Summary
Endocrine Therapy Resistant Estrogen Receptor Positive (ER+) Breast Cancer is the most common cause of breast cancer death. Miller et al. demonstrate that ligand-independent ER activity promotes the growth of breast cancer cells through CDK4/E2F. As an independent event the PI3K pathway is also up regulated in endocrine therapy resistant cells. Promising preclinical evidence by several groups for the combination of an inhibitor of ligand-independent ER, fulvestrant, with PI3K inhibition, has led to the activation of trials evaluating this concept.
Estrogen receptor positive (ER+), Human Epidermal Growth Factor Receptor 2 negative (HER2-) breast cancer is the most prevalent subtype and therefore most frequent cause of breast cancer death. While endocrine therapy improves outcome for all stages of the disease, prognosis is poor once therapy resistance develops. A major translational research goal is, therefore, to fully understand the mechanisms through which endocrine therapy resistance occurs so that effective interventions can be identified. One of the more poorly understood aspects of endocrine therapy resistance is ligand-independent ER activation. When this event occurs, the most common therapeutic approach, aromatase inhibition (AI) or other estrogen-deprivation therapies, become ineffective. However the ER destabilizing drug fulvestrant is modestly effective in this setting, particularly if adequate doses are used, providing clinical evidence that tumors can still depend on ER for growth even when tumors are apparently not estrogen-driven 1. While a number of investigators have focused on how ER becomes active in a ligand-independent manner through activation of growth factor signaling 2, relatively few have studied the signaling events distal to ER itself- a critical question from the perspective of therapeutic interventions.
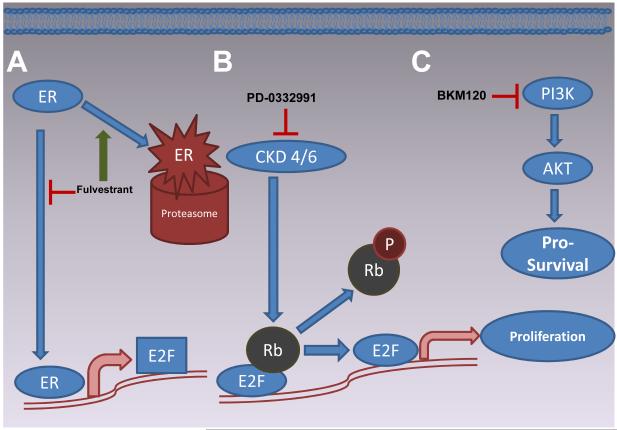
In this issue of Cancer Discovery, Miller et al. used fulvestrant as a probe to identify genes that are activated by ligand-independent ER in cell lines that had been selected in vitro to grow in estrogen depleted medium (Long-Term Estrogen Deprived – LTED) [ref]. They subsequently performed ChIP-seq to map the promoter elements associated with the fulvestrant modulated genes. By focusing on ER binding sites within 60KB of transcriptional start sites, they were able to confirm the identity of a number of targets for positive transcriptional regulation. Interestingly, they found that only about half of the ER binding regions were previously mapped canonical proximal estrogen response elements. Further transcriptional network analysis identified that 12 of the top 20 genes in the analysis harbored E2F binding sites within 2kb of the transcription start site. To provide further evidence for the role of these E2F regulated genes they examined 68 patient samples from a neoadjuvant AI clinical trial and found that an E2F gene expression signature was associated with higher levels of the proliferation marker Ki67 in the AI treated samples – thereby strengthening the association between the E2F pathway and estrogen-independent ER activation. This is a critical experiment as high levels of Ki67 in ER+ tumor samples despite treatment with an aromatase inhibitor is a reliable predictor of poor outcome 3. A kinome siRNA library screen in ER+ breast cancer cells selected in vitro to grow without estradiol suggested one proximal mediator of ligand independent ER mediated growth was CDK4 – consistent with the conclusion that E2F activation is a pivotal down-stream event. Thus one can envisage a relationship between ER and CDK4 regulated E2F outlined in Figure 1.
Figure 1.
By targeting both the survival and proliferation pathways, there is a synergistic effect on tumor cell death. A. Fulvestrant targets ER for proteosomal degradation which down regulates E2F transcription. B. Inhibition of CDK4 by PD-0332991 inhibits phosphorylation of Rb which in turn prevents the release of E2F from Rb repression. Both Fulvestrant and PD-0332991 accomplish the goal of down regulating E2F and down regulating the proliferation signal. C. Inhibition of PI3K by BMK120 decreases the pro-survial signal from PI3K.
While the experiments by Miller et al. are quite compelling, E2F activation is not the only signaling aberration in ligand independent ER positive breast cancer cells. The PI3K pathway also becomes hyper-activated upon the development of resistance to estrogen deprivation 4,5. Consistent with these pathways being independent events, PI3K inhibition did not alter ER-DNA binding and conversely fulvestrant did not alter phosphorylated AKT levels. Using immunocompromised mice bearing ER+ LTED tumor xenografts they demonstrated that both PI3K inhibition and fulvestrant delay tumor growth but in combination these inhibitors induce near-complete tumor regression. This is finding is consistent with the previously described synthetic lethal effect of combined ER and PI3K inhibition 6 and the finding fulvestrant sensitizes several LTED cell lines to the apoptotic effects of PI3K pathway targeting agents operating at the level of mTOR, PI3K catalytic activity or both 7.
A number of factors complicate clinical application of the therapeutic principles proposed by Miller and colleagues. First, is the heterogeneity of ER positive breast cancers – the models they study may represent biology that is unusual – i.e. in reality many tumors may not respond this way. The collection of cell lines used to model ER+ breast cancer is, after all, remarkably limited given how common this disease is. Many factors may ultimately determine the efficacy of fulvestrant PI3K inhibitor combination therapy – loss of ER, which is associated with pan-endocrine therapy resistance, lack of PI3K inhibitor sensitizing mutations and activation of other pathways are the obvious possibilities. Another very curious feature of ER+ tumors exhibiting ligand independence is that estradiol can paradoxically induce tumor regression. Indeed a recent report on the therapeutic effects of low dose estradiol for the treatment of aromatase inhibitor resistant breast cancer is worth considering in light of the mechanism under discussion 8. Could raising the estradiol levels to premenopausal levels somehow perturb the LTED mechanism described by Miller et al. through feedback suppression of E2F and/or PI3K, thereby restoring the ligand-dependent ER transcriptional pathway? After all, the very cell line they studied most intensely – LTED MCF7 cells – are highly sensitive to estradiol induced cell death 9,10.
A search of ClinicalTrials.gov reveals that multiple trials of various combinations of endocrine agents, PI3K inhibitors and CDK4 inhibitors are underway. However these investigations would be greatly enhanced by knowledge of the individual genomes in question. Within the next year most of the recurrent somatic mutations in ER+ HER2-breast cancer will have been identified by “Next Gen” sequencing. Application of this knowledge is, in the authors’ view, likely to generate a much clearer understanding of the tumors where these combinations will be effective. Given the model outlined in Figure 1, it will be interesting to examine copy number aberrations and somatic mutations in E2F targets, Rb, CDK4 regulators and in genes serving the PI3K pathway as potential modulators of drug efficacy. We should not be reticent about asking patients for metastatic disease biopsies and for consent for genomic analysis and data sharing in these studies, as therein we suspect will be found many of the answers to the complex puzzle of how these agents should be developed.
REFERENCES
- 1.Howell A, Sapunar F. Fulvestrant Revisited: Efficacy and Safety of the 500-mg Dose. Clin Breast Cancer. 2011 doi: 10.1016/j.clbc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7:4338s–4342s. discussion 4411s-4412s. [PubMed] [Google Scholar]
- 3.Ellis MJ, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller TW, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creighton CJ, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowder RJ, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez CG, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis MJ, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. Jama. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song RX, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JS, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]