Abstract
Objective
To provide family physicians with a practical clinical summary of the Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain, developed by the National Opioid Use Guideline Group.
Quality of evidence
Researchers for the guideline conducted a systematic review of the literature on the effectiveness and safety of opioids for chronic noncancer pain, and drafted a series of recommendations. A panel of 49 clinicians from across Canada reviewed the draft and achieved consensus on 24 recommendations.
Main message
Screening for addiction risk is recommended before prescribing opioids. Weak opioids (codeine and tramadol) are recommended for mild to moderate pain that has not responded to first-line treatments. Oxycodone, hydromorphone, and morphine can be tried in patients who have not responded to weaker opioids. A low initial dose and slow upward titration is recommended, with patient education and close monitoring. Physicians should watch for the development of complications such as sleep apnea. The optimal dose is one which improves function or decreases pain ratings by at least 30%. For by far most patients, the optimal dose will be well below a 200-mg morphine equivalent dose per day. Tapering is recommended for patients who have not responded to an adequate opioid trial.
Conclusion
Opioids play an important role in the management of chronic noncancer pain, but careful prescribing is needed to limit potential harms. The new Canadian guideline provides much-needed guidance to help physicians achieve a balance between optimal pain control and safety.
Opioid prescribing has increased dramatically in recent years. For example, oxycodone prescriptions among recipients of Ontario Drug Benefits rose by 850% from 1991 to 2007, from 23 prescriptions per 1000 individuals per year to 197 prescriptions per 1000 individuals per year.1 The average amount of oxycodone per prescription increased from 1830 mg to 2280 mg. Prescriptions of other opioids, particularly fentanyl, have also increased. These increases have been accompanied by increases in opioid-related harms such as addiction and overdose.2
Guideline development
In 2008, a research group selected by the Federation of Medical Regulatory Authorities of Canada conducted a systematic literature review on the effectiveness and adverse effects of opioids.3 The researchers drafted initial recommendations that were reviewed by the National Advisory Panel, a group of 49 experts in family medicine, physiatry, addiction medicine, and other disciplines. Through 4 rounds of review, the National Advisory Panel achieved consensus on 24 recommendations.
Quality of evidence
The research group relied on an update of a 2006 meta-analysis of controlled trials on the analgesic effectiveness of opioids for chronic noncancer pain (CNCP).4 In addition, several focused literature searches identified studies on long-term opioid effectiveness, medical complications, problematic opioid use, and opioid use within specific populations. In total, 10 798 studies were identified from the literature; 183 met inclusion criteria. The focused reviews included studies of any design that examined adult patients taking prescription opioids for CNCP. Final recommendations were graded according to level of evidence.
Main message
This paper is a brief clinical summary of the national guideline,5 including recommendations on opioid indications, selection, titration, precautions, and monitoring. The paper does not address other key components of CNCP treatment, such as nonopioid medications or physical and psychotherapeutic treatments. A companion paper (page 1269) summarizes the guideline’s recommendations for special populations.6 The complete guideline is available from nationalpaincentre.mcmaster.ca.5
Indications
Opioids should be reserved for patients who have not responded to nonopioid treatments and who have defined somatic or neuropathic pain conditions for which opioids have been shown to be effective. There is limited evidence to support the use of opioids for common pain conditions such as fibromyalgia and low back pain (Table 1).5 While tramadol (considered by many to be a weak opioid) is of modest benefit for fibromyalgia pain,7,8 strong opioids such as oxycodone or morphine have not been tested in this condition and are not recommended.9 Systematic reviews have concluded that opioids should not be used routinely for low back pain and other osteoarthritic conditions because of uncertainty about their long-term effectiveness,10 risk of misuse,11 and considerable side effects.12,13 Acetaminophen, nonsteroidal anti-inflammatory drugs, patient education, back exercises, and behavioural therapy are recommended for chronic low back pain.14
Table 1.
Evidence of opioid efficacy
|
EXAMPLES OF CNCP CONDITIONS FOR WHICH OPIOIDS WERE SHOWN TO BE EFFECTIVE IN PLACEBO-CONTROLLED TRIALS* |
EXAMPLES OF CNCP CONDITIONS THAT HAVE NOT BEEN STUDIED IN PLACEBO-CONTROLLED OPIOID TRIALS | |
|---|---|---|
| TRAMADOL ONLY | WEAK OR STRONG OPIOID | |
|
|
|
CNCP—chronic noncancer pain.
These trials were limited by the short duration of opioid therapy (maximum of 3 months).
Reprinted from the National Opioid Use Guideline Group.5
Baseline assessment
The physician should determine the cause and type of pain (neuropathic, nociceptive, or mixed) through a careful pain history, a physical examination, and appropriate investigations (Box 1).5 The physician should inquire about pain intensity (using an 11-point scale on which 0 represents no pain and 10 represents the worst possible pain), aggravating and relieving factors, and the effects of the pain on daily activities. Brief questionnaires, such as the short form of the McGill Pain Questionnaire15 or the Brief Pain Inventory (available online from www.algosresearch.org/PracticeTools/DxTestForms/index.html),16 can be useful for monitoring progress. Patients should be asked about personal and family histories of problematic substance use and about current use of alcohol, cannabis, opioids, benzodiazepines, sedating over-the-counter preparations, and street drugs. Screening questionnaires and urine drug screening can also help identify high-risk patients; additional resources and information are provided in the companion article on page 1269.6 Physicians should also inquire about mood and social support, as these can affect patients’ perceptions of pain.
Box 1. Baseline assessment of the chronic noncancer pain patient.
|
Reprinted from the National Opioid Use Guideline Group.5
Starting opioid therapy
Physicians should review the goals of opioid therapy and common side effects with patients before starting opioid therapy (Box 2).5 Physicians should emphasize that elimination of pain is unlikely; a realistic goal for opioid therapy is improved function or pain reduction of 30% or more. Potential medical complications should be reviewed, including sexual dysfunction,17,18 opioid-induced hyperalgesia,17,19 and sleep apnea.20–22 Patients should be warned to avoid alcohol and sedating drugs (particularly during titration), and to seek urgent medical attention if they experience early signs of overdose such as “nodding off” and slurred speech. Patients should also keep their opioid medication secure from friends or family. There is only weak evidence23 to support the use of opioid treatment agreements (Box 3),24 but they might be considered for patients who are not well known by the physician or who are at higher risk of misuse.
Box 2. Opioid information for patients: These messages could be used to create patient education materials.
Opioids are a group of similar medications that are used to help with pain—there is more than 1 type of opioid and they have different names (for example, Percocet, OxyContin, Tylenol No. 2, and Tramacet).
|
Reprinted from the National Opioid Use Guideline Group.5
Box 3. Sample opioid medication treatment agreement.
I understand that I am receiving opioid medication from Dr _______________ to treat my pain condition. I agree to the following:
I understand that if I break these conditions, Dr _______________ might choose to cease writing opiate prescriptions for me. |
Reprinted from Kahan et al.24
Benzodiazepines: Benzodiazepines are commonly implicated in opioid overdose deaths,25 and they considerably lower the lethal opioid dose.26 Therefore opioids should be titrated more slowly, with smaller dose increases, in patients taking benzodiazepines. Benzodiazepine tapering should be considered before initiating opioid therapy (Box 4),5,27 particularly in patients taking moderate to high daily doses (eg, 20 mg diazepam or 4 mg lorazepam or clonazepam).5 Benzodiazepines can be successfully tapered in the primary care setting28–31; psychiatric symptoms and sleep improve or remain stable with tapering.30,32–34 Similar precautions should be employed for other sedating drugs.
Box 4. Benzodiazepine tapering.
|
Reprinted from the National Opioid Use Guideline Group.5
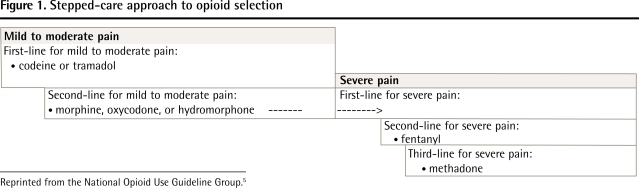
First-line opioids: If the physician decides to start opioid therapy, codeine or tramadol are suggested as first-line options for mild to moderate CNCP, as they have lower rates of overdose, misuse, and addiction than more potent opioids do,1,35–37 and controlled trials4 have shown that they are effective for CNCP (Figure 1).5
Figure 1.
Stepped-care approach to opioid selection
Reprinted from the National Opioid Use Guideline Group.5
Second-line opioids: If an adequate trial of codeine or tramadol fails to produce substantial pain relief or generates considerable side effects, then morphine, oxycodone, and hydromorphone are recommended as second-line treatments. Morphine should be used with caution in patients with renal impairment38 (Table 2).5, 27 Oxycodone and hydromorphone should be used with caution in patients at high risk of opioid misuse and addiction (see companion paper on page 1269).6 The starting dose of transdermal fentanyl (25 μg/h) can cause overdose in patients who are not fully tolerant to opioids. Therefore fentanyl should only be used in patients who have taken an opioid dosage of at least a 60- to 100-mg morphine equivalent dose (MED) daily for at least 2 weeks. Patients should not be switched directly from codeine to fentanyl, as up to 10% of white patients lack the enzyme P450 2D6 (CYP 2D6) that converts codeine to morphine and, therefore, they might not have developed a tolerance to opioids.39–41 Methadone has a high risk of overdose because of its long half-life42,43; it can be prescribed for pain only by physicians with a special exemption from Health Canada. Meperidine does not have a role in CNCP management; it has poor oral bioavailability, and parenteral meperidine can cause seizures and other neurologic events.44
Table 2.
Safety issues to consider when selecting opioids
| AGENT | SAFETY ISSUES* |
|---|---|
| Codeine | Prescribe for no more than 4 days in breastfeeding women: some women rapidly convert codeine to morphine, causing neonatal toxicity Overall lower risk of overdose and addiction than with stronger opioids |
| Tramadol | Associated with seizures in patients at high risk of seizure or when combined with medications that increase serotonin levels (eg, SSRIs) Lower risk of overdose and addiction than with stronger opioids |
| Morphine | A metabolite of morphine can accumulate to toxic levels in patients with renal impairment |
| Oxycodone, hydromorphone | Use with caution for patients at higher risk of opioid misuse and addiction |
| Fentanyl | Before prescribing fentanyl, ask about opioid use within the past 2 weeks; to ensure full tolerance, the patient should be taking a daily, scheduled dose of at least a 60- to 90-mg MED for at least 2 weeks, at least twice daily for CR opioids and at least 4 times daily for IR opioids Do not switch from codeine to fentanyl, regardless of the codeine dose; some patients have little or no opioid tolerance even with regular codeine use Maintain the initial dose for at least 6 days; use extra caution with patients at higher risk of overdose (eg, elderly patients, those taking benzodiazepines) Advise the patient as follows:
|
| Methadone | Use methadone to treat pain only if you hold a written Health Canada exemption Titration is hazardous because of its very long half-life, which leads to bioaccumulation |
| Meperidine | Not recommended for CNCP:
|
| Acetaminophenopioid combinations | Use with caution to avoid acetaminophen toxicity: no more than 3.2 g of acetaminophen for adults, which is equal to 10 tablets a day for codeine-acetaminophen or oxycodone-acetaminophen combinations; no more than 8 tablets a day for tramadol-acetaminophen combinations Warn heavy drinkers to not mix alcohol use with acetaminophen |
| CR formulations | Titrate with caution; CR tablets contain higher opioid doses than IR formulations do and can easily be converted to IR by biting or crushing the tablet |
| Parenteral opioids | Not recommended for CNCP:
|
Immediate-release (IR) opioids: Immediate-release opioids are used for initial titration and for breakthrough pain. They can also be used instead of controlled-release (CR) opioids for recurrent pain that lasts a few hours or less and for activity-related pain.
Controlled-release opioids: For patients who experience pain throughout the day, CR opioids might provide more constant pain relief than IR opioids do. Caution is required with CR tablets, as they contain much higher opioid doses than acetaminophen-opioid combinations do (eg, 1 80-mg OxyContin tablet is the equivalent of 16 Percocet tablets). Opioid euphoria, sedation, and overdose are dose-related.45–47
Initial titration: Opioids should be titrated slowly in CNCP patients in order to minimize the risk of acute toxicity (Table 3).5 An initial dosage of no more than a 5- to 10-mg MED 4 times daily is suggested, with dose increases of no more than a 5- to 10-mg MED per week. Elderly patients should be titrated more slowly than younger patients because they are at higher risk of acute toxicity (see companion paper on page 1269).6
Table 3.
Suggested initial opioid dose and titration based on oral dosing for CNCP
| OPIOID | INITIAL DOSE | RECOMMENDED TIME INTERVAL FOR INCREASE | SUGGESTED DOSE INCREASE | MINIMUM DAILY DOSE BEFORE CONVERTING IR TO CR |
|---|---|---|---|---|
| Codeine | 15–30 mg every 6 h | 7 d | 15–30 mg/d up to 600 mg/d | 100 mg |
| CR codeine | 50 mg every 12 h | 2 d | 50 mg/d up to maximum of 300 mg every 12 h | NA |
| Tramadol-acetaminophen | • 37.5/325 mg • 1 tablet every 4–6 h up to 4/d |
7 d | 1 tablet every 4–6 h up to 8 tablets/d | 3 tablets |
| CR tramadol | ||||
| • Zytram | 150 mg every 24 h | 7 d | 400 mg/d maximum | NA |
| • Tridural | 100 mg every 24 h | 2 d | 300 mg/d maximum | NA |
| • Ralivia | 100 mg every 24 h | 5 d | 300 mg/d maximum | NA |
| IR morphine | • 5–10 mg every 4–6 h • maximum 40 mg/d |
7 d | 5–10 mg/d | 20–30 mg |
| CR morphine | • 10–20 mg: once, twice, or 3 times daily • maximum 40 mg/d |
14 d | 5–10 mg/d | NA |
| IR oxycodone | • 5 mg every 4–6 h • maximum 30 mg/d |
7 d | 5 mg/d | 20 mg |
| CR oxycodone | • 10 mg 2–3 times daily • maximum 30 mg/d |
14 d | 10 mg/d | NA |
| IR hydromorphone | • 1–2 mg every 4–6 h • maximum 8 mg/d |
7 d | 1–2 mg/d | 6 mg |
| CR hydromorphone (Hydromorph Contin) | • 3 mg 2–3 times daily • maximum 9 mg/d |
14 d | 2–4 mg/d | NA |
CNCP—chronic noncancer pain, CR—controlled release, IR—immediate release, NA—not applicable.
Adapted from the National Opioid Use Guideline Group.5
Breakthrough doses: In most cases daily breakthrough doses should be no more than 10% to 20% of the total daily dose. The CR opioid can be increased if the patient consistently uses breakthrough doses multiple times during the day. Multiple daily breakthrough doses are usually not necessary unless the patient has severe neuropathic pain with unpredictable exacerbations. Additionally, IR opioids can be used just before activities that predictably cause severe exacerbations of pain; activity modification and pacing should also be employed.
Office visits: At each office visit during titration, pain intensity should be assessed using the 11-point (0 to 10) numeric rating scale. Opioids show a graded analgesic response, so if the pain is opioid-responsive, the patient will experience a small reduction in pain intensity with each dose increase. For nociceptive pain, the pain intensity should be recorded at rest and with activity. The physician should also inquire at each visit about side effects, compliance, and changes in mood and daily activities. The Brief Pain Inventory can help in the assessment.16
Optimal dose: The optimal dose is reached when the patient experiences improved function or at least a 30% pain reduction (about 2 points on the 11-point scale), with minimal analgesic benefit from 1 or 2 additional dose increases and no serious side effects or complications.48 For by far most CNCP patients, the optimal dosage will be well below a 200-mg MED daily (Table 4).5,27,49
Table 4.
Oral opioid analgesic conversion table based on oral dosing for chronic noncancer pain: A) Equivalence to 30 mg of oral morphine; B) Equivalence between oral morphine and transdermal fentanyl.
| A) | |||
|---|---|---|---|
| OPIOID | EQUIVALENCE TO 30 MG ORAL MORPHINE | TO CONVERT TO ORAL MORPHINE EQUIVALENT MULTIPLY BY ... | TO CONVERT FROM ORAL MORPHINE MULTIPLY BY ... |
| Morphine | 30 mg | 1 | 1 |
| Codeine | 200 mg | 0.15 | 6.67 |
| Oxycodone | 20 mg | 1.5 | 0.667 |
| Hydromorphone | 6 mg | 5 | 0.2 |
| Meperidine | 300 mg | 0.1 | 10 |
| Methadone and tramadol | Morphine dose equivalence not reliably established | ||
| B) TRANSDERMAL FENTANYL* | MORPHINE |
|---|---|
| 25 μg/h | 60–134 mg |
| 37 μg/h | 135–179 mg |
| 50 μg/h | 180–224 mg |
| 62 μg/h | 225–269 mg |
| 75 μg/h | 270–314 mg |
| 87 μg/h | 315–359 mg |
| 100 μg/h | 360–404 mg |
Formulations include 12-, 25-, 50-, 75-, and 100-μg/h patches, but the 12-μg/h patch is generally used for dose adjustment rather than initiation of fentanyl treatment.
Adapted from the National Opioid Use Guideline Group.5 Data from the Compendium of Pharmaceutical and Specialties27 and Pereira et al.49 Wide ranges have been reported in the literature. These equivalences refer to analgesic strength of oral opioids and not psychoactive effects or effectiveness in relieving withdrawal symptoms.
Doses above a 200-mg MED: The guideline suggests a careful reassessment if the dose approaches a 200-mg MED (the “watchful dose”). The physician should review the underlying diagnoses, the need for further investigation or consultation, the patient’s response to opioids, and dose-related medical complications. The 200-mg MED threshold was chosen because most patients require doses substantially below this; the average opioid dose used in controlled trials4 was 66 mg for oxycodone and 57 mg for morphine in nociceptive pain, and 81 mg for oxycodone and 92 mg for morphine in neuropathic pain (Table 5).5 Also, evidence suggests that dose-response relationships exist for medical complications of opioid therapy, including sexual dysfunction,50 sleep apnea,51–53 opioid-induced hyperalgesia,54 and falls and fractures.55,56 Recent evidence has identified a strong relationship between prescribed dose and risk of nonfatal and fatal overdoses.47 Among Ontario public drug plan recipients between 2003 and 2008, 2-year opioid-related mortality rates were 1.6, 7.9, and 9.9 per 1000 population for those prescribed less than a 200-mg MED, a 200- to 400-mg MED, and a greater than 400-mg MED, respectively.57
Table 5.
Morphine equivalents for strong opioids used in randomized trials
| DRUG | PAIN TYPE | MEQ MINIMUM | MEQ AVERAGE | MEQ MAXIMUM | NO. OF STUDIES |
|---|---|---|---|---|---|
| CR oxycodone | Nociceptive | 20 mg | 65.7 mg | 146.7 mg | 6 |
| Neuropathic | 40 mg | 81.3 mg | 173.3 mg | 3 | |
| CR morphine | Nociceptive | 25 mg | 56.8 mg | 120 mg | 2 |
| Neuropathic | 28.75 mg | 91.7 mg | 202.5 mg | 5 |
CR—controlled release, MEQ—morphine equivalent.
Adapted from the National Opioid Use Guideline Group.5
Furthermore, long-term observational studies found that patients taking high opioid doses tended to have greater disability and higher pain ratings than patients taking lower doses did, even after controlling for the severity of the underlying pain condition.58,59 Recent literature also suggests that physicians prescribe higher opioid doses to patients in greater psychological distress.60,61 These studies raise concerns about the safety and effectiveness of long-term, high-dose opioid therapy.
Switching opioids: Patients who have not responded to or who have had side effects with one opioid will sometimes benefit from switching to a different opioid.62 Because of unpredictable and incomplete cross-tolerance, the initial opioid dose of the new opioid should be no more than 50% of the previous dose if the latter is higher (ie, above a 75-mg MED), or 60% to 75% of the previous dose if the dose of the previous opioid was moderate (ie, below a 75-mg MED). There is no evidence to support the practice of combining different types of opioids.
Tapering
Patients whose pain has not responded to an adequate trial of several different opioids should have their doses tapered and discontinued. Observational studies have demonstrated that patients in severe pain despite high opioid doses experience reduced pain and improved mood with opioid tapering.63–68 It is not known why tapering might improve pain perception. Tapering might work by relieving hyperalgesia and withdrawal symptoms (withdrawal at the end of a dosing interval is more severe with high doses than low doses). Tapering might also improve mood by reducing opioid-induced sedation and dysphoria.61 Additionally, some of the benefits of tapering could be due to psychological cointerventions that accompanied tapering in these studies. Both physician and patient should approach the taper with positive expectations, as tapering is associated with improved mood and pain. Tapering is also indicated for patients experiencing side effects or dose-related medical complications. Box 5 outlines a tapering protocol.5
Box 5. Opioid tapering.
|
Adapted from the National Opioid Use Guideline Group.5
Consultation with specialists
Although evidence is weak, patients with severe pain and pain-related disability appear to have better outcomes when managed by multidisciplinary pain clinics.65 Access to multidisciplinary pain programs is limited in most parts of Canada.69 Pain clinics vary greatly in philosophy and treatment approaches, and family physicians should carefully select the clinics and practitioners to whom they refer their patients. Family physicians should let consultants know if they have specific concerns about patients’ opioid use. Family physicians are not obliged to prescribe opioids according to consultants’ recommendations. They should do so only if, in their clinical judgment, the consultants’ recommendations are safe, likely to be beneficial for the patients in question, and consistent with the guideline.
Opioid patients transferred from other clinics
Family physicians should carefully explain their opioid-prescribing policies to CNCP patients who are new to their practices but who are already taking long-term opioid therapy prescribed by their previous physicians. Patients taking inappropriate doses should be advised that the dose will be tapered in the near future. Patients who are unwilling to comply with the taper should be encouraged to seek medical care elsewhere. Currently, prescribing practices vary widely among family physicians and pain physicians. Therefore physicians must prescribe opioids according to their best judgment, even if this goes against the wishes of patients, the recommendations of consultants, or the practices of patients’ previous physicians.
Acute care settings
Physicians in acute care settings such as walk-in clinics or emergency departments should reach consensus on opioid prescribing policies for CNCP patients. One option is to simply refuse to prescribe in these settings; patients have a responsibility to ensure that they do not run out, and it is unsafe to prescribe opioids without knowing a patient’s full medical history. Another option is to prescribe a supply that will last until the family physician is available. If the latter policy is chosen, the clinic should institute the safeguards listed in Box 6.
Box 6. Precautions for prescribing opioids to CNCP patients in acute care settings.
Keep the following precautions in mind when prescribing opioids for CNCP in emergency departments or walk-in clinics:
|
CNCP—chronic noncancer pain.
Conclusion
Opioids play an important role in the management of CNCP, but careful prescribing is needed to limit potential harms. Opioid therapy should be reserved for pain conditions that have not responded to nonopioid therapies and for which opioids have been shown to be effective. Opioids should be combined with other pharmacologic and nonpharmacologic treatments. The dose should be titrated slowly and with close monitoring, particularly for patients at high risk of overdose and misuse. The optimal dose is one which improves function or decreases pain ratings by at least 30%. For by far most patients, this dose will be well below a 200-mg MED. Tapering is recommended for patients who have not responded to an adequate opioid trial.
KEY POINTS
Opioid prescribing has increased in Canada, accompanied by an increase in opioid-related harms such as overdose and addiction. To date, Canadian physicians have lacked evidence-based prescribing guidelines to help them reduce these harms. This review provides a brief clinical summary of the recently released Canadian guidelines for the general population.
A companion paper summarizes recommendations for special populations. Opioids play an important role in the management of chronic noncancer pain, but careful prescribing is needed. Opioid therapy should be reserved for pain conditions that have not responded to nonopioid therapies and for which opioids have been shown to be effective, and opioids should be combined with other pharmacologic and nonpharmacologic treatments.
Footnotes
This article has been peer reviewed.
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro du novembre 2011 à la page e407.
Contributors
All the authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
Three of the authors were members of the core guideline research group. However, all statements in this article are the sole responsibility of the authors, and the summary was not reviewed by the National Opioid Use Guideline Group.
References
- 1.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181(12):891–6. doi: 10.1503/cmaj.090784. Epub 2009 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249–51. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Furlan AD, Reardon R, Weppler C. Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182(9):923–30. doi: 10.1503/cmaj.100187. Epub 2010 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Opioid Use Guideline Group . Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Hamilton, ON: McMaster University; 2010. Available from: http://nationalpaincentre.mcmaster.ca/opioid/cgop_a00_executive_summary.html. Accessed 2011 Sep 20. [Google Scholar]
- 6.Kahan M, Wilson L, Mailis-Gagnon A, Srivastava A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain. Clinical summary for family physicians. Part 2: special populations. Can Fam Physician. 2011;57:1269–76. e419–28. (Eng), (Fr). [PMC free article] [PubMed] [Google Scholar]
- 7.Russell IJ, Kamin M, Bennett RM, Schnitzer TJ, Green JA, Katz WA. Efficacy of tramadol in treatment of pain in fibromyalgia. J Clin Rheumatol. 2000;6(5):250–7. doi: 10.1097/00124743-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–45. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 9.Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536–41. doi: 10.1136/ard.2007.071522. Epub 2007 Jul 20. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande A, Furlan A, Mailis-Gagnon A, Atlas S, Turk D. Opioids for chronic low-back pain. Cochrane Database Syst Rev. 2007;(3):CD004959. doi: 10.1002/14651858.CD004959.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Chou R. Pharmacological management of low back pain. Drugs. 2010;70(4):387–402. doi: 10.2165/11318690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Weinsheimer N, Akbar M, Schiltenwolf M. [Altered pain thresholds during and after opioid withdrawal in patients with chronic low back pain.] Article in German. Schmerz. 2010;24(3):257–61. doi: 10.1007/s00482-010-0912-4. [DOI] [PubMed] [Google Scholar]
- 13.Nuesch E, Rutjes AW, Husni E, Welch V, Juni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115. doi: 10.1002/14651858.CD003115.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J. 2010;10(6):514–29. doi: 10.1016/j.spinee.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. Eur J Pain. 2008;12(7):917–25. doi: 10.1016/j.ejpain.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 16.McCaffery M, Pasero C. Pain. Clinical manual. 2nd ed. St Louis, MO: Mosby Inc; 1999. [Google Scholar]
- 17.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 18.Daniell HW. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J Pain. 2006;7(12):901–7. doi: 10.1016/j.jpain.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7(1):43–8. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Zgierska A, Brown RT, Zuelsdorff M, Brown D, Zhang Z, Fleming MF. Sleep and daytime sleepiness problems among patients with chronic noncancerous pain receiving long-term opioid therapy: a cross-sectional study. J Opioid Manag. 2007;3(6):317–27. doi: 10.5055/jom.2007.0020. [DOI] [PubMed] [Google Scholar]
- 21.Mogri M, Khan MI, Grant BJ, Mador MJ. Central sleep apnea induced by acute ingestion of opioids. Chest. 2008;133(6):1484–8. doi: 10.1378/chest.07-1891. [DOI] [PubMed] [Google Scholar]
- 22.Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123(2):632–9. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- 23.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–20. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kahan M, Srivastava A, Wilson L, Mailis-Gagnon A, Midmer D. Opioids for managing chronic non-malignant pain: safe and effective prescribing. Can Fam Physician. 2006;52(9):1091–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Mirakbari SM, Innes GD, Christenson J, Tilley J, Wong H. Do co-intoxicants increase adverse event rates in the first 24 hours in patients resuscitated from acute opioid overdose? J Toxicol Clin Toxicol. 2003;41(7):947–53. doi: 10.1081/clt-120026516. [DOI] [PubMed] [Google Scholar]
- 26.Cone EJ, Fant RV, Rohay JM, Caplan YH, Ballina M, Reder RF, et al. Oxycodone involvement in drug abuse deaths: a DAWN-based classification scheme applied to an oxycodone postmortem database containing over 1000 cases. J Anal Toxicol. 2003;27(2):57–67. doi: 10.1093/jat/27.2.57. [DOI] [PubMed] [Google Scholar]
- 27.Repchinsky C Editor-in-Chief, editor. Compendium of pharmaceuticals and specialties. The Canadian drug reference for health professionals. Ottawa, ON: Canadian Pharmacists Association; 2008. [Google Scholar]
- 28.Schreiber S, Peles E, Adelson M. Association between improvement in depression, reduced benzodiazepine (BDZ) abuse, and increased psycho-tropic medication use in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2008;92(1–3):79–85. doi: 10.1016/j.drugalcdep.2007.06.016. Epub 2007 Aug 13. [DOI] [PubMed] [Google Scholar]
- 29.Baillargeon L, Landreville P, Verreault R, Beauchemin JP, Grégoire JP, Morin CM. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial. CMAJ. 2003;169(10):1015–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Gosselin P, Ladouceur R, Morin CM, Dugas MJ, Baillargeon L. Benzodiazepine discontinuation among adults with GAD: a randomized trial of cognitive-behavioral therapy. J Consult Clin Psychol. 2006;74(5):908–19. doi: 10.1037/0022-006X.74.5.908. [DOI] [PubMed] [Google Scholar]
- 31.Vicens C, Fiol F, Llobera J, Campoamor F, Mateu C, Alegret S, et al. Withdrawal from long-term benzodiazepine use: randomised trial in family practice. Br J Gen Pract. 2006;56(533):958–63. [PMC free article] [PubMed] [Google Scholar]
- 32.Moroz G, Rosenbaum JF. Efficacy, safety, and gradual discontinuation of clonazepam in panic disorder: a placebo-controlled, multicenter study using optimized dosages. J Clin Psychiatry. 1999;60(9):604–12. doi: 10.4088/jcp.v60n0907. [DOI] [PubMed] [Google Scholar]
- 33.Cook JM, Biyanova T, Thompson R, Coyne JC. Older primary care patients’ willingness to consider discontinuation of chronic benzodiazepines. Gen Hosp Psychiatry. 2007;29(5):396–401. doi: 10.1016/j.genhosppsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta N, Kramer ED, Zalman MA, Carino S, Jr, Smith MY, Haddox JD, et al. Association between non-medical and prescriptive usage of opioids. Drug Alcohol Depend. 2006;82(2):135–42. doi: 10.1016/j.drugalcdep.2005.08.019. Epub 2005 Oct 19. [DOI] [PubMed] [Google Scholar]
- 36.Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27(1):7–17. doi: 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- 37.Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, et al. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf. 2005;14(12):851–9. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- 38.Peterson GM, Randall CT, Paterson J. Plasma levels of morphine and morphine glucuronides in the treatment of cancer pain: relationship to renal function and route of administration. Eur J Clin Pharmacol. 1990;38(2):121–4. doi: 10.1007/BF00265969. [DOI] [PubMed] [Google Scholar]
- 39.Tyndale RF, Droll KP, Sellers EM. Genetically deficient CYP2D6 metabolism provides protection against oral opiate dependence. Pharmacogenetics. 1997;7(5):375–9. doi: 10.1097/00008571-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Romach MK, Otton SV, Somer G, Tyndale RF, Sellers EM. Cytochrome P450 2D6 and treatment of codeine dependence. J Clin Psychopharmacol. 2000;20(1):43–5. doi: 10.1097/00004714-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Howard LA, Sellers EM, Tyndale RF. The role of pharmacogenetically-variable cytochrome P450 enzymes in. Pharmacogenomics. 2002;3(2):185–99. doi: 10.1517/14622416.3.2.185. [DOI] [PubMed] [Google Scholar]
- 42.Shields LB, Hunsaker JC, 3rd, Corey TS, Ward MK, Stewart D. Methadone toxicity fatalities: a review of medical examiner cases in a large metropolitan area. J Forensic Sci. 2007;52(6):1389–95. doi: 10.1111/j.1556-4029.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 43.Sims SA, Snow LA, Porucznik CA. Surveillance of methadone-related adverse drug events using multiple public health data sources. J Biomed Inform. 2007;40(4):382–9. doi: 10.1016/j.jbi.2006.10.004. Epub 2006 Nov 1. [DOI] [PubMed] [Google Scholar]
- 44.Seifert CF, Kennedy S. Meperidine is alive and well in the new millennium: evaluation of meperidine usage patterns and frequency of adverse drug reactions. Pharmacotherapy. 2004;24(6):776–83. doi: 10.1592/phco.24.8.776.36066. [DOI] [PubMed] [Google Scholar]
- 45.Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berl) 2008;196(1):105–16. doi: 10.1007/s00213-007-0937-2. Epub 2007 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamas X, Farré M, Moreno V, Camí J. Effects of morphine in post addict humans: a meta-analysis. Drug Alcohol Depend. 1994;36(2):147–52. doi: 10.1016/0376-8716(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 47.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 49.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 50.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Hypogonadism and sexual dysfunction in male cancer survivors receiving chronic opioid therapy. J Pain Symptom Manage. 2003;26(5):1055–61. doi: 10.1016/s0885-3924(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 51.Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3(5):455–61. Erratum in: J Clin Sleep Med 2007;3(6):table of contents. [PMC free article] [PubMed] [Google Scholar]
- 52.Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425–32. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 53.Alattar MA, Scharf SM. Opioid-associated central sleep apnea: a case series. Sleep Breath. 2009;13(2):201–6. doi: 10.1007/s11325-008-0221-7. Epub 2008 Sep 20. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SP, Christo PJ, Wang S, Chen L, Stojanovic MP, Shields CH, et al. The effect of opioid dose and treatment duration on the perception of a painful standardized clinical stimulus. Reg Anesth Pain Med. 2008;33(3):199–206. doi: 10.1016/j.rapm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 56.Hass B, Lungershausen J, Hertel N, Poulsen Nautrup B, Kotowa W, Liedgens H. Cost-effectiveness of strong opioids focussing on the long-term effects of opioid-related fractures: a model approach. Eur J Health Econ. 2009;10(3):309–21. doi: 10.1007/s10198-008-0134-1. Epub 2008 Dec 21. [DOI] [PubMed] [Google Scholar]
- 57.Gomes T, Juurlink D, Dhalla I, Mailis-Gagnon A, Paterson M, Mamdani M. Trends in opioid use and dosing among the socioeconomically disadvantaged. Open Med. 2011;5(1):e13. [PMC free article] [PubMed] [Google Scholar]
- 58.Rome JD, Townsend CO, Bruce BK, Sletten CD, Luedtke CA, Hodgson JE. Chronic noncancer pain rehabilitation with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Mayo Clin Proc. 2004;79(6):759–68. doi: 10.4065/79.6.759. [DOI] [PubMed] [Google Scholar]
- 59.Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM, Disability Risk Identification Study Cohort Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine. 2008;33(2):199–204. doi: 10.1097/BRS.0b013e318160455c. [DOI] [PubMed] [Google Scholar]
- 60.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344–50. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 61.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, et al. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31(6):564–70. doi: 10.1016/j.genhosppsych.2009.07.003. Epub 2009 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quigley C. Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev. 2004;(3):CD004847. doi: 10.1002/14651858.CD004847. [DOI] [PubMed] [Google Scholar]
- 63.Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2(5):277–82. doi: 10.5055/jom.2006.0041. [DOI] [PubMed] [Google Scholar]
- 64.Becker N, Sjøgren P, Bech P, Olsen AK, Eriksen J. Treatment outcome of chronic non-malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Pain. 2000;84(2–3):203–11. doi: 10.1016/s0304-3959(99)00209-2. [DOI] [PubMed] [Google Scholar]
- 65.Miller NS, Swiney T, Barkin RL. Effects of opioid prescription medication dependence and detoxification on pain perceptions and self-reports. Am J Ther. 2006;13(5):436–44. doi: 10.1097/01.mjt.0000212894.35705.90. [DOI] [PubMed] [Google Scholar]
- 66.Ralphs JA, Williams AC, Richardson PH, Pither CE, Nicholas MK. Opiate reduction in chronic pain patients: a comparison of patient-controlled reduction and staff controlled cocktail methods. Pain. 1994;56(3):279–88. doi: 10.1016/0304-3959(94)90166-X. [DOI] [PubMed] [Google Scholar]
- 67.Crisostomo RA, Schmidt JE, Hooten WM, Kerkvliet JL, Townsend CO, Bruce BK. Withdrawal of analgesic medication for chronic low-back pain patients: improvement in outcomes of multidisciplinary rehabilitation regardless of surgical history. Am J Phys Med Rehabil. 2008;87(7):527–36. doi: 10.1097/PHM.0b013e31817c124f. [DOI] [PubMed] [Google Scholar]
- 68.Hooten WM, Townsend CO, Sletten CD, Bruce BK, Rome JD. Treatment outcomes after multidisciplinary pain rehabilitation with analgesic medication withdrawal for patients with fibromyalgia. Pain Med. 2007;8(1):8–16. doi: 10.1111/j.1526-4637.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 69.Peng P, Choiniere M, Dion D, Intrater H, Lefort S, Lynch M, et al. Challenges in accessing multidisciplinary pain treatment facilities in Canada. Can J Anaesth. 2007;54(12):977–84. doi: 10.1007/BF03016631. [DOI] [PubMed] [Google Scholar]