Abstract
Persistent infection with hepatitis C virus (HCV) is a major cause of chronic hepatitis in humans. In chronic carriers, the viral infection induces liver damage that predisposes the patient for cirrhosis and can lead to hepatocellular carcinoma. Current chemotherapies are limited to alpha interferon (IFN-α) used either alone or in combination with ribavirin (RBV). In addition to its limited efficacy, this treatment is frequently poorly tolerated because of its side effects. The urgently needed development of new drugs is made difficult by the lack of an in vitro or in vivo infectivity model, and no cell line has been found so far to reliably and reproducibly support HCV infection. For this reason, the closely related pestivirus bovine viral diarrhea virus (BVDV) has sometimes been used as a surrogate in vitro infectivity model. In this study we used an MDBK cell line persistently infected with noncytopathic BVDV to assess the antiviral effect of IFN-α and RBV, the two drugs currently in clinical use against HCV. The same system was then used to evaluate the potential of two classes of iminosugar derivates to clear noncytopathic BVDV infection from MDBK cells. We show that treatment with long-alkyl-chain deoxynojirimycin derivatives, which are inhibitors of the endoplasmic reticulum (ER)-resident α-glucosidases, can greatly reduce the amount of secreted enveloped viral RNA. Long-alkyl-chain deoxygalactonojirimycin derivatives, which do not inhibit ER α-glucosidases, were less potent but still more effective in this system than IFN-α or ribavirin.
Hepatitis C virus (HCV) causes persistent infection in approximately 70 to 80% of people infected and is responsible for liver injuries that can lead to cirrhosis and hepatocellular carcinoma (2, 34). While current therapeutic strategies against HCV infection rely mainly on either naked or pegylated alpha interferon (IFN-α) alone or in combination with ribavirin (RBV) (19, 28, 34, 37, 43), considerable efforts are being made to develop new molecules which show better efficacy, particularly for refractory patients, who represent on average 50% of patients treated (19, 28, 34, 42, 43). Depending on the HCV genotype, the percentage of refractory patient varies from 80% (genotype 1b) to 20% (genotype 3).
Molecules targeting viral activities, such as protease- or polymerase-specific inhibitors, are traditionally the most attractive candidates for drug development, though this strategy faces resistance problems, especially when dealing with fast-mutating viruses like HCV and human immunodeficiency virus. The problem of drug-resistant viral escape mutants has also been observed for hepatitis B virus (13, 18, 35, 36). Alternatively, a host cell target could be chosen which would have to be more important for the survival of the virus than for that of the host. Such a target would arguably be more difficult for the virus to mutate around.
The feasibility of such an approach has recently been investigated with deoxynojirimycin (DNJ)-based iminosugar derivatives as antiviral agents (6, 7, 17, 29, 30, 45). These glucose analogues competitively bind to the host cell-encoded endoplasmic reticulum (ER) α-glucosidases I and II and prevent them from performing the stepwise removal of three glucose residues attached to the N-linked glycans carried by newly synthesized polypeptides. This in turn prevents the polypeptides from interacting with the ER chaperones calnexin and calreticulin, which bind to monoglucosylated glycoproteins (22-25). Interaction with these ER chaperones is crucial for the correct folding of many viral glycoproteins (22-25). Potentially all viruses which encode glycoproteins that depend on calnexin interaction for proper folding could be targeted with ER α-glucosidase inhibitors (11, 17, 44, 45).
The antiviral mechanism of action of N-butyl-DNJ (NB-DNJ) and N-nonyl-DNJ (NN-DNJ), two alkylated derivatives of DNJ, was studied in more detail with bovine viral diarrhea virus (BVDV) as a surrogate model for HCV. The antiviral effect of these compounds was shown to be either fully (for the short-alkyl-chain derivative NB-DNJ) or partially (for the long-alkyl-chain derivative NN-DNJ) associated with their inhibition of the ER α-glucosidases I and II (8, 16). The two HCV envelope glycoproteins E1 and E2 also contain 5 or 6 and 11 N-glycosylation sites, respectively (14). Moreover, it has been shown that they interact with calnexin during their heterodimerization process, which is believed to lead to the productive virus formation pathway (10, 12, 15). It is therefore anticipated that inhibitors of ER α-glucosidases will disrupt the first step of HCV virion assembly and lead to inhibition of secretion, as has been shown for BVDV.
When a patient presents with viral hepatitis C, the chronic infection is usually already established before antiviral therapy starts. A persistently infected cell line therefore more closely resembles the in vivo situation. In the absence of a transient or persistent in vitro HCV infectivity model, the aim of our study was to analyze and compare the antiviral effect of two Food and Drug Administration (FDA)-approved anti-HCV drugs, IFN-α and ribavirin, with that of potential new anti-HCV molecules (long-alkyl-chain iminosugar derivatives) in the context of a persistent infection. To this end, we used a noncytopathic (ncp) strain of BVDV able to persistently infect cells in vitro.
MATERIALS AND METHODS
Viruses, cells, and inhibitors.
The ncp BVDV strain Pe515 (33) and the cytopathic (cp) BVDV strain NADL (National Animal Disease Laboratory) were used in this study. Madin-Darby bovine kidney (MDBK) cells were maintained in RPMI 1640 medium (Gibco-BRL) supplemented either with 10% fetal calf serum (FCS) (PAA Laboratories) which had been screened and found negative for the presence of BVDV and BVDV-specific antibodies or 10% horse serum (Sigma, St. Louis, Mo.) at 37°C in a humidified 5% CO2 atmosphere. IFN-α and ribavirin were purchased from Sigma. The two iminosugar derivatives N7-oxadecyl-DNJ (N7-DNJ) and N7-oxanonyl-6-deoxy-DGJ (N7-DGJ) were provided by Synergy Pharmaceuticals Inc.
Treatment of MDBK cells persistently infected with ncp BVDV with IFN, RBV, and iminosugar derivatives.
IFN-α was supplied at 106 U/ml. RBV and iminosugar derivatives were made up as stock solutions of 100 mM in water. All drugs were used diluted in medium to the final concentrations indicated in the text and figures. Drug treatment was started after the initial seeding of the cells (at 106 cells/35-mm dish) and renewed every 3 or 4 days depending on their confluency, when cells were passaged with fresh medium containing drugs.
Immunofluorescence analysis.
Cells grown on coverslips were fixed with a chilled (−20°C) solution of acetone-methanol (50:50, vol/vol) for 20 min at room temperature. Coverslips were air-dried and then rehydrated in phosphate-buffered saline (PBS) before being blocked overnight at 4°C in PBS containing 3% bovine serum albumin (BSA; Sigma) and 1% fish gelatin (Sigma). Two monoclonal antibodies, WB103/105 and WB166 (Veterinary Laboratory Agency, Weybridge, U.K.), that recognize the NS2/NS3 and E2 proteins, respectively, were used as primary antibodies, at a dilution of 1:100, for detection of BVDV infection. After probing with an anti-mouse-fluorescein isothiocyanate (FITC)-conjugated secondary antibody and extensive washing in PBS, the coverslips were dried and mounted with Vectashield reagent (Vector Laboratories, Burlingame, Calif.) containing 0.33 μg of 4′,6′-diaminido-2-phenylindole (DAPI) per ml. Stained cells were analyzed with an Axioskop 2 Plus Zeiss microscope.
Determination of inhibitory concentrations by plaque and yield assay.
MDBK cells were grown in six-well plates to 90% confluency and infected with cp or ncp BVDV for 1 h at 37°C. After removal of the inoculum, cells were washed with PBS and incubated in RPMI 1640 medium containing 10% FCS and inhibitors at different concentrations. After 2 or 3 days, the medium containing secreted virus was removed from the wells and spun at low speed to remove cellular debris. Different dilutions were used to infect a fresh monolayer of MDBK cells in six-well plates. For the titration of cp-BVDV, the plaques were counted by eye under the microscope after 2 days. For the titration of ncp-BVDV, an agarose overlay was used, and plaques were counted by peroxidase immunostaining after hybridization with an anti-BVDV antibody and a secondary antibody conjugated with peroxidase, as described previously (39). The 50% and 90% inhibitory concentrations (IC50 and IC90) were determined as the concentrations at which the number of plaques was halved or reduced by 90%, respectively, compared to untreated infected control cells.
Viral RNA purification and RT-PCR analysis.
The supernatants of cultured cells were harvested, spun at 5,000 × g for 5 min, and stored at −70°C before viral RNA (vRNA) purification. RNA from released viral particles was purified with the QIAamp Viral RNA purification kit (Qiagen, Crawley, U.K.) with 140 μl of supernatant as starting material. vRNA was eluted in 40 μl from the column; 5 μl of viral template RNA and primers P1 (5′-AACAAACATGGTTGGTGCAACTGGT-3′) and P2 (5′-CTTACACAGACATATTTGCCTAGGTTCCA-3′), which amplify an 826-bp region overlapping Ems and E1 (41), were used in the Titan One Tube reverse transcription (RT)-PCR system (Roche). After an incubation of 30 min at 50°C for the reverse transcription step, a total of 45 cycles (10 cycles of 30 s at 94°C, 1 min at 55°C, and 1 min at 68°C and 35 cycles of 30 s at 94°C, 1 min at 55°C, and 65 s at 68°C) of PCR were performed. The PCR products were separated on 2% agarose gels stained with ethidium bromide.
RESULTS
Generation and characterization of an MDBK cell line persistently infected with ncp BVDV.
Pestiviruses, including BVDV, can be divided into two biotypes depending on their effect on cultured cells. The cytopathic variant destroys infected cells, whereas the noncytopathic type replicates without visible damage to the host cell (26). Gong et al. (20) obtained stable cell lines from either bovine turbinate cells or lamb testis which contained a proportion of cells persistently infected with ncp BVDV. Typically between 30 and 50% of cells were infected, a level which neither increased nor decreased during successive passages.
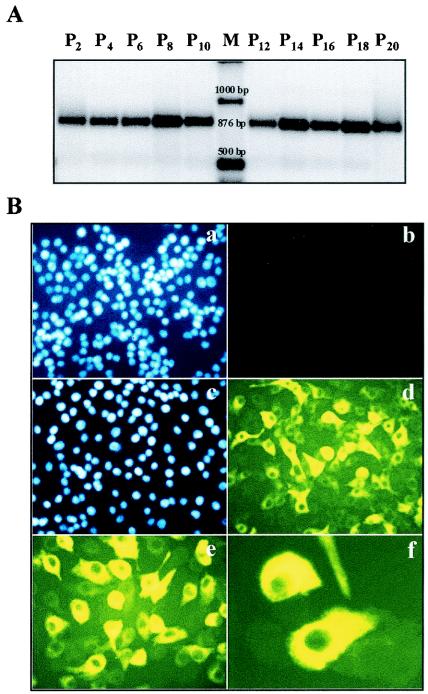
In order to study the ability of different antiviral molecules to clear a chronic-type infection, we established an MDBK cell line persistently infected with ncp BVDV by culturing BVDV-free MDBK cells in medium containing BVDV-positive FCS. After a few passages, which allowed the virus to establish the infection, we checked for the presence of vRNA in the supernatant culture by RT-PCR with 45 cycles of amplification (Fig. 1). A cDNA product of the expected size was observed, indicating successful infection with ncp BVDV. To demonstrate that the infection was persistent, we monitored the level of vRNA for successive cell passages and found that it was almost constant in intensity over the 20 passages tested (Fig. 1A). The viral titer was determined throughout the experiment and was found to be stable at around 104 PFU/ml.
FIG. 1.
Characterization of an MDBK cell line persistently infected with ncp BVDV. (A) Viral RNA was purified from the culture supernatants harvested every 3 days when the cells were passaged and subsequently used to perform an RT-PCR analysis; 20% of the RT-PCR product was loaded and run on a 2% agarose gel stained with ethidium bromide. (B) Noninfected cells (panels a and b) and the MDBK cell line, at passage 5 after establishment, persistently infected with ncp-BVDV (panels c to f) were fixed and probed with monoclonal antibodies directed against BVDV E2 and NS2/3 proteins, followed by incubation with an anti-mouse-FITC-conjugated secondary antibody (d to f). Nuclear staining with DAPI was performed to quantify the cells (a and c).
Next, we performed an analysis by immunofluorescence with a mixture of monoclonal antibodies directed against the E2 and NS2/3 proteins of BVDV to determine the proportion of cells infected (Fig. 1B). As expected, staining was associated with perinuclear membranes and the endoplasmic reticulum (Fig. 1B, panels e and f). As determined by counting and comparing cells stained with DAPI and fluorescence (panel c versus panel d), between 40 and 60% of cells were found positive, with various staining intensities, whereas only background fluorescence was detected in controls (panels a and b). Again, the proportion of BVDV-positive cells remained stable during passaging of the cells. We used this MDBK cell line persistently infected with ncp BVDV in the subsequent study.
Antiviral effect of IFN-α and RBV, alone and in combination, on persistent ncp BVDV infection in MDBK cells.
We first tested human IFN-α, one of the two drugs used clinically to fight HCV infection (19, 28, 34, 37, 43). Human IFN-α has been used previously against cp and ncp BVDV and was shown to be very active, with 103 U/ml being sufficient to suppress the replication of the virus (38). We examined the antiviral effect of IFN-α against the cp BVDV NADL strain and the ncp BVDV Pe515 strain and found that the IC50 was lower than 50 U/ml, confirming that this human cytokine was active against the bovine virus. The difference in potency observed compared to the published data (38) was most likely due to the method used for the evaluation of the inhibitory concentrations, which in our case is based on a yield assay (described in detail in Materials and Methods) rather than a conventional first-round plaque assay. This way of measuring inhibitory concentrations (after several rounds of replication) was proposed and used previously to evaluate the antiviral behavior of iminosugars (14), which, rather than impacting the first round of viral replication, interfere with the reinfection process by inducing the production of less infective viral particles.
We then tested RBV, which is also in clinical use (19, 28, 34, 37, 43). RBV is a broad-spectrum antiviral agent with activity against many DNA and RNA viruses (40). Against cp BVDV in bovine turbinate cells, RBV was shown to have an IC50 of 44.6 μM, with a corresponding CC50 (concentration which is toxic for 50% of the cells) of 500 μM (27). Using our method of evaluation, we found that RBV had an IC50 and IC90 of 8 and 15 μM, respectively, when a multiplicity of infection (MOI) of 1 was used for the first round of infection. Under the same conditions, the CC50 of RBV was determined to be approximately 50 to 75 μM.
To study the impact of both drugs on persistent infection, we cultured the infected MDBK cell line in the presence of different concentrations of each drug, ranging from 100 to 10,000 U/ml for IFN-α and from 2 to 8 μM for RBV. Cells were maintained in medium containing 10% BVDV-free FCS with or without drug and passaged every 3 days by a 1:6 dilution. The levels of vRNA present in the supernatant during successive passages were monitored by RT-PCR (Fig. 2).
FIG. 2.
Antiviral effect of IFN-α and RBV against ncp BVDV replicating in a persistently infected MDBK cell line. Viral RNA levels in the culture supernatants after either no treatment or treatment with different concentrations of (A) IFN-α or (B) RBV were measured by RT-PCR analysis over several passages; 20% of the RT-PCR product was loaded and run on a 2% agarose gel stained with ethidium bromide. Scan densitometry analysis of the gel is shown on the right-hand side of each gel.
IFN-α treatment caused a dose-dependent decrease in vRNA levels, confirming its in vitro antiviral properties. This antiviral effect was rapid but incomplete, as vRNA levels were already reduced to their lowest level at passage 2 but remained more or less stable during subsequent passages. Surprisingly, even at the highest concentration of IFN-α used, which is beyond physiologically encountered levels, the vRNA signal determined by RT-PCR did not entirely disappear. This result shows that under the conditions used, IFN-α alone was unable to clear the infection from a persistently infected MDBK cell line.
Although the FCS in the medium used during these experiments was PCR screened and found to be BVDV negative, we wanted to confidently rule out that the weak RT-PCR signal observed even with the highest IFN-α doses was the result of viral contamination originating from the addition of fresh FCS during the passaging of the cells. The experiment was repeated with horse serum (HS) instead of FCS to complement the medium. The results obtained in this second set of experiments were similar to those obtained in the first; the antiviral response was proportional to the dose of IFN-α used, but the vRNA in the supernatants could not be completely eliminated (data not shown). In conclusion, although IFN-α is able to induce a dose-dependent reduction in vRNA levels released from persistently infected MDBK cells, it does not eliminate the infection. Moreover, it is worth noting that we did not observe any rebound of the RT-PCR signal during passages under IFN-α pressure, suggesting that no resistant virus was selected within the time frame of this experiment.
Similar to IFN-α, ribavirin alone caused a dose-dependent and progressive decrease in the vRNA levels, suggesting an antiviral effect of this molecule in our model (Fig. 2B). However, compared to IFN-α, the effect of ribavirin was rather slow to appear at the concentrations tested. Under prolonged drug exposure, higher concentrations (e.g., 16 μM) of RBV had a visible impact on the cell growth rate and could not be used in this long-term study. RBV was most effective at 8 μM, but at passage 10 the RT-PCR signal was still observed, indicating that in this experimental setting RBV was unable to eliminate the RT-PCR signal from the cell line. However, we cannot completely exclude that the overall antiviral effect observed, as measured by the reduction of the RT-PCR signal, was partially due to toxicity caused by ribavirin.
Clinically, IFN-α and RBV are used in combination therapy, and a synergistic effect of the drugs has been observed. The mechanism of the synergistic effect of these drugs in vivo is not understood. The host immune system was thought to play an important role. However, a recent study has demonstrated that this synergistic effect could also be observed in a cell culture system (9), ruling out an exclusively immune system-mediated effect.
To confirm these data, we studied the impact of a combination of these drugs in the persistently infected MDBK cell line. Due to the complexity and time needed to perform even one experiment, only one drug combination, 100 U of IFN-α per ml and 2 μM RBV, was tested, as these concentrations were shown to be suboptimal in monotherapeutic treatment (Fig. 2). The combination of both drugs led to increased efficacy, with secreted vRNA disappearing after only two successive passages (Fig. 3). Although this experiment was not designed to study in detail a potential synergistic effect of these drugs in vitro, the result strongly suggests that the effect of using the combination is more than additive.
FIG. 3.
Antiviral effect of a combination of IFN-α and RBV against ncp BVDV replicating in a persistently infected MDBK cell line. Viral RNA levels present in the culture supernatants after treatment with no drug, 2 μM RBV, 100 U of IFN-α per ml, or 2 μM RBV plus 100 U of IFN-α per ml were measured by RT-PCR analysis over several passages (passage numbers indicated on the top of the gel); 20% of the RT-PCR product was loaded and run on a 2% agarose gel stained with ethidium bromide.
Antiviral effect of iminosugar derivatives.
In a previous study, we reported the in vitro antiviral effect of N7-oxadecyl-DNJ (N7-DNJ) and N7-oxanonyl-DGJ (N7-DGJ), alkylated derivatives of deoxynojirimycin (DNJ) and deoxygalactonojirimycin (DGJ), respectively, against a cp strain of BVDV (16). The antiviral features of these molecules are recapitulated in Table 1. The IC50 and IC90 values varied depending on the MOI used to perform the yield assay. However, for the three different cp strains (NADL, Pe515, and CP7) tested at the same MOI, these values were similar, indicating that the efficacy of these drugs was not dependent upon the strain (data not shown). Also, both cp and ncp strains seemed equally susceptible to iminosugars, as determined by using the cpPe515 and ncpPe515 strains.
TABLE 1.
Antiviral features of the two long-alkyl-chain iminosugar derivatives used in this studya
| Compound | IC50 (μM) at MOI:
|
IC90 (μM) at MOI:
|
Cytotoxic concn (μM)
|
SI50 (CC50/IC50) at MOI:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 0.01 | 0.1 | 1 | 10% | 30% | 50% | 0.01 | 0.1 | 1 | |
| N7-oxadecyl-DNJ | 17.5 ± 2.5 | 35 ± 5 | 80 ± 20 | 62.5 | 200 ± 20 | 1,000 ± 200 | >1,000 | >2,000 | >5,000 | >250 | >125 | >50 |
| N7-oxanonyl-6deoxy-DGJ | 2.5 ± 0.5 | 17.5 ± 2.5 | 125 ± 25 | 7.5 ± 2.5 | nr | nr | >3,000 | >3,500 | >4,000 | >1,333.33 | >200 | >26.66 |
Values indicated are the means of numbers obtained in at least three sets of experiments ± the standard deviation. nr, not reached. Cytotoxic concentrations were obtained as described in Materials and Methods.
Together with data published previously, the following trend was observed. Although long-alkyl-chain DGJ derivatives like N7-DGJ achieve lower IC50s than long-alkyl-chain DNJ derivatives like N7-DNJ when a low MOI (0.01) is used, they fail to achieve an IC90 at higher MOIs (0.1 and 1) (Fig. 4). This plateau effect is not observed with long-alkyl-chain DNJ derivatives, and an IC90 is obtained for these compounds. DGJ derivatives do not inhibit ER α-glucosidases. The mechanism of the antiviral action of long N-alkyl-chain iminosugar derivatives was studied previously and involved an abnormal accumulation of E2-E2 dimers in the ER of infected cells and subsequently in secreted virus particles (16). The E2 homodimer accumulation probably does not cause but rather reflects the underlying antiviral mechanism, which is currently being investigated, and we cannot explain the plateau effect for the time being. However, this effect was observed in short-term experiments involving cp and ncp strains of BVDV.
FIG. 4.
Antiviral properties of members of two classes of iminosugar derivatives. The antiviral effect of N7-oxadecyl-DNJ (squares) and N7-oxanonyl-6deoxy-DGJ (triangles) on the replication of cp BVDV was evaluated by yield assay as described in detail in Materials and Methods. Monolayers of MDBK cells were infected at an MOI of 0.1 and treated with increasing drug concentrations. After 2 days, plaques were counted by eye under the microscope. The percent replication relative to that of untreated infected cells is represented as a function of the drug concentration used. The mean value of three independent experiments is shown. Antiviral features obtained with the ncp BVDV strain were identical (data not shown).
We were interested in establishing whether an antiviral compound which does not eliminate vRNA in a few replication cycles can nevertheless control a persistent infection, given sufficient time. Therefore, the same experiment performed with IFN-α and RBV was repeated with N7-DNJ and N7-DGJ (Fig. 5 and 6, respectively). When treated with N7-DNJ, the virus-derived RT-PCR signal decreased with increasing drug concentrations, and this decrease was enhanced in subsequent passages, indicating a sustained dose-response effect. After four to six passages, treatment with 500 μM N7-DNJ led to the complete disappearance of vRNA from supernatants, a result that could not be achieved with either IFN-α or RBV. This result shows that, in this model, N7-DNJ has potent antiviral properties and that this class of ER α-glucosidase-inhibiting iminosugar derivatives is at least as potent as the molecules in clinical use, used here as a reference for antiviral potency. Treatment with N7-DGJ, which does not inhibit ER α-glucosidases and works via an antiviral mechanism associated with its long alkyl side chain, could not match the result obtained with N7-DNJ over 10 passages, even when used at a concentration of 1 mM (data not shown). This result led us to extend the time frame of the experiment from 10 to 18 passages.
FIG. 5.
Antiviral effect of N7-oxadecyl-DNJ on the replication of ncp BVDV persistently infecting an MDBK cell line. Viral RNA levels present in culture supernatants after either no treatment or treatment with various concentrations of N7-DNJ were measured by RT-PCR analysis over several passages (passage numbers indicated on the top of the gel); 20% of the RT-PCR product was loaded and run on a 2% agarose gel stained with ethidium bromide.
FIG. 6.
Antiviral effect of N7-oxanonyl-6deoxy-DGJ on the replication of ncp BVDV persistently infecting an MDBK cell line. Viral RNA levels present in culture supernatants after either no treatment or treatment with various concentrations of N7-oxanonyl-6deoxy-DGJ was measured by RT-PCR analysis over several passages (passage numbers indicated on the top of the gels); 20% of the RT-PCR product was loaded and run on a 2% agarose gel stained with ethidium bromide. (A) First set of experiments. (B) Second set of experiments. A scan densitometry analysis of the gels is shown on the right-hand side of each gel.
In a first set of experiment, infected cells were treated with four different concentrations of N7-DGJ ranging from 31 μM to 250 μM, and secreted vRNA levels were monitored during successive passages. Figure 6A shows that this treatment caused a decrease in vRNA levels. The decrease in vRNA levels secreted from ncp BVDV-infected cells was very similar at all dose ranges, around 50% at passage 14. This result correlated with the plateau effect observed in the short-term experiments with a cp strain. To confirm this result, we repeated the experiment with higher N7-DGJ concentrations (Fig. 6B). In this second experiment, N7-DGJ was unable to clear the infection even at 1 mM. However, scan densitometry analysis of the results revealed that after 18 passages of treatment with 1 mM N7-DGJ, the reduction in vRNA levels was greater than 95% compared to the control. Although this reduction was not as convincing as that obtained with N7-DNJ, it was at least as good as that obtained with IFN-α (90% reduction; Fig. 2A) and better than that obtained with RBV alone (80% reduction; Fig. 2B).
DISCUSSION
As no in vitro cell culture system that is able to support HCV infection is available (3, 4), we established and used an MDBK cell line persistently infected with ncp BVDV to compare the ability of antiviral molecules to combat a chronic infection over serial passages. Two FDA-approved anti-HCV drugs, RBV and INF-α, as well as two classes of iminosugar derivatives, N7-NDJ and N7-DGJ, were evaluated in this model.
Treatment with either RBV or IFN-α led to a decrease in secreted vRNA in the culture supernatants after serial passages of the cells. This reduction was dose dependent and increased with the length of treatment. However, neither RBV nor IFN-α was able to eliminate the infection from the MDBK cell line when used in monotherapy, despite the very high concentrations of IFN-α used.
Previous studies of cells infected in vitro with BVDV have shown that IFN-α is induced following infection with cp BVDV but not ncp BVDV (1) and that ncp BVDV inhibits endogenous induction of IFN-α by other viruses (31). However, exogenous IFN-α inhibits replication of both cp and ncp BVDV in vitro (5, 38). The results obtained in this study confirm the latter, but the fact that IFN-α alone was unable to clear the infection may be partially explained by the former.
Recently, the anti-HCV effect of IFN-α has been studied in Huh7 cell lines maintaining subgenomic HCV replicons (21). Interestingly, although IFN-α was able to inhibit viral replication to a great extent, it was found that HCV replicons could persist in Huh7 cells despite the long and potent treatment. In that study, and similar to what we observed after 40 days of IFN-α treatment, no IFN-α-resistant variants were selected during the course of the long-term (125 days) treatment.
Interestingly, we found that a combination of 2 μM RBV and 100 U of IFN-α per ml successfully eliminated the RT-PCR signal from the infected cell line, suggesting a more than additive effect. However, further experiments with various combinations of drug concentrations are necessary to demonstrate whether the effect observed is due to synergy between these drugs, and our cell culture model could be useful to examine further some molecular aspects of a potentially synergistic effect at the cellular level.
The main purpose of our study was to evaluate in our model of chronic infection the antiviral effect of two classes of iminosugar derivatives and compare it to that of standards (IFN-α and RBV). The antiviral properties of glucose or galactose analogue-derived alkylated iminosugars have been reported previously, and their mechanism of action has been partially elucidated (16). Treatment with DNJ derivatives (which inhibit ER α-glucosidases) reduces the secretion of RNA-containing virus particles, while no decrease in the level of secreted enveloped viral RNA occurs with DGJ-based derivatives (which do not inhibit ER α-glucosidases). Furthermore, long-alkyl-chain iminosugar derivatives have an additional antiviral effect unrelated to ER α-glucosidase inhibition. The two molecules used in this study, N7-oxadecyl-DNJ, and N7-oxanonyl-6-deoxy-DGJ, therefore have different patterns of activity against BVDV, as illustrated in Fig. 4 and summarized in Table 1. Although DNJ-based compounds are more promising in terms of efficacy, because of their known and potential side effects (17), a DGJ derivative would be preferable for the long-term treatment necessitated by a chronic disease like HCV-induced hepatitis.
Two main questions motivated this study. Are iminosugars, both DNJ and DGJ derivatives, able to control a persistent infection from a cell line? How do they compare to IFN-α and RBV in this context? We have shown that at nontoxic doses, the DNJ-based compound N7-DNJ eliminated the detectable virus-derived RT-PCR signal from the culture supernatants, indicating that it was a more potent antiviral than IFN-α or RBV in the BVBV/MDBK system used. Although it caused a reduction of the signal comparable to that obtained with IFN-α used at 10,000 U/ml, the DGJ derivative N7-DGJ did not eliminate the RT-PCR signal from the culture supernatant, indicating that this drug could be insufficient in antiviral monotherapy.
In the persistently infected surrogate BVDV/MDBK model, iminosugar derivatives are at least as efficient as if not better (in the case of N7-DNJ) than the two drugs used to clinically combat HCV. They are also much less toxic than IFN-α and RBV in vivo and, because of their unrelated antiviral mechanism, may be able to show efficacy against viral escape mutants likely to emerge during treatment with currently used drugs. Recently, an in vitro combination study with IFN-α and NB-DNJ against BVDV showed a synergistic effect (32). Our findings support the further study and development of iminosugar derivatives as potential anti-HCV therapeutics most likely to perform best in combination therapy.
Acknowledgments
N.Z. is a Royal Society Dorothy Hodgkin Fellow and Research Fellow of Wolfson College, Oxford. N.B.-N. is supported by the Wellcome Trust.
This work was supported by Synergy Pharmaceuticals and the Oxford Glycobiology Institute Endowment.
REFERENCES
- 1.Adler, B., H. Adler, H. Pfister, T. W. Jungi, and E. Peterhans. 1997. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 71:3255-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager, R., and V. Lohmann. 2001. Novel cell culture systems for the hepatitis C virus. Antiviral Res. 52:1-17. [DOI] [PubMed] [Google Scholar]
- 4.Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. [DOI] [PubMed] [Google Scholar]
- 5.Bielefeldt Ohmann, H., and L. A. Babiuk. 1988. Influence of interferons alpha I1 and gamma and of tumour necrosis factor on persistent infection with bovine viral diarrhoea virus in vitro. J. Gen. Virol. 69:1399-1403. [DOI] [PubMed] [Google Scholar]
- 6.Block, T. M., and R. Jordan. 2001. Iminosugars as possible broad spectrum anti hepatitis, virus agents: the glucovirs and alkovirs. Antivir. Chem. Chemother. 12:317-325. [DOI] [PubMed] [Google Scholar]
- 7.Block, T. M., X. Lu, A. S. Mehta, B. S. Blumberg, B. Tennant, M. Ebling, B. Korba, D. M. Lansky, G. S. Jacob, and R. A. Dwek. 1998. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat. Med 4:610-614. [DOI] [PubMed] [Google Scholar]
- 8.Branza-Nichita, N., D. Durantel, S. Carrouee-Durantel, R. A. Dwek, and N. Zitzmann. 2001. Antiviral effect of N-butyldeoxynojirimycin against bovine viral diarrhea virus correlates with misfolding of E2 envelope proteins and impairment of their association into E1-E2 heterodimers. J. Virol. 75:3527-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckwold, V. E., J. Wei, M. Wenzel-Mathers, and J. Russell. 2003. Synergistic in vitro interactions between alpha interferon and ribavirin against bovine viral diarrhea virus and yellow fever virus as surrogate models of hepatitis C virus replication. Antimicrob. Agents Chemother. 47:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choukhi, A., S. Ung, C. Wychowski, and J. Dubuisson. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courageot, M. P., M. P. Frenkiel, C. D. Dos Santos, V. Deubel, and P. Despres. 2000. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 74:564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deleersnyder, V., A. Pillez, C. Wychowski, K. Blight, J. Xu, Y. S. Hahn, C. M. Rice, and J. Dubuisson. 1997. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 71:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doo, E., and T. J. Liang. 2001. Molecular anatomy and pathophysiologic implications of drug resistance in hepatitis B virus infection. Gastroenterology 120:1000-1008. [DOI] [PubMed] [Google Scholar]
- 14.Dubuisson, J. 2000. Folding, assembly and subcellular localization of hepatitis C virus glycoproteins. Curr. Top. Microbiol. Immunol. 242:135-148. [DOI] [PubMed] [Google Scholar]
- 15.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durantel, D., N. Branza-Nichita, S. Carrouee-Durantel, T. D. Butters, R. A. Dwek, and N. Zitzmann. 2001. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 75:8987-8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwek, R. A., T. D. Butters, F. M. Platt, and N. Zitzmann. 2002. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 1:65-75. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, K. P., K. S. Gutfreund, and D. L. Tyrrell. 2001. Lamivudine resistance in hepatitis B: mechanisms and clinical implications. Drug Resist. Updates 4:118-128. [DOI] [PubMed] [Google Scholar]
- 19.Foster, G. R., and H. C. Thomas. 2000. Therapeutic options for HCV-management of the infected individual. Baillieres Best Pract. Res. Clin. Gastroenterol. 14:255-264. [DOI] [PubMed] [Google Scholar]
- 20.Gong, Y., R. Trowbridge, S. Mackintosh, A. Shannon, and E. J. Gowans. 1998. A stable cell line with a proportion of cells persistently infected with bovine viral diarrhoea virus. Vet. Microbiol. 63:117-124. [DOI] [PubMed] [Google Scholar]
- 21.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond, C., I. Braakman, and A. Helenius. 1994. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 91:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond, C., and A. Helenius. 1994. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science 266:456-458. [DOI] [PubMed] [Google Scholar]
- 24.Hebert, D. N., B. Foellmer, and A. Helenius. 1995. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81:425-433. [DOI] [PubMed] [Google Scholar]
- 25.Hebert, D. N., J. F. Simons, J. R. Peterson, and A. Helenius. 1995. Calnexin, calreticulin, and Bip/Kar2p in protein folding. Cold Spring Harb. Symp. Quant. Biol. 60:405-415. [DOI] [PubMed] [Google Scholar]
- 26.Kummerer, B. M., N. Tautz, P. Becher, H. Thiel, and G. Meyers. 2000. The genetic basis for cytopathogenicity of pestiviruses. Vet. Microbiol. 77:117-128. [DOI] [PubMed] [Google Scholar]
- 27.Markland, W., T. J. McQuaid, J. Jain, and A. D. Kwong. 2000. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 44:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHutchison, J. G., and T. Poynard. 1999. Combination therapy with interferon plus ribavirin for the initial treatment of chronic hepatitis C. Semin. Liver Dis. 19(Suppl. 1):57-65. [PubMed] [Google Scholar]
- 29.Mehta, A., S. Carrouee, B. Conyers, R. Jordan, T. Butters, R. A. Dwek, and T. M. Block. 2001. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology 33:1488-1495. [DOI] [PubMed] [Google Scholar]
- 30.Mehta, A., N. Zitzmann, P. M. Rudd, T. M. Block, and R. A. Dwek. 1998. Alpha-glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 430:17-22. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura, S., T. Shimazaki, K. Sakamoto, A. Fukusho, Y. Inoue, and N. Ogawa. 1995. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 57:677-681. [DOI] [PubMed] [Google Scholar]
- 32.Ouzounov, S., A. Mehta, R. A. Dwek, T. M. Block, and R. Jordan. 2002. The combination of interferon [alpha]-2b and n-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: implications for hepatitis C virus (HCV) therapy. Antiviral Res. 55:425-435. [DOI] [PubMed] [Google Scholar]
- 33.Pocock, D. H., C. J. Howard, M. C. Clarke, and J. Brownlie. 1987. Variation in the intracellular polypeptide profiles from different isolates of bovine virus diarrhoea virus. Arch. Virol. 94:43-53. [DOI] [PubMed] [Google Scholar]
- 34.Poynard, T., V. Ratziu, Y. Benhamou, P. Opolon, P. Cacoub, and P. Bedossa. 2000. Natural history of HCV infection. Baillieres Best Pract. Res. Clin. Gastroenterol. 14:211-228. [DOI] [PubMed] [Google Scholar]
- 35.Ren, S., and E. J. Lien. 2001. Development of HIV protease inhibitors: a survey. Prog. Drug Res. Spec. No. 1-34. [PubMed]
- 36.Richman, D. D. 2001. HIV chemotherapy. Nature 410:995-1001. [DOI] [PubMed] [Google Scholar]
- 37.Scott, L. J., and C. M. Perry. 2002. Interferon-alpha-2b plus ribavirin: a review of its use in the management of chronic hepatitis C. Drugs 62:507-556. [DOI] [PubMed] [Google Scholar]
- 38.Sentsui, H., R. Takami, T. Nishimori, K. Murakami, T. Yokoyama, and Y. Yokomizo. 1998. Anti-viral effect of interferon-alpha on bovine viral diarrhea virus. J. Vet. Med. Sci. 60:1329-1333. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu, M., K. Satou, N. Nishioka, T. Yoshino, E. Momotani, and Y. Ishikawa. 1989. Serological characterization of viruses isolated from experimental mucosal disease. Vet. Microbiol. 19:13-21. [DOI] [PubMed] [Google Scholar]
- 40.Snell, N. J. 2001. Ribavirin—current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2:1317-1324. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan, D. G., and R. K. Akkina. 1995. A nested polymerase chain reaction assay to differentiate pestiviruses. Virus Res. 38:231-239. [DOI] [PubMed] [Google Scholar]
- 42.Trepo, C. 2000. Genotype and viral load as prognostic indicators in the treatment of hepatitis C. J. Viral. Hepat. 7:250-257. [DOI] [PubMed] [Google Scholar]
- 43.Weiland, O. 2000. Interferon and ribavirin combination therapy: indications and schedules. Forum (Genova) 10:22-28. [PubMed] [Google Scholar]
- 44.Wu, S. F., C. J. Lee, C. L. Liao, R. A. Dwek, N. Zitzmann, and Y. L. Lin. 2002. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 76:3596-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zitzmann, N., A. S. Mehta, S. Carrouee, T. D. Butters, F. M. Platt, J. McCauley, B. S. Blumberg, R. A. Dwek, and T. M. Block. 1999. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA 96:11878-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]