins D. A., Wei X., Wu J. W., Runnels J. M., Cote D., Means T. K., Luster A. D., Scadden D. T., Lin C. P. (2005). In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Clevers H. C. (2005). WNT signalling and haematopoiesis: a WNT-WNT situation. Nat. Rev. Immunol. 5, 21-30 [DOI] [PubMed] [Google Scholar]
- Stier S., Ko Y., Forkert R., Lutz C., Neuhaus T., Grunewald E., Cheng T., Dombkowski D., Calvi L. M., Rittling S. R., et al. (2005). Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977-988 [DOI] [PubMed] [Google Scholar]
- Tesio M., Golan K., Corso S., Giordano S., Schajnovitz A., Vagima Y., Shivtiel S., Kalinkovich A., Caione L., Gammaitoni L., et al. (2011). Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117, 419-428 [DOI] [PubMed] [Google Scholar]
- Trumpp A., Essers M., Wilson A. (2010). Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 10, 201-209 [DOI] [PubMed] [Google Scholar]
- Ulyanova T., Priestley G. V., Nakamoto B., Jiang Y., Papayannopoulou T. (2007). VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp. Hematol. 35, 565-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B., Purton L. E., Yu M., Brashem-Stein C., Flowers D., Staats S., Moore K. A., Le Roux I., Mann R., Gray G., et al. (1998). The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 91, 4084-4091 [PubMed] [Google Scholar]
- Visnjic D., Kalajzic Z., Rowe D. W., Katavic V., Lorenzo J., Aguila H. L. (2004). Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258-3264 [DOI] [PubMed] [Google Scholar]
- Walkley C. R., Olsen G. H., Dworkin S., Fabb S. A., Swann J., McArthur G. A., Westmoreland S. V., Chambon P., Scadden D. T., Purton L. E. (2007a). A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129, 1097-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley C. R., Shea J. M., Sims N. A., Purton L. E., Orkin S. H. (2007b). Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C., Madan V., Bartels S., Costa C., Blasig R., Rodewald H. R. (2009). Hematopoietic stem cell transplantation without irradiation. Nat. Methods 6, 267-269 [DOI] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., et al. (2008). Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118-1129 [DOI] [PubMed] [Google Scholar]
- Winkler I. G., Sims N. A., Pettit A. R., Barbier V., Nowlan B., Helwani F., Poulton I. J., van Rooijen N., Alexander K. A., Raggatt L. J., et al. (2010). Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815-4828 [DOI] [PubMed] [Google Scholar]
- Wright D. E., Wagers A. J., Gulati A. P., Johnson F. L., Weissman I. L. (2001). Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933-6 [DOI] [PubMed] [Google Scholar]
- Xie Y., Yin T., Wiegraebe W., He X. C., Miller D., Stark D., Perko K., Alexander R., Schwartz J., Grindley J. C., et al. (2009). Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457, 97-101 [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Iwama A., Takayanagi S., Eto K., Ema H., Nakauchi H. (2009). TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 113, 1250-1256 [DOI] [PubMed] [Google Scholar]
- Yoshihara H., Arai F., Hosokawa K., Hagiwara T., Takubo K., Nakamura Y., Gomei Y., Iwasaki H., Matsuoka S., Miyamoto K., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685-697 [DOI] [PubMed] [Google Scholar]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W. G., Ross J., Haug J., Johnson T., Feng J. Q., et al. (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836-841 [DOI] [PubMed] [Google Scholar]
- Zheng J., Huynh H., Umikawa M., Silvany R., Zhang C. C. (2011). Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood 117, 470-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.