Abstract
Background
Eosinophils play an important role in the pathogenesis of bronchial asthma and its exacerbation. Recent reports suggest the involvement of IFN-γ-inducible protein of 10 kDa (IP-10) in virus-induced asthma exacerbation. The objective of this study was to examine whether CXCR3 ligands including IP-10 modify the effector functions of eosinophils.
Methods
Eosinophils isolated from the blood of healthy donors were stimulated with CXCR3 ligands and their adhesion to rh-ICAM-1 was then measured using eosinophil peroxidase assays. The generation of eosinophil superoxide anion (O2-) was examined based on the superoxide dismutase-inhibitable reduction of cytochrome C. Eosinophil-derived neurotoxin (EDN) release was evaluated to determine whether CXCR3 ligands induced eosinophil degranulation. Cytokine and chemokine production by eosinophils was examined using a Bio-plex assay.
Results
Eosinophil adhesion to ICAM-1 was significantly enhanced by IP-10, which also significantly induced eosinophil O2- generation in the presence of ICAM-1. Both the enhanced adhesion and O2- generation were inhibited by an anti-β2 integrin mAb or an anti-CXCR3 mAb. Other CXCR3 ligands, such as monokine induced by IFN-γ (Mig) and IFN-inducible T cell α chemoattractant (I-TAC), also induced eosinophil adhesion and O2- generation in the presence of ICAM-1. IP-10, but not Mig or I-TAC, increased the release of EDN. IP-10 increased the production of a number of cytokines and chemokines by eosinophils.
Conclusions
These findings suggest that CXCR3 ligands such as IP-10 can directly upregulate the effector functions of eosinophils. These effects might be involved in the activation and infiltration of eosinophils in the airway of asthma, especially in virus-induced asthma exacerbation.
Keywords: asthma, acute exacerbation, ICAM-1, IP-10, rhinovirus
Background
Bronchial asthma is a chronic disorder characterized by airway inflammation, reversible airway obstruction, mucus hypersecretion, and airway hyperresponsiveness [1]. A variety of cells, including eosinophils, T lymphocytes, mast cells, neutrophils, and dendritic cells, are involved in the process of airway inflammation of asthma. Of these cells, eosinophils preferentially accumulate at sites of allergic inflammation and are believed to play important roles in the pathophysiology of asthma through the release of a variety of inflammatory mediators, including major basic protein, cysteinyl leukotrienes (cysLTs), reactive oxygen species and cytokines [2,3]. Green et al. reported that a treatment strategy directed at normalization of the induced sputum eosinophil count reduces asthma exacerbations [4], suggesting an important role for eosinophils in the pathogenesis of asthma exacerbation.
Acute respiratory infections are a major cause of asthma exacerbation [5,6]. Clinical data suggest that not only the numbers of neutrophils, but also that of eosinophils increases in asthmatic airways during, or following, viral infection [7-9]. For example, experimental viral infection increases eosinophil counts in the epithelium of patients with allergic asthma [7]. The sputum of asthmatic patients with confirmed viral infection contains high levels of eosinophilic cationic protein [8]. These findings suggest that eosinophils are indeed activated and infiltrate asthmatic airways during or following viral infection.
The initial steps of eosinophil accumulation in the asthmatic airway are adhesion to and subsequent migration across endothelial cells. Eosinophil adhesion molecules, such as α4 integrins including α4β1 (VLA-4, CD49d/CD29) and β2 integrins including αLβ2 (LFA-1, CD11a/CD18) and αMβ2 (Mac-1, CD11b/CD18), play roles in the adhesion to and transmigration through the endothelium [10]. Furthermore, eosinophil adhesion to adhesion molecules, such as vascular cell adhesion molecule (VCAM)-1 (a ligand for α4 integrins) and intercellular adhesion molecule (ICAM)-1 (a ligand for β2 integrins) may augment eosinophil function [11-13]. For example, the adhesion of eosinophils to VCAM-1 stimulates eosinophil superoxide anion (O2-) generation [11]. Similarly, adhesion of eosinophils to ICAM-1 augments both leukotriene (LT) C4 generation and the release of eosinophil-derived neurotoxin (EDN) [12]. Therefore, interaction between eosinophils and adhesion molecules would contribute to the development of airway inflammation in bronchial asthma.
IFN-γ-inducible protein of 10 kDa (IP-10) belongs to the CXC chemokine subfamily that binds to the common receptor, CXCR3, and it is produced by various cell types in response to IFN-γ [14]. CXCR3 is expressed at high levels on Th0 and Th1 lymphocytes. Jinquan et al. reported that CXCR3 is also expressed on eosinophils [15]. Recently, the role of IP-10 in virus-induced asthma exacerbation has been highlighted [16-18]. However, whether IP-10 actually modifies allergic airway inflammation, including the accumulation or activation of eosinophils, has not been fully investigated.
Monokine induced by IFN-γ (Mig) and IFN-inducible T cell α chemoattractant (I-TAC) are also CXC chemokines [19]. However, the roles of Mig or I-TAC in the pathogenesis of asthma and their effects on eosinophils have not been elucidated in detail.
Here, we examined the effect of the CXCR3 ligands IP-10, Mig and I-TAC on eosinophil functions such as their adhesion properties, O2- generation, EDN release and cytokine production, in the presence or absence of adhesion molecules such as ICAM-1.
Methods
Preparation of eosinophils
Eosinophils were isolated from peripheral blood specimens collected from non-atopic healthy donors with a peripheral blood differential eosinophil count of < 5%. The numbers of males and females, ranging in age from 24 to 42 years, were comparable among the donors. Written, informed consent was obtained from each donor before collecting blood samples. Eosinophils were isolated by negative selection using immunomagnetic beads as described [11-13,20-23]. Over 98% of the cells were eosinophils, as determined by morphologic criteria using May-Grünwald-Giemsa staining. We also confirmed by flow cytometry that over 98% of the cells were CD16-negative/CD14-negative (data not shown). Eosinophil viability was > 99%, as determined by Trypan blue dye exclusion. Eosinophils were resuspended in Hank's balanced salt solution (HBSS) supplemented with gelatin to a final concentration of 0.1% (HBSS/gel).
Eosinophil adhesion assay
Eosinophil adhesion to rh-ICAM-1- or rh-VCAM-1-coated plates was assessed based on the residual eosinophil peroxidase (EPO) activity of adherent eosinophils as described [11-13,20]. Eosinophils (100 μl of 1 × 105 cells/ml in HBSS/gel) were incubated at 37°C for 20 min in rh-ICAM-1- or rh-VCAM-1-coated plates in the presence or absence of the CXCR3 ligands IP-10 (R&D Systems, Minneapolis, MN), Mig (R&D Systems), or I-TAC (R&D Systems). In selected experiments, eosinophils were incubated with anti-α4-integrin mAb (3 μg/ml; clone HP1/2; Cosmo Bio Co. Ltd, Tokyo, Japan), anti-β2-integrin mAb (3 μg/ml; clone L130; Becton Dickinson, Franklin Lakes, NJ), or anti-CXCR3 mAb (3 μg/ml; clone 49801; R&D Systems, Minneapolis, MN) before assay. The plates were washed with HBSS and 100 μl of HBSS/fetal calf serum (FCS) was then added to the wells. Standards comprised of 100 μl of serially diluted cell suspensions (1 × 103, 3 × 103, 1 × 104, 3 × 104, and 1 × 105 cells/ml) were added to empty wells. The EPO substrate (1 mM o-phenylenediamine, 1 mM H2O2, and 0.1% Triton X-100 in Tris buffer, pH 8.0) was then added to all wells and the plates were incubated for 30 min at room temperature. The reaction was stopped by adding 50 μl of 4 M H2SO4 and absorbance was measured at 490 nm. Each experiment was performed in quadruplicate using eosinophils from a single donor, and percentage eosinophil adhesion was determined from mean values that were calculated from log dose-response curves. Eosinophil viability after incubation was > 98%, as determined by Trypan blue dye exclusion.
Eosinophil superoxide anion generation
Eosinophil O2- generation was measured in 96-well plates (Corning Inc.) as described based on the superoxide dismutase (SOD)-inhibitable reduction of cytochrome C [11-13]. We initially added SOD (0.2 mg/ml in HBSS/gel; 20 μl) to SOD control wells and then HBSS/gel to all wells to bring the final volume to 100 μl. The eosinophil density was adjusted to 1.25 × 106 cells/ml of HBSS/gel mixed 4:1 with cytochrome C (12 mg/ml of HBSS/gel), and 100 μl of eosinophil suspension was then added to all wells. Immediately after adding CXCR3 ligand, the absorbance of the cell suspensions in the wells was measured at 550 nm in an Immuno-Mini (NJ-2300; Japan Intermed Co., Tokyo, Japan), followed by repeated measurements over the next 240 min. In selected experiments, eosinophils were incubated with anti-β2-integrin mAb (3 μg/ml) or anti-CXCR3 mAb (3 μg/ml) before assay. The plates were incubated in a 5% CO2 incubator at 37°C between measurements. Each reaction was evaluated in duplicate against the control reaction in wells containing 20 μg/ml of SOD. The results were adjusted for a 1-ml reaction volume, and O2- generation was calculated at an extinction coefficient of 21.1 mM-1cm-1, as nanomoles of cytochrome C reduced per 1.0 × 106 cells/ml minus the SOD control [9-11]. The maximum value during the incubation time was examined to evaluate the effects of various factors on eosinophil O2- generation. Cell viability, determined by Trypan blue exclusion at the end of each experiment, remained at 95% after a 240-min incubation with the activator.
Eosinophil Degranulation
Eosinophils (1 × 106 cells/ml) in 96-well plates were incubated for the 240 min that were required for measurement of O2- generation, and were then immediately centrifuged (700 g) at 4°C for 15 min. Recovered cell-free supernatants were subjected to EDN analysis, as described previously [21]. Levels of EDN were quantified using ELISA kits (Medical and Biological Laboratory Co Ltd, Nagoya, Japan).
Cytokine and chemokine production
Eosinophils (1 × 106 cells/ml) were resuspended in RPMI 1640 containing 10% FCS. Cells were incubated with CXCR3 ligands in 96-well tissue-culture plates for 24 h at 37°C and 5% CO2 [24]. After culture, the supernatants were collected, and cytokine and chemokine concentrations were measured by using Bio-plex assay kits (Bio-Rad, Mississauga, Canada).
Statistical analysis
Values are expressed as means ± SEM. Two groups were compared using Student's t test and more than two groups were compared using a repeated-measures analysis of variance (ANOVA) with Tukey's test of multiple comparisons. Values of P < 0.05 were considered statistically significant.
Results
Effect of IP-10 on eosinophil adhesiveness
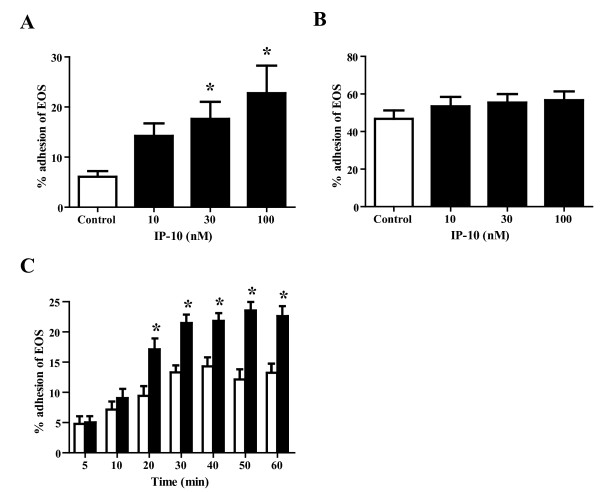
We initially examined whether or not IP-10 directly modifies eosinophil adhesiveness. We found that IP-10 (100 nM) significantly enhanced the adhesiveness of eosinophils to plastic plates compared with controls (data not shown), suggesting that IP-10 modifies adhesive interactions between eosinophils and adhesion molecules. To test this hypothesis, we investigated the effect of IP-10 on eosinophil adhesion to recombinant adhesion molecules. Eosinophils were stimulated with rh-IP-10 (10-100 nM) in the presence of immobilized rh-ICAM-1 or rh-VCAM-1, and adhesion was then evaluated. Under these conditions, IP-10 did not significantly increase eosinophil adhesion to ICAM-1 at a concentration of 10 nM, but did significantly enhance adhesion to ICAM-1 at a concentration of 30 and 100 nM, compared with the control (14.3 ± 2.5%, 17.6 ± 3.4% and 22.8 ± 5.5% vs. 6.1 ± 1.1%; NS, P < 0.05 and P < 0.05, respectively; n = 5 each; Figure 1A). In contrast, IP-10 (100 nM) did not enhance eosinophil adhesion to either VCAM-1 or fibronectin, which are counter ligands for eosinophil α4 integrin, compared with controls (46.8 ± 4.5% vs. 56.7 ± 4.6% and 20.4 ± 2.8% vs. 20.2 ± 1.8%, respectively; both NS; n = 5 each; Figure 1B and data not shown). We evaluated the effects of incubation with IP-10 on the adhesiveness of eosinophils to ICAM-1 over time. Eosinophils were incubated with or without IP-10 (100 nM) for periods ranging from 5 to 60 min, washed, and then adhesion to ICAM-1 was evaluated. The results showed that IP-10 (100 nM) augmented eosinophil adhesiveness started from 20 min and lasted for 60 min (P < 0.05 for all time points between 20 and 60 min, n = 6; Figure 1C).
Figure 1.
IP-10 significantly enhances the adhesiveness of eosinophils. (A) IP-10 significantly augments eosinophil adhesion to rh-ICAM-1-coated plates. Eosinophils (100 μl of 1 × 105 cells/ml in HBSS/gel) obtained from different healthy donors, were incubated with IP-10 (10-100 nM) at 37°C for 20 min in rh-ICAM-1-coated plates. The adhesiveness of the eosinophils was then assessed by assay of residual EPO activity. Data are shown as means ± SEM of 5 experiments using cells from different donors. (B) IP-10 does not modify eosinophil adhesion to rh-VCAM-1-coated plates. Eosinophils were incubated with IP-10 (10-100 nM) in rh-VCAM-1-coated plates, and the adhesiveness of the eosinophils was examined. Data are shown as means ± SEM of 5 experiments using cells from different donors. (C) Time course of IP-10 induction of eosinophil adhesion to ICAM-1 coated plates. Eosinophils were incubated with or without IP-10 (100 nM) for periods ranging from 5 to 60 min. The kinetics of eosinophil adhesion to ICAM-1 coated plates was determined by aspiration of non-adhered cells at the indicated times, followed by washing every 5 min for the next 10 min and then every 10 min over the next 50 min, following which adhesion was assayed. Data are shown as means ± SEM of 6 experiments using cells from different donors. Open and filled bars, eosinophil adhesion without and with IP-10, respectively. *P < 0.05 versus without IP-10.
Effects of anti-integrin mAbs on eosinophil adhesion enhanced by ICAM-1 and IP-10
To identify the eosinophil integrin(s) involved in IP-10-induced eosinophil adhesion to ICAM-1, we incubated eosinophils with anti-β2 integrin mAb, anti-α4 integrin mAb, or an isotype-matched control mouse IgG1 at ambient temperature for 15 min, and then evaluated adhesion. Eosinophil adhesion that was enhanced by ICAM-1 plus IP-10 was inhibited by anti-β2 integrin mAb (mouse IgG1 vs. anti-β2 integrin mAb: 36.8 ± 3.2% vs. 21.1 ± 2.7%, P < 0.01; n = 6 each; Figure 2A). Anti-α4 integrin mAb did not modify the ICAM-1 and IP-10-enhanced eosinophil adhesion (mouse IgG1 vs. anti-α4 integrin mAb: 36.8 ± 3.2% vs. 33.3 ± 3.2%, NS; n = 6 each; Figure 2A). Neither anti-α4 integrin mAb nor anti-β2 integrin mAb modified spontaneous eosinophil adhesion (Figure 2A).
Figure 2.
Anti-β2 integrin mAb and anti-CXCR3 mAb suppress eosinophil adhesion enhanced by ICAM-1 and IP-10. (A) IP-10-enhanced eosinophil adhesion to ICAM-1 is inhibited by anti-β2 integrin mAb. Eosinophils were pre-incubated with either anti-integrin mAb (3 μg/ml) or control IgG for 15 min prior to analysis of adhesion in the presence or absence of IP-10. Data are shown as means ± SEM of 6 experiments using cells from different donors. (B) IP-10-enhanced eosinophil adhesion to ICAM-1 is inhibited by anti-CXCR3 mAb. Eosinophils were pre-incubated with either anti-CXCR3 mAb (3 μg/ml) or control IgG for 15 min prior to analysis of adhesion in the presence or absence of IP-10. Data are shown as means ± SEM of six experiments using cells from different donors. Open and filled bars, eosinophil adhesion without and with IP-10, respectively. **P < 0.01 versus without IP-10. ##P < 0.01, ###P < 0.001 versus mouse IgG1.
Effect of anti-CXCR3 mAb on eosinophil adhesion enhanced by ICAM-1 and IP-10
We examined whether CXCR3, a major receptor for CXC chemokines, is involved in IP-10-induced eosinophil adhesion to ICAM-1. Eosinophils were incubated with either anti-CXCR3 mAb or an isotype-matched control mouse IgG1 at ambient temperature for 15 min before adhesion assays. Anti-CXCR3 mAb inhibited the eosinophil adhesion that was enhanced by ICAM-1 plus IP-10 (mouse IgG1 vs. anti-CXCR3 mAb: 28.5 ± 2.2 vs. 16.5 ± 1.2%; P < 0.001; n = 6; Figure 2B), suggesting that IP-10 could directly upregulate eosinophil adhesion via CXCR3.
Effect of IP-10 on eosinophil O2- generation in the presence of ICAM-1
We next examined whether IP-10 modifies O2- generation of eosinophils in the presence of ICAM-1. IP-10 did not affect O2- generation by eosinophils at a concentration of 30 nM (Figure 3A). However, at a concentration of 100 nM, IP-10 significantly activated eosinophil O2- generation in wells coated with ICAM-1 compared with controls (5.2 ± 1.8 vs. 8.5 ± 3.0 nmol/106 cells, P < 0.05; n = 6; Figure 3A), although the effect of IP-10 on eosinophil O2- generation was of small magnitude.
Figure 3.
IP-10 significantly enhances eosinophil O2- generation. (A) IP-10 significantly enhances eosinophil O2- generation in the presence of ICAM-1. Eosinophil cell density was adjusted to 1.25 × 106 cells/ml of HBSS/gel mixed 4:1 with cytochrome C, and the eosinophil suspension was then added to ICAM-1 coated 96-well plates. Immediately after adding IP-10 (30 or 100 nM), eosinophil O2- generation was measured based on the SOD-inhibitable reduction of cytochrome C. Data are shown as means ± SEM of 5 experiments using cells from different donors. (B) Eosinophil O2- generation induced by the combination of IP-10 + ICAM-1 is blocked by anti-β2 integrin mAb. Eosinophils were pre-incubated with anti-β2 integrin mAb (3 μg/ml) or control IgG for 15 min prior to analysis of O2- generation of eosinophils as in (A). Data are shown as means ± SEM of 7 experiments using cells from different donors. (C) Eosinophil O2- generation induced by a combination of IP-10 + ICAM-1 is blocked by anti-CXCR3 mAb. Eosinophils were pre-incubated with anti-CXCR3 mAb (3 μg/ml) or control IgG for 15 min prior to analysis of O2- generation of eosinophils as in (A). Data are shown as means ± SEM of 7 experiments using cells from different donors. Open and filled bars, eosinophil O2- generation without and with IP-10, respectively. *P < 0.05 versus without IP-10. #P < 0.05 versus mouse IgG1.
Effect of anti-β2 integrin or anti-CXCR3 mAb on eosinophil O2- generation induced by a combination of ICAM-1 and IP-10
We investigated whether β2integrin or CXCR3 is involved in the enhanced eosinophil O2- generation induced by ICAM-1 plus IP-10, by assay of the effect of pre-incubating eosinophils with anti-β2 integrin mAb, anti-CXCR3 mAb, or an isotype-matched control mouse IgG1 at ambient temperature for 15 min prior to analysis. Eosinophil O2- generation induced by the combination of ICAM-1 and IP-10 was blocked by both anti-β2 integrin and anti-CXCR3 mAbs compared with mouse IgG1 (4.9 ± 0.9 vs. 0.5 ± 0.9 and 4.0 ± 0.9 vs. 1.0 ± 0.6 nmol/106 cells, respectively; n = 7 each; P < 0.05 for both; Figures 3B and 3C). Neither anti-β2 integrin mAb nor anti-CXCR3 mAb modified spontaneous O2- generation by eosinophils.
Effects of other CXCR3 ligands on adhesion and O2- generation of eosinophils
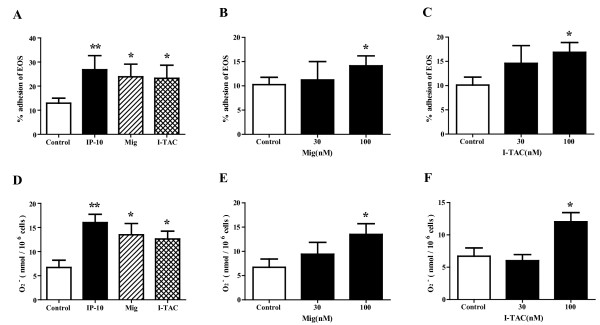
We examined whether CXCR3 ligands other than IP-10 modify eosinophil adhesion and O2- generation by stimulation of eosinophils with IP-10, Mig or I-TAC in the presence of ICAM-1. At a concentration of 100 nM, all of these CXCR3 ligands enhanced eosinophil adhesion to ICAM-1 versus control (IP-10, Mig and I-TAC: 26.8 ± 5.8%, 23.9 ± 5.2% and 23.2 ± 5.4% respectively vs. 12.9 ± 2.1%; P < 0.01, P < 0.05 and P < 0.05; n = 5 each; Figure 4A). However, in contrast to IP-10, 30 nM of Mig or I-TAC did not increase eosinophil adhesion to ICAM-1 (Figures 4B and 4C), suggesting that the effect of Mig or I-TAC on eosinophil adhesion was weaker than that of IP-10. All of the CXCR3 ligands augmented eosinophil O2- generation in the presence of ICAM-1 at a concentration of 100 nM, but not at a concentration of 30 nM (IP-10, Mig and I-TAC: 16.0 ± 1.7, 13.5 ± 2.2 and 12.0 ± 1.4, respectively, vs. 6.7 ± 1.5 nmol/106 cells; P < 0.01, P < 0.05 and P < 0.05; n = 5 each; Figures 4D-F).
Figure 4.
Other CXCR3 ligands also enhance eosinophil adhesion and eosinophil O2- generation. (A-C) All tested CXCR3 ligands significantly augmented eosinophil adhesion to ICAM-1-coated plates. Eosinophils were incubated with or without CXCR3 ligands for 20 min, and adhesion of eosinophils was then examined. (A) All of the CXCR3 ligands (100 nM) augmented eosinophil adhesion to ICAM-1. (B) Dose-response relationship between Mig and eosinophil adhesion. (C) Dose-response relationship between I-TAC and eosinophil adhesion. Data shown are means ± SEM of 5 experiments using cells from different donors. (D-F) All tested CXCR3 ligands significantly induced eosinophil O2- generation in the presence of ICAM-1. Eosinophil O2- generation was measured with or without CXCR3 ligands. (D) All of the CXCR3 ligands (100 nM) induced eosinophil O2- generation. (E) Dose-response relationship between Mig and eosinophil O2- generation. (F) Dose-response relationship between I-TAC and eosinophil O2- generation. Data shown are means ± SEM of 5 experiments using cells from different donors. *P < 0.05, **P < 0.01 versus without CXCR3 ligands.
Effect of CXCR3 ligands on EDN release or cytokine/chemokine production by eosinophils
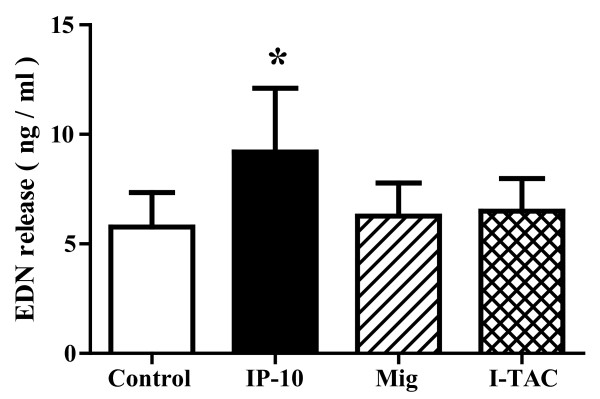
We examined the effect of CXCR3 ligands on the degranulation of eosinophils as well as the production of cytokines and chemokines by eosinophils. Eosinophils were incubated with CXCR3 ligands and eosinophil degranulation was evaluated by measurement of the concentration of eosinophil-derived neurotoxin (EDN) in the supernatant. At a concentration of 100 nM, IP-10, but not Mig or I-TAC, increased EDN release (IP-10, Mig and I-TAC: 9.2 ± 2.9, 6.3 ± 1.5 and 6.5 ± 1.5, respectively, vs. 5.8 ± 1.6 ng/ml; P < 0.05, NS and NS; n = 6 each; Figure 5), suggesting that only IP-10 induced the degranulation of eosinophils. IP-10 did not increase EDN release at a concentration of 30 nM (data not shown). We next measured the concentrations of cytokines and chemokines in supernatant using a Bio-plex assay. As shown in Table 1, IP-10 at a concentration of 100 nM significantly increased the production of several cytokines/chemokines, including proinflammatory cytokines and Th1/Th2 cytokines, by eosinophils. Although Mig or I-TAC increased MIP-1β or IL-9 respectively at a concentration of 100 nM, almost every cytokine/chemokine was not upregulated by stimulation with Mig or I-TAC. To confirm the reliability of the Bio-plex assay kits, we measured IL-6 and IL-8 concentrations by ELISA, and similar results were obtained (data not shown).
Figure 5.
IP-10, but not Mig or I-TAC, induces eosinophil release of EDN. Eosinophils (1 × 106 cells/ml) in 96-well plates were incubated for the 240-min required to measure O2- generation, immediately centrifuged and the concentration of EDN in cell-free supernatants was then quantified using ELISA. Data shown are means ± SEM of 6 experiments using cells from different donors. *P < 0.05 versus without CXCR3 ligands.
Table 1.
Production of cytokines and chemokines by eosinophils stimulated with CXCR3 ligands.
| Cytokine | Control (pg/ml) | IP-10 (pg/ml) | Mig (pg/ml) | I-TAC (pg/ml) |
|---|---|---|---|---|
| IL-1ra | 1.8 ± 0.7 | 25.0 ± 2.1* | 3.8 ± 1.3 | 3.9 ± 0.9 |
| IL-1β | 0.3 ± 0.1 | 6.5 ± 2.5* | 2.6 ± 1.0 | 1.8 ± 0.9 |
| IL-2 | 0.0 ± 0.0 | 2.0 ± 0.1* | 0.1 ± 0.1 | 0.1 ± 0.0 |
| IL-4 | 0.1 ± 0.0 | 1.1 ± 0.1* | 0.1 ± 0.0 | 0.1 ± 0.0 |
| IL-5 | 0.0 ± 0.0 | 0.3 ± 0.1* | 0.0 ± 0.0 | 0.0 ± 0.0 |
| IL-6 | 9.2 ± 4.9 | 80.5 ± 27.4* | 40.5 ± 17.4 | 29.5 ± 14.8 |
| IL-7 | 0.1 ± 0.0 | 0.9 ± 0.1* | 0.2 ± 0.1 | 0.2 ± 0.0 |
| IL-8 | 186.8 ± 89.8 | 404.7 ± 73.3* | 315.3 ± 79.7 | 251.7 ± 76.5 |
| IL-9 | 1.1 ± 0.2 | 4.4 ± 0.4* | 1.8 ± 0.3 | 2.2 ± 0.3* |
| IL-10 | 0.7 ± 0.2 | 2.1 ± 0.4* | 1.2 ± 0.4 | 1.7 ± 0.6 |
| IL-12(p70) | 0.3 ± 0.1 | 0.9 ± 0.1* | 0.4 ± 0.1 | 0.3 ± 0.1 |
| IL-13 | 1.1 ± 0.3 | 1.8 ± 0.2* | 1.4 ± 0.2 | 1.4 ± 0.2 |
| IL-15 | 0.2 ± 0.0 | 0.5 ± 0.1* | 0.3 ± 0.1 | 0.3 ± 0.1 |
| IL-17 | 0.8 ± 0.2 | 2.5 ± 0.7* | 0.6 ± 0.2 | 0.7 ± 0.4 |
| Eotaxin | 1.7 ± 0.6 | 23.2 ± 0.5* | 2.4 ± 1.1 | 2.4 ± 0.9 |
| FGF | 1.4 ± 0.5 | 5.6 ± 1.3* | 1.1 ± 0.6 | 3.1 ± 1.1 |
| G-CSF | 0.2 ± 0.1 | 2.5 ± 0.4* | 0.5 ± 0.2 | 0.3 ± 0.2 |
| GM-CSF | 0.8 ± 0.3 | 9.4 ± 1.0* | 1.6 ± 0.4 | 1.7 ± 0.5 |
| IFN-γ | 2.6 ± 0.6 | 107.0 ± 10.3* | 5.4 ± 2.1 | 5.6 ± 2.0 |
| MCP-1 | 32.6 ± 14.3 | 86.5 ± 28.7* | 65.5 ± 24.6 | 53.5 ± 22.1 |
| MIP-1α | 6.4 ± 2.8 | 30.7 ± 10.4* | 17.8 ± 8.9 | 14.5 ± 7.9 |
| MIP-1β | 93.7 ± 31.0 | 183.5 ± 19.1* | 160.0 ± 24.5* | 135.5 ± 25.7 |
| PDGF | 0.6 ± 0.3 | 2.2 ± 0.5* | 0.4 ± 0.1 | 1.2 ± 0.3 |
| RANTES | 6.5 ± 0.9 | 20.3 ± 1.0* | 7.8 ± 1.1 | 7.9 ± 1.0 |
| TNF-α | 4.0 ± 1.9 | 19.2 ± 4.5* | 5.3 ± 3.3 | 2.7 ± 1.0 |
| VEGF | 7.8 ± 2.6 | 13.3 ± 2.5* | 10.5 ± 3.3 | 10.4 ± 2.8 |
Eosinophils were incubated with CXCR3 ligands for 24 h, and the concentrations of cytokines and chemokines in cell-free supernatants were measured using Bio-plex assay kits. *P < 0.05 versus without CXCR3 ligands.
Discussion
We found that IP-10, a CXCR3 ligand, augments eosinophil adhesiveness. This effect occurred when the counter-adhesion protein was ICAM-1, but not VCAM-1. We also found that IP-10 activates eosinophil O2- generation in the presence of ICAM-1. Anti-β2 integrin or anti-CXCR3 mAb inhibited the induction of eosinophil adhesion or O2- generation in the presence of ICAM-1, suggesting that the effect of CXCR3 ligands involves eosinophil β2 integrin and CXCR3. The other CXCR3 ligands, Mig and I-TAC, also significantly enhanced eosinophil adhesion to ICAM-1 and induced eosinophil O2- generation in the presence of ICAM-1. Furthermore, IP-10 increased the release of EDN and the production of a number of cytokines and chemokines. These results suggest novel roles for CXCR3 ligands including IP-10 as activators of eosinophils through β2 integrin and CXCR3.
Although allergic airway inflammation is generally considered to be a process that is mediated by the Th2-type response, recent reports suggest that the Th1-type response also plays an important role. For example, the passive transfer of OVA-specific Th1 cells exacerbates OVA-induced airway eosinophilia in a mouse model [25]. Because IP-10 is originally generated by IFN-γ, and CXCR3 is predominantly expressed on Th1 cells [26], IP-10 is thought to play an important role in the Th1-mediated immune response. However, similar to Th1 cells, IP-10 itself can exacerbate Th2-mediated airway inflammation. For example, IP-10 overexpression in the lung of a mouse model of allergic airway inflammation increases airway hyperreactivity, eosinophilia, and IL-4 levels [27]. Furthermore, IP-10 deletion attenuated Th2-type allergic airway inflammation in a mouse model of asthma [27]. These results demonstrated that the Th1-type chemokine IP-10 rather enhances Th2-mediated eosinophilic inflammation. The mechanism through which IP-10 exacerbates Th2-mediated inflammation remains obscure.
Most asthma exacerbation in a clinical setting is induced by viral respiratory infections, in particular those caused by the rhinovirus (RV) [5]. Although eosinophils, as well as neutrophils, are increased in asthmatic airways during or after viral infection [7-9], their mechanisms of action have not been fully clarified. Recent evidence suggests that IP-10 is involved in RV-induced asthma exacerbation [16-18]. For example, RV infection induces bronchial epithelial cells to produce IP-10 in vitro and in vivo [16]. Monocytic cells also play an important role as a major source of IP-10 during RV infection [17,28]. Serum IP-10 concentrations are specifically increased in RV-induced asthma [18]. Furthermore, increased levels of IP-10 correlate with clinical disease severity during RV-induced exacerbation. These data suggest that IP-10 is likely to play a role in the pathogenesis of virus-induced asthma exacerbation.
Our results suggest that IP-10 induces eosinophil adhesiveness to ICAM-1, but not to VCAM-1 (Figure 1). Anti-CXCR3 Ab inhibited the induction of eosinophil adhesion (Figure 2B). Jinquan et al. reported that CXCR3 is expressed on eosinophils and that IP-10 induces chemotaxis [16]. Therefore, IP-10 could directly upregulate eosinophil functions via CXCR3. Indeed, in the present study, a combination of IP-10 and ICAM-1 activated eosinophil functions, such as O2- generation (Figure 3A). Furthermore, IP-10 induced eosinophil degranulation (Figure 5) and the production of a number of cytokines/chemokines (Table 1). ICAM-1 is not only an adhesion molecule that plays an important role in inflammatory cell recruitment, but is also a cellular receptor for the majority (90%) of RVs [29]. Furthermore, RV infection increases ICAM-1 expression on epithelial cells [30,31]. Therefore, it is likely that eosinophil adhesion to epithelial cells through ICAM-1 would be enhanced during RV-induced asthma exacerbation. Moreover, eosinophil adhesion to ICAM-1 could activate eosinophil functions [11-13]. Since both IP-10 and ICAM-1 can be upregulated in RV-induced asthma exacerbation as described above, our results indicate that IP-10 interaction with ICAM-1 in the airway may play an important role in the activation and infiltration of eosinophils during RV-induced asthma exacerbation.
Not only CXCR3, but also other chemokine receptors are expressed on eosinophils. To date, constitutive and/or inducible expression of CCR1, CCR3, CXCR1, CXCR2, CXCR3, and CXCR4 has been reported in human eosinophils [15,32-34]. Of these receptors, high levels of CCR3 are constitutively expressed on eosinophils [35]. The CCR3 ligand, eotaxin, is a potent chemoattractant and activator of eosinophils, and thus the eotaxin/CCR3 axis presumably plays an essential role in the development of allergic inflammation. However, recent evidence indicates that chemokine receptor expression on eosinophils in peripheral blood differs from that on eosinophils in bronchoalveolar lavage fluid (BALF) [36-38], suggesting that chemokine receptors other than CCR3 also play important roles in eosinophil functions in the airway. For example, Liu et al. reported that eosinophils in the airway express more CXCR3 and less CCR3 than those in peripheral blood [36]. Katoh et al. found higher ratios of CXCR3-expressing eosinophils in BALF than in peripheral blood [37]. Nagase et al. reported that less CCR3 is expressed by eosinophils in BALF than in peripheral blood [38]. These findings suggest that eosinophils in the airways express more CXCR3 and less CCR3 than those in the peripheral circulation. The present study demonstrated that IP-10 activated the functions of eosinophils from peripheral blood. Therefore, CCR3 and CXCR3 might play similar roles in the activation and infiltration of eosinophils in the asthmatic airway. Since IP-10 is less sensitive to corticosteroid [18], we speculate that regulation of the CXCR3 ligand/CXCR3 axis would be important for regulating eosinophilic airway inflammation under specific conditions, such as viral infections.
In contrast to IP-10, the role of Mig or of I-TAC in the pathogenesis of asthma has not been fully clarified. Pillete et al. reported that segmental allergen challenge in asthmatic individuals induced a significant increase in IP-10, but not in Mig or I-TAC, in BALF [39]. Fulkerson et al. reported that Mig inhibits eosinophil responses to diverse stimuli [40], suggesting an immune suppressive role of Mig in some circumstances. The present study demonstrated that both Mig and I-TAC upregulate eosinophil functions such as adhesion to rh-ICAM-1 and O2- generation (Figure 4). Moreover, Mig and I-TAC-enhanced eosinophil adhesion and respiratory burst were suppressed by anti-CXCR3 Ab. These results suggested that both Mig and I-TAC could directly activate eosinophils through CXCR3. However, neither Mig nor I-TAC modified the release of EDN by eosinophils, and their effect on eosinophil adhesion or cytokine/chemokine production was weaker than that of IP-10 (Figures 4 and 5, and Table 1). Therefore, Mig or I-TAC might have lesser significance for the pathogenesis of eosinophilic airway inflammation than IP-10.
Conclusions
In conclusion, our results indicate that CXCR3 ligands upregulate eosinophil functions such as adhesion and O2- generation through CXCR3 and β2 integrin. Furthermore, IP-10 increased eosinophil release of EDN and eosinophil production of a number of cytokines/chemokines. Therefore, inhibition of the CXCR3 ligand/CXCR3 axis might serve as a strategy to suppress eosinophilic airway inflammation, especially in virus-induced asthma exacerbation.
List of abbreviations
ANOVA: analysis of variance; BALF: bronchoalveolar lavage fluid; cysLT: cysteinyl leukotriene; EDN: eosinophil-derived neurotoxin; EPO: eosinophil peroxidase; FCS: fetal calf serum; HBSS: Hank's balanced salt solution; ICAM: intercellular adhesion molecule; IP-10: IFN-γ-inducible protein of 10 kDa; I-TAC: IFN-inducible T cell α chemoattractant; LT: leukotriene; Mig: monokine induced by IFN-γ; O2-: superoxide anion; RV: rhinovirus; SOD: superoxide dismutase; VCAM: vascular cell adhesion molecule.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YT carried out the experiments, analyzed the data, and drafted the manuscript. KN participated in direction of the study, analyzed the data, and wrote the manuscript. TK carried out the eosinophil experiments. KH and MK participated in the data analyses. MN participated in direction of the study and edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Yotaro Takaku, Email: taka-q@paw.hi-ho.ne.jp.
Kazuyuki Nakagome, Email: nakagomek-tky@umin.ac.jp.
Takehito Kobayashi, Email: kobatake@saitama-med.ac.jp.
Koichi Hagiwara, Email: hagiwark@saitama-med.ac.jp.
Minoru Kanazawa, Email: mkanazawa@saitama-med.ac.jp.
Makoto Nagata, Email: favre4mn@saitama-med.ac.jp.
Acknowledgements
We would like to thank Akemi Yokote for excellent technical assistance. This study was financially supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labor and Welfare of Japan.
References
- Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- Weller PF. Human eosinophils. J Allergy Clin Immunol. 2000;100:283–287. doi: 10.1016/s0091-6749(97)70237-9. [DOI] [PubMed] [Google Scholar]
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005;116:267–273. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, Cunningham A, Robinson BS, Myint SH, Ward ME, Tyrrell DA, Holgate ST. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds. Inflammatory indices induced sputum; a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- Fraenkel DJ, Bardin PG, Sanderson G, Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- Grünberg K, Smits HH, Timmers MC, de Klerk EP, Dolhain RJ, Dick EC, Hiemstra PS, Sterk PJ. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Schleimer RP. The role of adhesion molecules in human eosinophil and basophil recruitment. J Allergy Clin Immunol. 1994;94:427–438. doi: 10.1016/0091-6749(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- Mori M, Takaku Y, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. Eosinophil superoxide anion generation induced by adhesion molecules and leukotriene D4. Int Arch Allergy Immunol. 2009;149(Suppl 1):31–38. doi: 10.1159/000210651. [DOI] [PubMed] [Google Scholar]
- Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, Gould SL, Springer MS, DeMartino JA. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288–18291. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, Hansen JB, Dissing S, Malling HJ, Skov PS, Poulsen LK. CXCR3 expression and activation of eosinophils: role of IFN-γ-inducible protein-10 and monokine induced by IFN-γ. J Immunol. 2000;165:1548–1556. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- Korpi-Steiner NL, Valkenaar SM, Bates ME, Evans MD, Gern JE, Bertics PJ. Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection. Clin Exp Allergy. 2010;40:1203–1213. doi: 10.1111/j.1365-2222.2010.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, Zummo G, Holgate ST, Attia J, Thakkinstian A, Davies DE. IFN-γ-induced protein 10 is novel biomarker of rhinovirus induced asthma exacerbation. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Nagata M, Saito K, Tsuchiya K, Sakamoto Y. Leukotriene D4 upregulates eosinophil adhesion via the cysteinyl leukotriene 1 receptor. J Allergy Clin Immunol. 2002;109:676–680. doi: 10.1067/mai.2002.122841. [DOI] [PubMed] [Google Scholar]
- Saito K, Nagata M, Kikuchi I, Sakamoto Y. Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via β2 integrin. Ann Allergy Asthma Immunol. 2004;93:594–600. doi: 10.1016/S1081-1206(10)61269-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Nagata M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol. 2006;34:760–765. doi: 10.1165/rcmb.2005-0303OC. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Takaku Y, Yokote A, Miyazawa H, Soma T, Hagiwara K, Kanazawa M, Nagata M. Interferon-β augments eosinophil adhesion inducing activity of endothelial cells. Eur Respir J. 2008;32:1540–1547. doi: 10.1183/09031936.00059507. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184:6350–6358. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, Dufour JH, Luster AD. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, Linn MJ, McHenry CL, Gosangi B, Bentley JK, Tsai WC, Sajjan US, Lukacs NW, Hershenson MB. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol. 2010;185:2525–2535. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule1 (ICAM-1) via increased NF-kβ-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- Grünberg K, Sharon RF, Hiltermann TJ, Brahim JJ, Dick EC, Sterk PJ, Van Krieken JH. Experimental rhinovirus 16 infection increases intercellular adhesion molecule-1 expression in bronchial epithelium of asthmatics regardless of inhaled steroid treatment. Clin Exp Allergy. 2000;30:1015–1023. doi: 10.1046/j.1365-2222.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering H, Götze O, Kimmig D, Smolarski R, Kapp A, Elsner J. The biologic role of interleukin-8: functional analysis and expression of CXCR1 and CXCR2 on human eosinophils. Blood. 1999;93:694–702. [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Fujisawa T, Ohta K, Yamamoto K, Morita Y, Hirai K. Expression of CXCR4 in eosinophils: functional analyses and cytokine-mediated regulation. J Immunol. 2000;164:5935–5943. doi: 10.4049/jimmunol.164.11.5935. [DOI] [PubMed] [Google Scholar]
- Teran LM. Chemokines and IL-5: major players of eosinophil recruitment in asthma. Clin Exp Allergy. 1999;29:287–290. doi: 10.1046/j.1365-2222.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/S0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- Katoh S, Fukushima K, Matsumoto N, Ehara N, Matsumoto K, Yamauchi A, Hirashima M. Accumulation of CXCR3-Expressing Eosinophils and increased concentration of its ligands (IP-10 and Mig) in bronchoalveolar lavage fluid of patients with chronic eosinophilic pneumonia. Int Arch Allergy Immunol. 2005;137:229–235. doi: 10.1159/000086335. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: Decreased CCR3 and increased CXCR4 expression by lung eosinophils. J Allergy Clin Immunol. 2001;108:563–569. doi: 10.1067/mai.2001.118292. [DOI] [PubMed] [Google Scholar]
- Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- Fulkerson PC, Zimmermann N, Brandt EB, Muntel EE, Doepker MP, Kavanaugh JL, Mishra A, Witte DP, Zhang H, Farber JM, Yang M, Foster PS, Rothenberg ME. Negative regulation of eosinophil recruitment to the lung by the chemokine monokine induced by IFN-gamma (Mig, CXCL9) Proc Natl Acad Sci USA. 2004;101:1987–1992. doi: 10.1073/pnas.0308544100. [DOI] [PMC free article] [PubMed] [Google Scholar]