To the Editor
Two papers recently published in Nature Medicine provide evidence that hypoxia-inducible factor-2α (HIF-2α) is crucial for articular surface homeostasis through mechanisms that involve, at least in part, regulation of genes such as Col10a1 (encoding the α1 chain of type X collagen, Col10A1), Mmp13 (encoding matrix metallo-proteinase-13, MMP-13) and Vegfa (encoding vascular endothelial growth factor-A, VEGF)1,2. Interestingly, Saito et al.1 also report a mild delay of chondrocyte hypertrophy in fetal growth plates of mice heterozygous for genetic knockout of HIF-2α (encoded by Epas1). The key implication of this finding is that homozygous loss of HIF-2α would markedly delay chondrocyte hypertrophy and replacement of cartilage by bone. However, as shown below, we found that this was not the case.
HIF-2 is a heterodimer of two proteins, HIF-2α and HIF-1β3. HIF-1β is constitutively expressed, whereas HIF-2α is the hypoxia-responsive component of the complex. The other HIF-α isoform is HIF-1α. Both isoforms are regulated by oxygen in a similar fashion. However, the two α subunits partially differ in their biological functions and in their molecular targets3.
The vast majority of skeletal bones are derived from the replacement of a chondrocyte mold by bone tissue according to a well-defined temporal and spatial sequence of events4,5. Undifferentiated mesenchymal cells condense and become highly proliferating chondrocytes. Proliferative chondrocytes synthesize type II collagen (Col2A1) and form a columnar layer; they then stop proliferating and differentiate into postmitotic hypertrophic cells. Hypertrophic chondrocytes predominantly synthesize Col10A1 and mineralize their surrounding matrix. This unique differentiation process, which is called endochondral bone development, is followed by death of hypertrophic chondrocytes, blood vessel invasion and, finally, replacement of the cartilaginous matrix with bone. HIF-1 has an essential and nonredundant role in endochondral bone development4,5.
To unequivocally prove a role for HIF-2α in fetal cartilage development and particularly in chondrocyte hypertrophy, we conditionally inactivated HIF-2α in limb bud mesenchyme using a Prx1 promoter– driven Cre–transgenic mouse and a mouse homozygous for a floxed Epas1 allele (Epas1fl/fl) or a double heterozygote for a floxed Epas1 allele and a universal null Epas1 allele (Epas1fl/−), respectively. Each of the three mouse lines has been extensively characterized and used in previous studies5.
At birth and throughout their weaning period, Prx1-Cre; Epas1fl/fl and Prx1-Cre; Epas1fl/− mutant mice looked indistinguishable from Prx1-Cre; Epas1fl/+, Prx1-Cre; Epas1+/− and Epas1fl/fl control mice (data not shown). PCR analysis of genomic DNA extracted from newborn Prx1-Cre; Epas1fl/fl and control chondrocytes showed that recombination of the Epas1 in mutant chondrocytes was virtually complete (Supplementary Fig. 1a). In addition, accumulation of HIF-2α protein was severely decreased in embryonic day 15.5 (E15.5) Prx1-Cre; Epas1fl/− hindlimb paws when compared to controls (Supplementary Fig. 1b); the very modest residual signal observed in mutant specimens was probably the result of background staining.
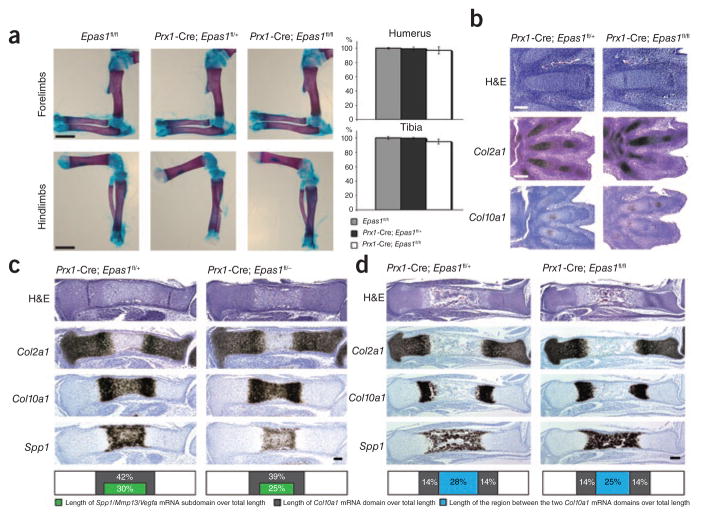
Whole-mount alizarin red S and alcian blue staining of mutant skeletons at birth revealed no pattern defect and no difference in length of the long bones when compared to control specimens (Fig. 1a). BrdU analysis performed at E15.5 did not show any change of chondrocyte proliferation rate in growth plates lacking HIF-2α (Supplementary Fig. 1c); moreover, we did not observe any difference in TUNEL staining in mutants versus controls (data not shown).
Figure 1.
Phenotypical analysis of Prx1-Cre; Epas1fl/fl and control littermates in embryonic and neonatal stages. (a) Whole-mount alizarin red S and alcian blue staining of hindlimb and forelimb zeugopods and stylopods isolated from newborn mutant and control mice. Scale bar, 1 mm. Length of tibia and humerus is shown as percentage of Epas1fl/fl controls ± s.d. The bar graph is a quantification of the data in the images. Differences between mutants and controls are not statistically significant. (b) H&E staining and in situ hybridization analysis of E13.5 mutant and control autopods. Bright-field images are shown. Scale bars: 100 μm (top) and 200 μm (bottom two rows). (c) H&E staining and in situ hybridization analysis of E15.5 mutant and control tibias. Bright-field images of one representative experiment are shown. Scale bar, 100 μm. Lengths of Col10a1 mRNA subdomains and of Col10a1/Spp1/Mmp13/Vegfa mRNAs subdomains are expressed as percentage of specimen total length (bottom). The difference in length between the mutant subdomain expressing Col10a1, Spp1, Mmp13 and Vegfa mRNAs and control (bottom) is statistically significant (P < 0.05). (d) H&E staining and in situ hybridization analysis of E17.5 mutant and control tibias. Bright-field images of one representative experiment are shown. Scale bar, 200 μm. Lengths of Col10a1 mRNA subdomains and distance between the two Col10a1 mRNA domains are expressed as percentage of specimen total length (bottom); differences in length between mutants and controls are not statistically significant.
We then pursued a systematic analysis of tibias and paws at various stages of fetal and postnatal development by routine histology and in situ hybridization. E13.5 mutant paws were histologically indistinguishable from controls; in particular, the appearance of Col10a1 mRNA–expressing cells was not delayed by loss of HIF-2α (Fig. 1b and data not shown). These findings indicate that HIF-2α is dispensable for mesenchymal condensations, for differentiation of mesenchymal cells into chondrocytes and for Col10a1 mRNA expression, at least at early stages of cartilage formation. Of note, during endochondral bone development, accumulation of detectable levels of Col10a1 mRNA consistently preceded the appearance of hypertrophic chondrocytes (Fig. 1b).
At E15.5, mutant tibia length was comparable to control (Fig. 1c and data not shown), as was extension of the domain of expression of Col2a1 mRNA in the growth plate, whereas the extension of the domain of expression of Col10a1 mRNA and, in particular, of the subdomain expressing osteopontin (Spp1), Mmp13 and Vegfa mRNAs, was shorter in mutants, although the difference was statistically significant only for the subdomain (Fig. 1c and data not shown). Osteopontin, MMP13 and VEGF are produced by late hypertrophic cells; that is, by those chondrocytes whose appearance immediately precedes blood vessel invasion and replacement of cartilage by bone. All in all, our findings thus indicate that, whereas appearance of Col10a1 mRNA–expressing cells is not affected by loss of HIF-2α, as shown by analysis of E13.5 hindlimb paw, differentiation of hypertrophic, Col10a1 mRNA–producing cells into late hypertrophic cells expressing Col10a1, Spp1, Mmp13 and Vegfa mRNAs is modestly, though significantly, impaired by lack of this transcription factor.
Consistent with these data, E17.5 mutant tibias were slightly shorter than controls; moreover, the distance between the two Col10a1 mRNA domains and, thus, the extension of the bone marrow cavity, were modestly decreased (Fig. 1d), features that were all probably consequences of the delayed appearance of late hypertrophic chondrocytes and, therefore, of the delayed replacement of cartilage by bone. Of note, however, none of these differences reached statistical significance, which highlights the overall extremely modest nature of the phenotype in the mutants. Indeed, postnatally, mutants and controls were virtually indistinguishable, as shown by whole-mount alizarin red S and alcian blue staining, routine histology and in situ hybridization (Fig. 1a, Supplementary Fig. 1d and data not shown).
In summary, differently from what we might have predicted on the basis of the findings in Epas1+/− mice reported by Saito et al.3, homozygous loss of HIF-2α in limb bud mesenchyme achieved by conditional knockout causes only a modest delay of endochondral bone development. More important, differently from what Saito et al.3 suggests, this delay is not due to impairment of Col10A1 accumulation, as Col10A1 expression is very robust and occurs in a timely manner in growth plates lacking HIF-2α, but rather is secondary to a very modest impairment of differentiation of hypertrophic cells into late hypertrophic chondrocytes. Last, the delay of endochondral bone development in mutants is transient and no longer detectable in postnatal life.
In conclusion, the role of HIF-2α in growth plate development and, particularly, in expression of Col10a1 mRNA, is not as crucial as it is in articular surface homeostasis. Identification of the molecular and cellular mechanisms responsible for this difference requires further studies.
Acknowledgments
This paper was supported by US National Institutes of Health grant RO1 AR048191-06 to E.S.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Saito T, et al. Nat Med. 2010;16:678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, et al. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JD, Simon M. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araldi E, Schipani E. Bone. 2010;47:190–196. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provot S, et al. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]