Abstract
Background
Fatty acid amide hydrolase (FAAH) is a key enzyme in regulating endocannabinoid (eCB) signaling. A common single nucleotide polymorphism (C385A) in the human FAAH gene has been associated with increased risk for addiction and obesity.
Methods
Using imaging genetics in 82 healthy adult volunteers, we examined the effects of FAAH C385A on threat- and reward-related human brain function.
Results
Carriers of FAAH 385A, associated with reduced enzyme and, possibly, increased eCB signaling, had decreased threat-related amygdala reactivity but increased reward-related ventral striatal reactivity in comparison to C385 homozygotes. Similar divergent effects of FAAH C385A genotype were manifest at the level of brain-behavior relationships. 385A carriers showed decreased correlation between amygdala reactivity and trait anxiety but increased correlation between ventral striatal reactivity and delay discounting, an index of impulsivity.
Conclusions
Our results parallel pharmacologic and genetic dissection of eCB signaling, are consistent with the psychotropic effects of Δ9-tetrahydrocannabinol and highlight specific neural mechanisms through which variability in eCB signaling impacts complex behavioral processes related to risk for addiction and obesity.
Introduction
Prompted by the well-described psychotropic effects of Δ9-tetrahydrocannabinol (THC), the active chemical of the medicinal plant Cannabis, modern neuroscience methodologies have greatly advanced our understanding of the intrinsic mechanisms mediating and regulating cannabinoid signaling in the CNS (1). Such endogenous cannabinoid or endocannabinoid (eCB) signaling has emerged as a potent modulator of neural circuitries mediating both basic physiological (2; 3) and advanced behavioral responses (4–6). Experimental manipulation of these mechanisms has revealed significant behavioral effects, especially in threat- and reward-related domains, which are generally consistent with the effects of Cannabis intoxication in humans (7). Furthermore, robust effects of eCB signaling on complex emotion- and reward-related behaviors and their underlying neural substrates, which are often abnormal in diseases such as addiction, depression, anxiety and obesity, have spurred efforts to develop novel therapeutic agents targeting this neuromodulatory system (8; 9).
After their biosynthesis from arachidonic acid, eCBs such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) typically modulate synaptic neurotransmission through stimulation of CB1, the principal CNS cannabinoid receptor widely expressed on multiple neuronal subtypes and their distributed circuitries. In turn, the duration and intensity of eCB signaling, especially for AEA, is regulated by two complementary mechanisms: enzymatic degradation via fatty acid amide hydrolase (10) and active synaptic clearance via the AEA transporter (11). The psychotropic and THC-like effects of AEA, however, appear to be coupled with fatty acid amide hydrolase (FAAH), but not AEA transporter function (12). Thus, FAAH, an integral membrane enzyme, may uniquely regulate behaviorally-relevant eCB signaling by mediating the hydrolytic breakdown of AEA into arachidonic acid and ethanolamine.
The elucidation of these molecular mechanisms has motivated attempts to understand its possible contribution to the emergence of stable individual differences in behavioral attributes (e.g., anxious or impulsive temperament) associated with increased risk for psychiatric disorders. Common genetic variation (i.e., polymorphisms) affecting the functioning of components involved in eCB neurotransmission (e.g., AEA, CB1, FAAH) may represent a significant potential source of inter-individual variability in eCB signaling that mediates emergent differences in emotion- and reward-related behaviors (13). In the last five years, significant progress has been made in describing the contributions of such common genetic polymorphisms to individual differences in complex behavioral phenotypes – in particular, by identifying effects of functional genetic variation on the neural processes that mediate behavioral responses to environmental challenge (14; 15).
In the current study, we used an imaging genetics (16; 17) strategy to explore the contribution of genetic variation affecting eCB signaling to inter-individual variability in threat- and reward-related human brain function. Because of its critical role in regulating the signaling duration and intensity of AEA (10), and its selective contribution to the psychotropic effects of AEA (12), we focused on a common variant in the human gene for FAAH. Specifically, we examined a functional nonsynonymous single nucleotide polymorphism (C385A; rs324420) resulting in the conversion of a conserved proline residue to threonine (P129T) in the amino acid sequence of FAAH (18). In human lymphocytes, FAAH 385A, with an allele frequency of ~25% in populations of Caucasian ancestry, is associated with normal catalytic properties, but reduced cellular expression of FAAH, possibly through enhanced sensitivity to proteolytic degradation (19; 18). Moreover, the C385A is the only common mutation in FAAH (20) and the 385A, which putatively augments AEA signaling via decreased enzymatic degradation, has been associated with reward-related pathologies including street drug use and problem drug/alcohol abuse, as well as being overweight and obese (20; 18).
In animal models, both pharmacologic and genetic disruption of FAAH function result in decreased anxiety-like behaviors, as well as increased consumption and preference for ethanol (21–24; 12). Consistent with these divergent effects, we hypothesized that the FAAH 385A would be associated with relatively decreased threat-related amygdala reactivity, but increased reward-related reactivity in the ventral striatum (VS). Blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) was employed to assay threat-related amygdala and reward-related VS reactivity using challenge paradigms that have successfully identified the impact of genetically-driven variability in serotonin and dopamine neurotransmission on these same brain functions (25–29).
Materials & Methods
Participants
A total of 103 participants were recruited from the Adult Health and Behavior (AHAB) project, an archival database encompassing detailed measures of behavioral and biological traits among a community sample of 1,379 non-patient, middle-aged volunteers. Written informed consent according to the guidelines of the University of Pittsburgh Institutional Review Board was provided by all participants prior to their participation in our neuroimaging subcomponent of AHAB. All participants included in our analyses were in good general health and free of study exclusions (see supplementary materials). In the current study, overlapping FAAH genotype and threat-related amygdala reactivity data were available in 82 adult Caucasian volunteers, while overlapping genotype and reward-related VS reactivity were available in 71 of these same volunteers.
Genotyping
FAAH C385A was genotyped using published methods (see supplementary materials). In addition, the 5-HTTLPR, MAOA 30-bp VNTR, TPH2 G(−844)T, DAT1, DRD2 −141C Ins/Del and DRD4 exon3 48-bp VNTR were all genotyped using published protocols (25–29). All of these genotypes were scored by two independent readers by comparison to sequence verified standards and all call rates were >95%. No additional polymorphisms in FAAH or any other eCB-related genes (e.g., CB1) were examined in our study.
Consistent with analyses in the parent study from which our subjects were recruited (30), we used the program STRUCTURE (31) to evaluate presence of genetic substructure in the sample using 15 ancestry informative markers (see supplementary materials for details).
Amygdala Reactivity Paradigm
The experimental fMRI paradigm consisted of four blocks of a face processing task interleaved with 5 blocks of a sensorimotor control task (32; 25; 33; 34). Subject performance (accuracy and reaction time) was monitored during all scans. Details of our paradigm are provided as supplementary material.
Ventral Striatum Reactivity Paradigm
Our blocked-design paradigm consisted of pseudorandom presentation of trials wherein participants played a card guessing game and received positive or negative (i.e., win or loss) feedback for each trial (27; 35). Details of our paradigm are provided as supplementary material.
BOLD fMRI Data Acquisition, Processing & Analysis
Each participant was scanned using a Siemens 3T MAGNETOM Allegra developed specifically for advanced brain imaging applications and characterized by increased T2* sensitivity and fast gradients which minimize echo-spacing thereby reducing EPI geometric distortions and improving image quality. BOLD functional images were acquired with a gradient echo EPI sequence (TR/TE=2000/25 msec, FOV=20 cm, matrix=64×64) which covered 34 inter-leaved axial slices (3mm slice thickness) aligned with the AC-PC plane and encompassing the entire cerebrum and the majority of the cerebellum (see supplementary materials).
Whole brain image analysis was completed using SPM2. Following preprocessing (see supplementary materials), linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (i.e., Faces>Shapes) blood oxygen level-dependent activation for each individual and scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine FAAH C385A genotype effects on condition-specific regional responses (see supplementary materials). All analyses were thresholded at a voxel level of p<0.05, FDR corrected for multiple comparisons within an inclusive mask of activations of interest, and an extent threshold of at least 10 contiguous voxels. These statistical thresholds have recently been demonstrated to effectively limit “false positive” associations in imaging genetics studies below 5% (0.2–4.1%) and are, in fact, conservative (36).
Both our amygdala and VS regions of interest (ROI) were constructed using the WFU PickAtlas Tool (v1.04). Our bilateral amygdala ROI was based on the Talairach Daemon option of the PickAtlas, but with additional 3D dilation (1x) to encompass the dorsal extended amygdala. The VS ROI was defined as a sphere of 20mm in radius, centered on the Talairach coordinates of x = 0, y = 10, z = −10. Visual inspection confirmed that this ROI encompassed the entire VS as well as adjacent regions of the caudate nucleus (i.e., head of caudate) in both right and left hemispheres. All neuroimaging data are reported using the coordinate system of Talairach & Tournoux.
Behavioral Assessments
Spielberger State-Trait Anxiety Inventory (STAI) Trait version was used to assess the general tendency with which individuals perceive encountered situations to be threatening and to respond to such situations with subjective feelings of apprehension and tension (37). Delay discounting (DD) is a well-characterized behavioral measure of preference for immediate over delayed rewards and provides an index of impulsive tendencies in humans (38). The DD task used in our current study has been previously described in detail (35). Barratt Impulsiveness Scale (BIS-10-R) was used to assess tendencies to act without thinking, to make decisions “on the spur of the moment” and to fail to plan ahead (39).
Data Analyses
Because there were only 2 385A allele homozygotes, analyses were conducted using two genotype groups: 385A carriers (n=39) and C385 homozygotes (n=43). FAAH genotype effects on threat- and reward-related amygdala and VS reactivity, respectively, were examined using ANCOVA with sex as a covariate in all condition-specific analyses. Moderation of brain-behavior relationships by FAAH genotype was explored using genotype-specific correlations and multiple regression.
Results
Sample Demographics (Table)
Table.
Sample and FAAH C385A genotype demographics.
| FAAH 385A Carriers | FAAH C385 Homozygotes | |||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| Total N | 17 | 22 | 39 | 26 | 17 | 43 |
| Age (Mean ± SD) | 44.6 ± 6.8 | 45.1 ± 6.0 | 44.9 ± 6.2 | 45.6 ± 7.1 | 43.7 ± 7.2 | 44.8 ± 7.1 |
FAAH C385A allele frequencies (C=0.75 & A=0.25) in our sample of 82 healthy adult Caucasian volunteers were consistent with prior reports. The distribution of our observed genotypes (C/C=43, C/A=37 & A/A=2) did not deviate from Hardy-Weinberg equilibrium (χ2=3.2, P=0.21). This was confirmed with Fisher’s exact test (P=0.08) implemented in GENEPOP (v1.2). STRUCTURE analyses revealed no evidence of genetic substructure in our sample (log probability of data for k = 1, 2, 3 and 4 subpopulations in STRUCTURE were − 1292.6, −1304.6, −1287.6 and −1296.3, respectively) and thus, no further adjustments were made to control for type I or type II errors attributable to genetic stratification. Moreover, the two genotype groups did not differ in the distribution of multiple functional polymorphisms (all χ2s<2.1, all P’s>0.15) in both serotonin (i.e., 5-HTTLPR, MAOA 30-bp VNTR, TPH2 G(-844)T) and dopamine (i.e., DAT1, DRD2 −141C Ins/Del and DRD4 exon3 48-bp VNTR) related genes previously linked with variability in either amygdala or VS reactivity (25–29). Genotype groups also did not differ (all P’s>0.45) with respect to age, sex distribution, performance on either task (with all subjects at or near ceiling) or any behavioral measures (i.e., trait anxiety, delay discounting and impulsivity). The two groups also did not differ (all P’s>0.20) with respect to the occurrence of any prior Axis I pathology (data available upon request from corresponding author). Finally, neither past cannabis abuse nor dependence (defined using DSM-IV criteria) differed as a function of FAAH C385A genotype (C385 homozygotes: 7=abuse, 2=dependence; 385A carriers: 6=abuse, 1=dependence; F≤0.21, P≥0.65). Thus, our observed genotype effects are not confounded by current (all subjects were free of any substance use) or past cannabis exposure. Furthermore, removal of these 16 subjects did not affect the pattern of results reported below.
FAAH Genotype Effects on Amygdala Reactivity
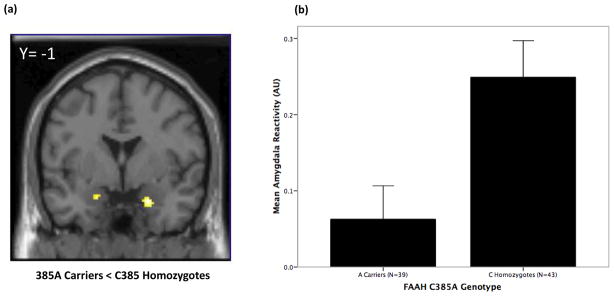
There was a significant effect of FAAH C385A genotype on amygdala reactivity to all threat-related expressions (right: Z=2.75, P=0.035; left: Z=2.54, P=0.044), angry expressions (left: Z=2.54, P=0.042) and fearful expressions (right: Z=2.83, P=0.029; left: Z=2.47, P=0.049). Consistent with our hypothesis, the 385A was associated with significantly decreased amygdala reactivity in all comparisons (Fig. 1).
Figure 1.
(a) Statistical parametric map illustrating significantly less fear-related amygdala reactivity in FAAH 385A carriers in comparison with C385 homozygotes (right amygdala: 22, −1, −20; Z = 2.83, P = 0.029, 34 voxels; left amygdala: −20, 1, −17; Z = 2.47, P = 0.049, 18 voxels). (b) Mean (±SEM) fear-related amygdala reactivity from maximum voxel in the right amygdala cluster from (a) in arbitrary units (AU) as a function of FAAH genotype (F(1,79) = 8.46, P = 0.005).
FAAH Genotype Effects on Ventral Striatum Reactivity
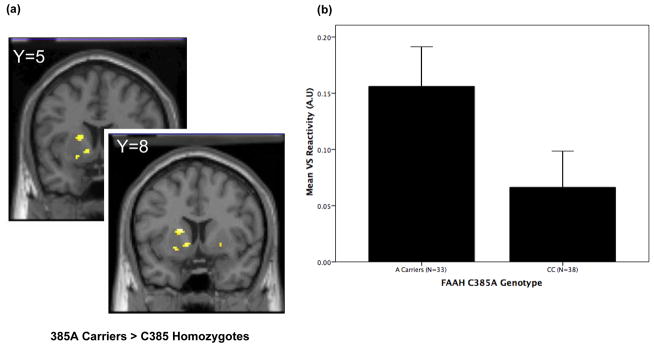
There was also an effect of FAAH C385A genotype on reward-related VS reactivity in the left hemisphere (Z=2.26, P=0.05). Again consistent with our hypothesis, the 385A was associated with significantly increased VS reactivity (Fig. 2).
Figure 2.
(a) Statistical parametric map illustrating significantly greater reward-related VS reactivity in FAAH 385A carriers in comparison with C385 homozygotes (left VS: −10, 8, −2; Z = 2.26, P =0.049, 24 voxels). Two coronal overlays are presented to illustrate the extent of the genotype effect on VS reactivity, which spanned a continuum from Y = 5 to Y = 11. (b) Mean (±SEM) reward-related VS reactivity from maximum voxel in the left VS cluster from (a) in arbitrary units (AU) as a function of FAAH genotype (F(1,69) = 5.32, P = 0.024).
FAAH Genotype Modulation of Brain-Behavior Correlations
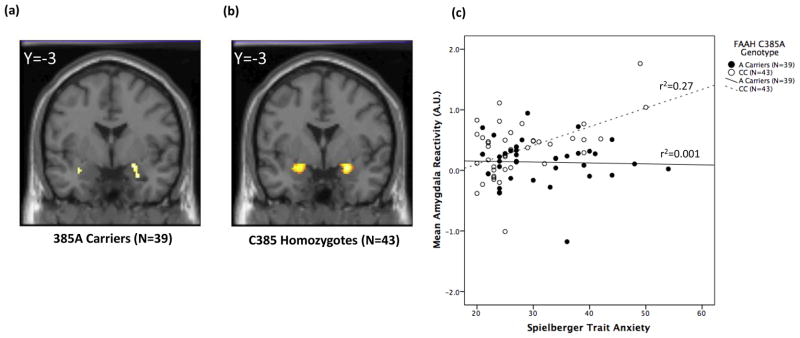
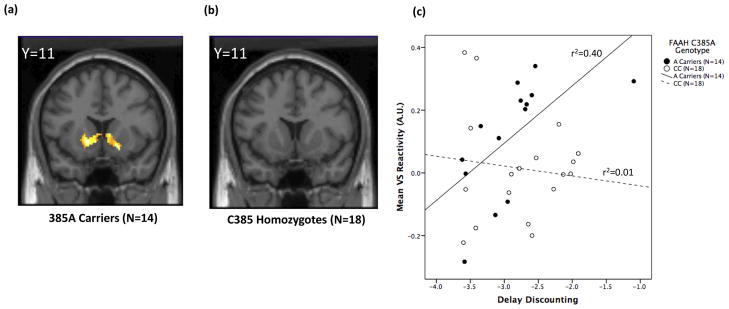
Given the divergent genotype effects on amygdala and VS reactivity, we explored the potential modulatory influence of the FAAH C385A on the relationships between underlying brain function and emergent threat- and reward-related behaviors using genotype-specific correlations. The relationship between bilateral amygdala reactivity to fearful expressions and trait anxiety, as assessed by the trait form of the Spielberger State-Trait Anxiety Inventory, in FAAH 385A carriers was nearly threefold lower in magnitude and tenfold lesser in extent relative to C385 homozygotes (Fig. 3a & b). In contrast, a significant relationship between bilateral VS reactivity and delay discounting, a behavioral index of an individual’s preference for smaller immediate over larger delayed rewards, was only present in FAAH 385A carriers (Fig. 4a & b). Although overlapping VS reactivity and delay discounting data were available in only a subset of subjects (N=32), a similar pattern was observed for the relationship between VS reactivity and self-reported impulsivity, as indexed by BIS, for which values were available in all subjects (385A Carriers: r=0.43, P= 01; C385 homozygotes: r=0.12, P=0.46). Multiple regression analyses were implemented to formally test the significance of these observed interactions. Main effects of both FAAH genotype and amygdala reactivity were regressed onto STAI-Trait followed by the interaction term of FAAH genotype-by-amygdala reactivity. This interaction term significantly predicted trait anxiety (β=0.38, P=0.03) indicating that FAAH C385A moderates the relationship between threat-related amygdala reactivity and trait anxiety (Fig. 3c). In identical analyses, main effects of both FAAH genotype and VS reactivity were regressed onto delay discounting followed by the interaction term of FAAH genotype-by-VS reactivity. The interaction term of FAAH genotype-by-VS reactivity significantly predicted delay discounting (β=−0.54, P=0.04) indicating that FAAH C385A also moderates the relationship between reward-related VS reactivity and impulsivity (Fig. 4c).
Figure 3.
Statistical parametric maps illustrating the correlation between fear-related amygdala reactivity and trait anxiety in (a) FAAH 385A carriers (right amygdala: 24, −3, −12; Z = 2.26, P =0.049, 46 voxels; left amygdala: −32, −3, −15; Z = 2.04, P = 0.046, 10 voxels) and (b) C385 homozygotes (right amygdala: 26, 1, −14; Z = 3.84, P = 0.004, 81 voxels; left amygdala: −20, −4, −10; Z = 3.82, P = 0.008, 184 voxels). (c) Plots of the differential correlation between fear-related amygdala reactivity (−22, 1, −15; Z = 3.48, P = 0.003, 13 voxels) and trait anxiety as a function of FAAH C385A genotype (β = 0.38, P = 0.03; 385A carriers: r = −0.03, P = 0.84; C385 homozygotes: r = 0.52, P < 0.001).
Figure 4.
Statistical parametric maps illustrating the correlation between reward-related VS reactivity and delay discounting in (a) FAAH 385A carriers (maximum voxel: −14, 11, −6; Z = 3.15, P = 0.03, 449 voxels) and (b) C385 homozygotes (no significant correlation). (c) Plots of the differential correlation between reward-related VS reactivity (−16, 15, −4; Z = 2.99, P = 0.025, 41 voxels) and delay discounting as a function of FAAH C385A genotype (β = −0.54, P = 0.04; 385A carriers: r = 0.63, P = 0.02; and C385 homozygotes: r = 0.11, P = 0.65).
Discussion
We report that a common functional polymorphism associated with relatively decreased FAAH availability has divergent effects on threat- and reward-related human brain function. Specifically, we found that carriers of the FAAH 385A, associated with reduced enzyme expression and, presumably, increased AEA signaling, have decreased threat-related amygdala reactivity. In contrast, carriers of the FAAH 385A exhibited increased reward-related VS reactivity in comparison to C385 homozygotes. Moreover, divergent effects of FAAH C385A genotype on brain function were manifest in a consistent manner at the level of brain-behavior relationships. Relative to C385 homozygotes, FAAH 385A carriers showed a diminished relationship between amygdala reactivity and trait anxiety. In contrast, 385A carriers exhibited a markedly increased relationship between VS reactivity and delay discounting, a behavioral index of impulsivity and reward sensitivity. Importantly, absence of occult genetic stratification and the independence of FAAH genotypes from other functional polymorphisms impacting these brain functions (25–29) indicate that these divergent effects are uniquely attributable to the FAAH C385A.
Decreased threat-related amygdala reactivity and associated trait anxiety may contribute to the emergence of pathologies such as addiction and obesity, previously associated with the FAAH 385A (20; 18; 40), by reducing the sensitivity of these individuals to potential environmental threat or harm. In fact, blunted amygdala reactivity has been reported in individuals at high familial risk for alcoholism and this has been interpreted as possibly contributing to decreased threat-sensitivity and subsequently increased risk-taking behaviors in these genetically predisposed individuals (41). An increase in reward-related VS reactivity and associated impulsivity (e.g., steeper discounting of future, relative to immediate rewards) may likewise contribute to disinhibitory psychopathologies through heightened reward-sensitivity and impulsive decision making. Studies in addicted patients have generally reported a sensitization of the neural circuitry for reward, including the VS (42). And, increased behavioral impulsivity and reward-sensitivity are significant risk factors for addiction (43). Thus, through divergent effects on both threat- and reward-related brain function, FAAH C385A may have a compound and accelerated effect on risk for related pathologies.
In animal models, increasing AEA signaling through pharmacologic or genetic disruption of FAAH has anxiolytic-like effects (23; 24). In contrast, pharmacologic or genetic disruption of CB1-mediated eCB signaling has anxiogenic- and depressive-like effects (44–46). Such effects of eCB signaling on anxiety-like behaviors are likely mediated through the amygdala, where CB1 receptors are densely expressed (47; 48), and both AEA and 2-AG concentrations are markedly elevated during the presentation of anxiogenic stimuli (49). Thus, our observed effects of decreased threat-related amygdala reactivity and diminished correlation between amygdala reactivity and trait anxiety in FAAH 385A carriers may reflect relatively increased AEA signaling in the amygdala. Consistent with our genetic effect, a recent study in human subjects has reported that acute oral administration of THC is associated with reduced amygdala reactivity to threat-related facial expressions of emotion (50). Available data from animal models indicate that such effects may be driven by relatively increased CB1 agonism leading to long-term depression of local inhibitory networks in the basolateral amygdala, and by greater inhibition of pyramidal output neurons in the central amygdala via GABAergic intercalated neurons (51; 47).
Analogous effects on reward-related behaviors have been associated with disruption of AEA signaling in animal models (52). FAAH knockout mice exhibit increased ethanol consumption and preference, as well as decreased ethanol intoxication (21; 22). CB1 knockouts, in contrast, exhibit hyposensitivity to cannabinoids, opiates, cocaine and morphine (53–55). Disruption of FAAH function also potentiates the ability of AEA to increase dopamine levels in the nucleus accumbens (12), suggesting that effects of eCB signaling on reward-related behaviors are mediated through this core region of the VS, and may be dependent on AEA regulation of dopamine. Similar effects may drive our observation of increased reward-related VS reactivity and an exaggerated relationship between such reactivity and delay discounting in FAAH 385A carriers, who presumably have increased AEA via decreased enzymatic degradation.
Despite the remarkable convergence of the above findings, the exact nature of the downstream signaling pathways through which FAAH C385A may modulate neuronal and neural circuit function cannot be determined from our current results. FAAH catalyses the hydrolysis of other biologically active endogenous fatty acid amides (e.g., oleamide & oleoylethanolamide), which impact threat- and reward-related behaviors independently of AEA. For example, oleamide administration produces anxiolytic-like effects in mice (56) and oleoylethanolamide (OEA) affects feeding in rats (57). Importantly, however, FAAH has high selectivity for AEA (58). Nevertheless, the effects of FAAH C385A cannot be specifically linked to AEA neurotransmission without additional data. If the neural and behavioral effects of FAAH C385A are mediated by genotype-driven differential availability of AEA, then these effects should be sensitive to manipulation of CB1 receptors. An interesting test of this putative mechanism would be to examine the impact of CB1 antagonists, such as rimonabant, on neural phenotypes associated with FAAH C385A genotype. If this polymorphism biases brain function through AEA stimulation of CB1, then antagonism of the receptor should eliminate the divergent effects on amygdala and VS reactivity documented here. If CB1 antagonism fails to abolish the differential effects of FAAH C385A, then these effects are likely mediated by non-eCB fatty acid amides. In addition to testing this mechanistic hypothesis with pharmacological fMRI, future studies with substantially increased sample sizes are necessary to model allele load effects of FAAH 385A, as well as potential FAAH interactions with functional genetic polymorphisms affecting other components of eCB neurotransmission. For example, a recent imaging genetics study identified significant associations between multiple polymorphisms, each of unknown functionality, in the human CB1 gene (CNR1) and striatal response to happy facial expressions (59).
It is important to note that there were no direct associations between FAAH genotype and behavioral phenotypes (i.e., anxiety or impulsivity) in our study. This is a common occurrence when working with relatively small samples, possibly reflecting the minimal effect the proximal biological impact associated with any genotype has on any distal behavioral phenotype (16; 17), as well as the importance of environmental stressors in unmasking genetically driven effects on behavior (14). The effect size of FAAH genotype related variability in amygdala and VS reactivity was δ=0.63 and δ=0.45, respectively, and in the “medium” range (60), particularly for the amygdala. The power to detect these effects using a t test in our sample ranges from 0.84 in the amygdala to 0.58 in the VS. However, it should be noted that these estimates are based on behavioral phenotypes (61) and that calculations of power are less certain and can be misleading given the large number of voxels being tested in fMRI-derived neural phenotypes (62). Moreover, use of a priori ROIs, magnet field strength, experimental paradigm used to elicit BOLD signal change, the statistical software used for data analysis, and the signal-to-noise ratio in ROIs are all likely to impact power and may be difficult to quantify. Clearly as the use of fMRI becomes more cost effective, the assessment of larger samples will allow for the detection of smaller effects, particularly in circumscribed a priori regions of interest.
Despite the lack of significant direct associations between genotype and behavior, we did observe robust differences in the relationships between regional brain function and complex behaviors as a function of FAAH C385A genotype. We speculate that our observed brain-behavior patterns may reflect the influence FAAH C385A associated differences in endogenous eCB tone on stimulus-driven neural circuit function mediating complex behavioral processes. Relatively higher levels of AEA in the amygdala of FAAH 385A carriers may reduce the responsivity of this structure to salient input (possibly through CB1-mediated potentiation of local GABAergic interneurons) and, as a consequence, lead to reduced anxiety-like behaviors predicted by amygdala function. In contrast, higher levels of AEA may increase the responsivity of the VS in FAAH 385A carriers (possibly through CB1-mediated increased dopamine release and potentiation of VS neuron activity) leading to increased reward-sensitivity predicted by VS function. Support for this speculation exists in studies reporting a failure of restraint stress to effect changes in amygdala activation in knockouts lacking FAAH or animals treated with FAAH inhibitors (63), and increased food-intake as a result of local FAAH inhibition in the nucleus accumbens (64). Thus, the endogenous state of eCB signaling associated with either constitutive genetic variation such as the FAAH C385A or acute pharmacologic manipulation likely biases the responsivity of neural circuits to behaviorally-relevant information and their subsequent regulation of complex behaviors.
The observed divergent effects of FAAH C385A on amygdala and VS reactivity may further reflect eCB related modulation of the direct functional dynamics of these two regions. However, comparative anatomy reveals dense glutamatergic projections from the amygdala to the VS, specifically the nucleus accumbens, as well as to regions of the medial prefrontal cortex that project to the nucleus accumbens (65). Through these pathways, the amygdala typically drives VS activity (66). Thus, available evidence indicates that activity of the VS, either directly or indirectly, is potentiated by the amygdala suggesting that our observed divergent effects reflect the modulatory influence of eCB signaling associated with the FAAH C385A on local circuit function and responsivity to behaviorally-relevant stimuli.
Finally, as our findings are in a sample of healthy adults, the utility of FAAH C385A driven variability in threat- and reward-related brain function as a predictive marker of vulnerability to neuropsychiatric disorder needs to be tested in longitudinal designs that account for the moderating effects of environmental stress and drug exposure in the emergence of clinical disorder (14). Such effects would be predicted by the existing positive associations between the FAAH 385A and reward-related pathologies such as drug abuse and obesity (20; 18). However, reported clinical associations, especially with drug abuse, have typically been limited to individuals homozygous for the 385A allele. As we had only two 385A homozygous individuals in our current sample we were unable to evaluate possible effects of 385A allele load on reward-related VS reactivity. Future studies with larger samples affording modeling of 385A allele load are necessary to determine if additional alterations in reward-related brain function may be present in 385A homozygotes. Moreover, while alterations in eCB signaling associated with FAAH function have been associated with anxiety-related behaviors in animal models and comparable effects on anxiety-related behaviors have been noted following Cannabis intoxication in humans, there are currently no reported associations between the FAAH C385A (or other eCB-related genetic polymorphisms) and complex anxiety-related behaviors in healthy volunteers or patients with anxiety disorders. Until such links are established, the importance of our observed effects of FAAH C385A on amygdala reactivity in mediating variability in anxiety-related behaviors, as well as clinical mood and anxiety disorders, is unclear.
Supplementary Material
Acknowledgments
We thank Sarah M. Brown for assistance with fMRI data collection and analyses. We also thank Patrick M. Fisher, Karen E. Muñoz and Janet Lower for assistance with data analyses.
Funding
This work was supported by NIH grants HL040962 to SBM & MH072837 to ARH, as well as a NARSAD Young Investigator Award to ARH. IH is supported by a Pittsburgh Mind Body Center postdoctoral fellowship. LHW is supported by the predoctoral Training Program in Behavioral Brain Research (GM081760). FD and DG are supported by the intramural research program of NIAAA.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 2.Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–3. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 3.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–81. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 4.Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–42. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Scherma M, Medalie J, Fratta W, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–40. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson P. Human studies of cannabinoids and medicinal cannabis. Handb Exp Pharmacol. 2005:719–56. doi: 10.1007/3-540-26573-2_25. [DOI] [PubMed] [Google Scholar]
- 8.Maccarrone M. Fatty acid amide hydrolase: a potential target for next generation therapeutics. Curr Pharm Des. 2006;12:759–72. doi: 10.2174/138161206775474279. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ. Pharmacological properties and therapeutic possibilities for drugs acting upon endocannabinoid receptors. Curr Drug Targets CNS Neurol Disord. 2005;4:685–96. doi: 10.2174/156800705774933041. [DOI] [PubMed] [Google Scholar]
- 10.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 11.Piomelli D, Beltramo M, Glasnapp S, et al. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci U S A. 1999;96:5802–7. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solinas M, Tanda G, Justinova Z, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–80. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- 13.Onaivi ES, Leonard CM, Ishiguro H, et al. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002;66:307–44. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 14.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–90. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 16.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–70. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 18.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–9. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum Genet. 2006;120:581–8. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- 21.Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–44. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- 23.Kathuria S, Gaetani S, Fegley D, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 24.Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–50. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Brown SM, Peet E, Manuck SB, et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:805. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- 26.Drabant EM, Hariri AR, Meyer-Lindenberg A, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 27.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 29.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder I, Muldoon MF, Ferrell RE, Manuck SB. Polymorphisms in the Serotonin Receptor 2A (HTR2A) gene are associated with the Metabolic Syndrome. Metabolic Syndrome and Related Disorders. 2007;5:323–30. doi: 10.1089/met.2007.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–45. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- 33.Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 2007;164:1613–4. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- 34.Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60:1155–62. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 35.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–7. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–61. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD. State-trait anger expression inventory. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- 38.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–92. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barratt ES. Impulsiveness and aggression. In: Monahan J, Steadman HJ, editors. Violence and mental disorder: Developments in risk assessment. Chicago: University of Chicago Press; 1994. pp. 61–79. [Google Scholar]
- 40.Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144:660–6. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- 41.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–9. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 43.de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- 44.Navarro M, Hernandez E, Munoz RM, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–6. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 45.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–87. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 46.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 47.Katona I, Rancz EA, Acsady L, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–52. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- 49.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 50.Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–9. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends in Molecular Medicine. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–34. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 53.Ledent C, Valverde O, Cossu G, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–4. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 54.Cossu G, Ledent C, Fattore L, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–5. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 55.Soria G, Mendizabal V, Tourino C, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–80. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- 56.Wei XY, Yang JY, Dong YX, Wu CF. Anxiolytic-like effects of oleamide in group-housed and socially isolated mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1189–1195. doi: 10.1016/j.pnpbp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 57.LoVerme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci. 2005;62:708–16. doi: 10.1007/s00018-004-4494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desarnaud F, Cadas H, Piomelli D. Anandamide Amidohydrolase Activity in Rat Brain Microsomes. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 59.Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur J Neurosci. 2006;23:1944–8. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- 60.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N. J: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 61.Soper DS. The Free Statistics Calculators Website. 2008. [Google Scholar]
- 62.Hayasaka S, Peiffer AM, Hugenschmidt CE, Laurienti PJ. Power and sample size calculation for neuroimaging studies by non-central random field theory. Neuroimage. 2007;37:721–30. doi: 10.1016/j.neuroimage.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- 64.Sorice-Gomez E, Matias I, Rueda-Orozco PE, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 66.McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cereb Cortex. 2008;18:1961–72. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.