Abstract
In this issue of Molecular Cell, Hirschey et al. demonstrate that loss of the NAD+-dependent deacetylase SIRT3 and resultant mitochondrial protein hyperacetylation play a critical role in the pathogenesis of metabolic syndrome, providing new insights into the therapeutic potential of SIRT3.
Mitochondria are essential organelles for cell survival because of their extraordinary ability to generate energy. Mitochondrial dysfunction has been implicated in various human diseases including metabolic disorders, neurodegenerative diseases, cancer, and other age-associated diseases. In recent years, it has become increasingly clear that reversible lysine acetylation is an important post-translational modification of mitochondrial proteins for the maintenance of their proper function. A number of proteomics studies have revealed that many key metabolic enzymes are acetylated in mitochondria and that their enzymatic activities are regulated by adaptive changes in acetylation in response to environmental stimuli (Guan and Xiong, 2011). In mammals, one such dynamic modification is mediated by the NAD+-dependent protein deacetylase SIRT3. SIRT3 is one of the seven mammalian sirtuins, an evolutionarily conserved family of enzymes (Imai and Guarente, 2010), and has been shown to control multiple key metabolic pathways through its deacetylase activity in response to nutrient deprivation (Guan and Xiong, 2011). For example, it has recently been reported that SIRT3 deacetylates long-chain acyl-CoA dehydrogenase (LCAD) during fasting and promotes mitochondrial fatty acid oxidation in the liver (Hirschey et al., 2010). In the present study, Hirschey et al. have now identified a new role of SIRT3-dependent mitochondrial protein deacetylation in the pathogenesis of metabolic syndrome (Hirschey et al., 2011).
The authors initially examined how SIRT3 function and mitochondrial protein acetylation are altered by high-fat diet (HFD) feeding. They found that hepatic SIRT3 protein expression decreases in chronically HFD-fed mice (13 weeks on a HFD), compared to standard diet (SD)-fed mice. The observed loss of SIRT3 protein expression appears to be caused by the reduction of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), a known regulator of SIRT3. Consequently, HFD feeding enhances acetylation levels of hepatic mitochondrial proteins including LCAD. Increased LCAD acetylation results in the suppression of its enzymatic activity in the liver of HFD-fed mice, compared to SD-fed mice. Furthermore, as previously reported (Hirschey et al., 2010), SIRT3 knockout (SIRT3KO) mice display increased mitochondrial protein acetylation and decreased LCAD enzymatic activity in the liver under SD feeding, but do not exacerbate these changes under HFD feeding, implicating the direct link between HFD, SIRT3, and mitochondrial protein acetylation.
Interestingly, under HFD feeding, SIRT3KO mice show lower energy expenditure, greater adiposity, and higher insulin resistance than wild-type (WT) mice. To investigate the effects of SIRT3 deficiency on the development of insulin resistance, HFD-fed SIRT3KO mice were analyzed before they displayed severe obese phenotypes. Importantly, HFD-fed SIRT3KO mice at 3 months of age develop higher insulin levels and more severe insulin resistance than HFD-fed WT mice. Furthermore, HFD-fed SIRT3KO mice have more severe hepatosteatosis, higher inflammatory cytokine levels, and higher plasma lipid levels. Microarray data demonstrate that stearoyl-CoA desaturase 1 (SCD1), a key lipogenic enzyme, is most highly induced in the liver of SIRT3KO mice, compared to WT mice. Consistent with this finding, SIRT3KO mice display higher desaturation index due to the higher enzymatic activity of SCD1. Indeed, HFD-induced metabolic complications observed in SIRT3KO mice are significantly attenuated in SIRT3/SCD1 double knockout mice, suggesting that SCD1 plays a direct role for the pathogenesis of HFD-fed SIRT3KO phenotypes. Taken together, these findings indicate that the defect in hepatic SIRT3 function contributes to metabolic phenotypes in HFD-fed SIRT3KO mice. Nonetheless, it is still important to examine the tissue-dependent role of SIRT3 in metabolic disorders observed in HFD-fed SIRT3KO mice. For example, it has recently been shown that SIRT3 expression in skeletal muscle is reduced in models of type 1 and type 2 diabetes, leading to the development of insulin resistance (Jing et al., 2011). In pancreatic β-cells, mitochondrial signals affected by SIRT3 deficiency might contribute to pathophysiological changes in insulin secretion stimulated by various insulin secretagogues (Jitrapakdee et al., 2010). Thus, generation of tissue-specific SIRT3 transgenic and/or knockout mice will be required to fully assess this critical problem.
Lastly, the authors show association between a single nucleoside polymorphism (SNP) in the SIRT3 gene, namely rs11246020, and human metabolic syndrome. The point mutation encoded by rs11246020 results in the valine-to-isoleucine alteration at the position 208 (V208I) within the catalytic domain of SIRT3. The in vitro kinetics assays demonstrate that recombinant SIRT3-V208I shows reduced enzymatic activity compared to WT-SIRT3, indicating that the SIRT3-V208I mutant might increase susceptibility to the development of metabolic syndrome in humans. To investigate how this point mutation influences glucose and lipid metabolism in vivo, it will be interesting to analyze metabolic phenotypes in SIRT3-V208I mutant knock-in mice.
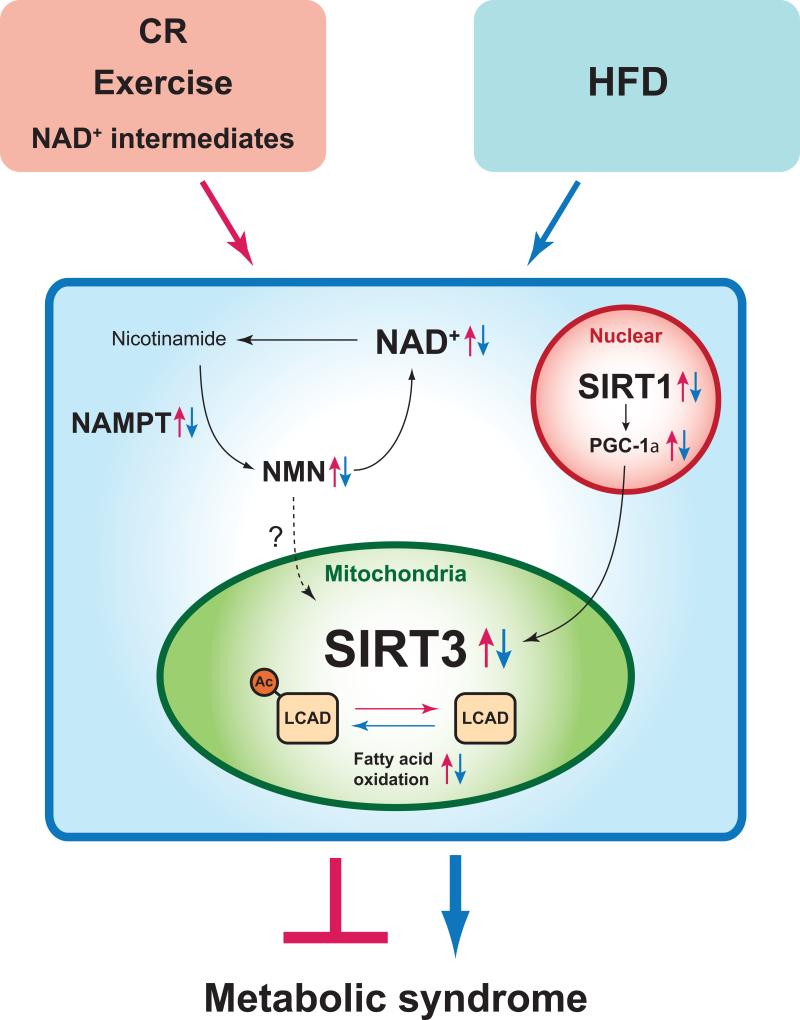
The findings reported by Hirschey et al. (Hirschey et al., 2011) provide critical insights into a new intervention against metabolic disorders and possibly other age-associated diseases (Figure 1). An accumulating body of evidence has shown that calorie restriction (CR) increases SIRT3 expression and its deacetylase activity, which might contribute to many beneficial effects of CR. Of particular note, CR protects age-related hearing loss and oxidative damage by increasing SIRT3-mediated deacetylation of isocitrate dehydrogenase 2 in mice (Someya et al., 2010). It has also been reported that regular endurance exercise enhances SIRT3 protein levels in skeletal muscle and normalizes age-associated mitochondrial dysfunction in humans (Lanza et al., 2008). Intriguingly, CR and fasting enhance NAD+ levels in the liver mitochondria, possibly through the induction of the key NAD+ biosynthetic enzyme, nicotinamide phosphoribosyltransferase (NAMPT) and its reaction product, nicotinamide mononulceotide (NMN) (Guan and Xiong, 2011; Nakagawa et al., 2009). Therefore, it will be of great importance to examine if the supplementation of key NAD+ intermediates such as NMN and nicotinamide riboside (NR) can boost mitochondrial NAD+ biosynthesis and be effective to treat and/or prevent metabolic disorders (Yoshino et al., 2011). It should be noted that activation of the nuclear sirtuin SIRT1 also exerts a protective function against diet-induced obesity and diabetes in mice (Imai and Guarente, 2010). Provided that PGC-1α is deacetylated and activated by SIRT1, it is possible that metabolic benefits from SIRT1 activation could be mediated, at least in part, by the PGC-1α-SIRT3 axis. Further investigation is expected to dissect the system dynamics of possible interplays among mitochondrial SIRT3, nuclear SIRT1, and NAMPT-mediated NAD+ biosynthesis (Figure 1). The present study will surely fuel more enthusiasm to the field of mitochondrial metabolism research and accelerate the development of new SIRT3-targeted treatments against metabolic syndrome and other age-associated diseases.
Figure 1. Therapeutic potential of mitochondrial SIRT3 for metabolic syndrome.
High-fat diet (HFD) feeding suppresses SIRT3 activity and NAMPT-mediated NAD+ biosynthesis (Yoshino et al., 2011), contributing to the pathogenesis of metabolic syndrome (blue arrows). SIRT3 activation is expected to protect against metabolic syndrome by deacetylating key mitochondrial enzymes such as long-chain acyl-CoA dehydrogenase (LCAD). Calorie restriction (CR), exercise, and key NAD+ intermediates are likely able to enhance SIRT3 dosage/activity (red arrows). Of note, Hirschey et al. (2011) also point out an interesting possibility that nuclear SIRT1 positively regulates SIRT3 expression levels through modulating the activity of PGC-1α.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2011;36:108–116. doi: 10.1016/j.tibs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, et al. SIRT3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol Cell. 2011 doi: 10.1016/j.molcel.2011.07.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:1019–1032. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills K, Yoon M, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]