Abstract
Streptococcus pyogenes with null mutations in the csrRS regulatory locus are highly virulent in mice due to derepression of hyaluronic acid capsule synthesis and exotoxins, e.g., streptolysin S (SLS) and pyrogenic exotoxin B (SpeB). We generated derivatives of a ΔcsrRS strain that also carry deletions in hasAB (leading to an acapsular phenotype) or in sagA (phenotypically SLS−) or an interruption of speB (SpeB−) to test the relative contributions of these factors to the development of necrotic skin lesions. Inoculation of 2 × 106 to 4 × 106 CFU of either acapsular or SLS− strains into hairless mice resulted in lesions ∼70% smaller than those of the ΔcsrRS parent strain. Elimination of SLS also reduced lethality from 100% to 0% at this inoculum (P < 10−7; Fisher exact test). In contrast, SLS+ SpeB− mutants yielded lesions that were only 41% smaller than the parent strain (t = 2.2; P = 0.04), but only 3 the 17 lesions had dermal sloughing (P = 10−5). The nonulcerative lesions associated with SpeB− strains appeared pale with surrounding erythema. We conclude that capsule and SLS contribute to the subcutaneous spread of S. pyogenes and to a fatal outcome of infection. SpeB facilitates early dermal ulceration but has minor influence on lesion size and mortality. Large ulcerative lesions are observed only when both toxins are present.
Enhanced virulence of Streptococcus pyogenes csrR mutants is associated with increased expression of the hyaluronic acid capsule and several exotoxins, most notably streptolysin S (SLS) and pyrogenic exotoxin B (SpeB, or cysteine proteinase) (12). In the hairless mouse model of streptococcal skin infection, csrR mutants produce rapidly expanding, necrotic lesions, whereas the CsrR+ wild-type M1 strain from which they are derived induces only small abscesses or self-limited erythema.
There is little disagreement about the importance of the hyaluronic acid capsule in streptococcal pathogenesis; however, significant controversy exists concerning the importance of many of the other CsrRS-regulated gene products in virulence. Several research groups have characterized SLS as a key factor contributing to virulence (4, 17, 28). In contrast, discrepancies have been reported concerning the importance of cysteine proteinase in studies with speB mutants (2, 3, 21, 24, 32, 34). We hypothesized that the incremental pathogenicity associated with the mutations in csrR depends on the expression of these regulated virulence factors. Accordingly, we characterized here the impact of these toxins on murine infection by deleting them individually or in combination in S. pyogenes strains lacking CsrR. We show an additive effect of hyaluronic acid capsule, SLS, and SpeB on mouse virulence, and we observe that these factors contribute in different ways to the character, extent, and lethality of the skin lesions.
MATERIALS AND METHODS
All experiments used derivatives of a type M1 isolate, MGAS166 (27). Streptococci were stored at −70°C and passaged minimally before and after genetic manipulations. Todd-Hewitt broth (Difco, Detroit, Mich.) supplemented with 0.2% yeast extract (THYB) was used for liquid cultures and Todd-Hewitt yeast agar plates (THYA) was used for colony isolation.
Site-directed mutations were constructed by cloning a fragment of the target gene with the desired deletion into the temperature-sensitive plasmid pJRS233, electroporating streptococcal strains, and plating for erythromycin resistance at 30°C (29). Transformants were picked and passaged at 37°C to select for plasmid integration. Strains that became erythromycin sensitive with nonselective passage were analyzed by PCR and DNA sequencing of the target region to confirm that the intended allelic exchange had occurred. Table 1 includes a list of strains used in the present study with abbreviated designations used in other tables and figures.
TABLE 1.
Streptococcal strains used in this study
| Strain | Descriptiona | Abbreviation | Source or reference |
|---|---|---|---|
| MGAS166 | M1 clinical isolate with spontaneous Strr | 27 | |
| UMAA2392 | MGAS166 ΔcsrRS (441-bp in-frame deletion of csrR with a spontaneous mutation of the CsrS initiation codon from ATG to ACG) | RS | 12 |
| UMAA2526 | ΔcsrRS ΔhasAB | 12 | |
| UMAA2630 | MGAS166 ΔhasAB | This study | |
| UMAA2820 | MGAS166 ΔsagA | This study | |
| UMAA2840 | ΔcsrRS ΔsagA | RSA | This study |
| UMAA3268 | ΔcsrRS speB::Ωkm-2 | RSB | This study |
| UMAA3136 | ΔcsrRS ΔsagA, speB::Ωkm-2 | RSAB | This study |
| UMAA2966 | ΔcsrRS ΔhasAB, ΔsagA | This study |
Strr, streptomycin resistance.
The deletion in hasAB results in an in-frame fusion from the reading frame of HasA to HasB. This deletion removes the 66 C-terminal HasA residues and 304 residues from the N terminus of HasB. The plasmid carrying this deletion was constructed by using methods and primers that were previously described (12). The deletion in sagA eliminates 60 bp from the reading frame that encodes GGCCCCCTTCCFSIATGSGN, the cysteine-rich region of the peptide just downstream of the putative signal sequence cleavage site. The deleted base pairs were replaced with 15 nucleotides encoding five random amino acids. The shuttle plasmid carrying this mutant allele was constructed by using PCR with overlapping extension (15). Two sets of primers were used: (i) upstream-forward (5′-CAGGTAGGGATCAAGCGAGCAG-3′) and upstream-reverse (5′-GGAGCAACTTGAGTTGTTTCAGC-3′) and (ii) downstream-forward (5′-TTCTCAAGGTGGGTAGCGGAAGTT-3′) and downstream-reverse (5′-GCAACGGCGGAATCTGTAAA-3′).
ΔhasAB mutants produced matte colonies and had minimal cell-associated uronic acid detected by using the Stains-All method (26). Similarly, ΔsagA mutants showed no detectable cholesterol-resistant hemolysin activity when aliquots of broth culture were tested in exponential and stationary phases (1). Cysteine proteinase-deficient mutants were generated by exchange of the wild-type speB gene with a speB fragment inactivated by insertion of an ΩKm-2 interposon (kindly provided by Ulf Sjobring, Lund, Sweden) (34). The resulting speB mutant strains are kanamycin resistant.
For animal experiments, all strains were grown to mid-exponential phase (ABS600 = 0.6) and 2 × 106 to 4 × 106 CFU was administered in a 100-μl volume of THYB subcutaneously into the flanks of hairless 4-week-old male crl:SKH1(hrhr) Br mice (Charles River, Wilmington, Mass.). Total lesions (induration, abscess, ulcer, and erythema) and any regions of necrosis were measured daily, and the lesion area was calculated as follows: area = (L × W)π/4, where L is the long diameter and W is the short diameter of the lesion. Large irregular lesions were traced on paper to determine more accurately the lesion area. Mice that were immobilized by large lesions were sacrificed and considered to have had lethal outcomes.
RESULTS
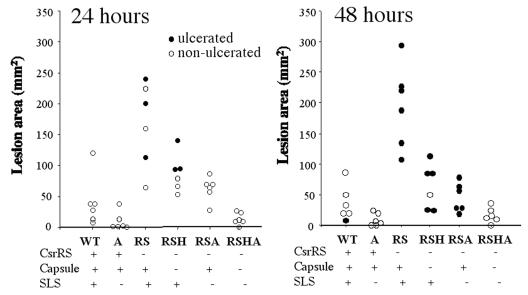
To determine the relative contribution of hyaluronic acid capsule and SLS to the virulence in our skin infection model, we injected groups of mice with ∼106 CFU of wild-type and various mutant strains. The mean area and appearance of the resulting lesions at 24 and 48 h are depicted in Fig. 1. Note that lesions produced by the wild-type bacteria (MGAS166) were relatively small (∼50 mm2) with minimal ulceration. Deletion of sagA in this background produced a notable decreased in the size of the lesions and no areas of ulceration at 48 h (P = 0.057; Student t test). A similar but more significant effect was observed comparing a more virulent ΔcsrRS mutant and its ΔsagA derivative (P = 0.0005), since the amount of ulceration in the ΔcsrRS mutant is consistently greater than in the CsrR+ parental strain. Deletion of hasAB produced a similar reduction in the size and area of necrosis of the lesions (P = 0.002). Moreover, although all six animals inoculated with the CsrR− parental strain died at 72 h, none of the animals inoculated with either SLS− or capsule-negative derivatives died by 72 h.
FIG. 1.
Areas of skin lesions and dermal necrosis in mice at 24 and 48 h after subcutaneous inoculation with 106 CFU of a type M1 S. pyogenes strain or derivative mutants with deletions in csrRS, sagA, or hasAB. Strains tested are designated by letter codes indicating which mutated genes they possess (i.e., RS, ΔcsrRS, A, ΔsagA; H, ΔhasAB).
The deletion of both sagA and hasAB resulted a strain (RSHA) that was less virulent than either individual deletion strain (P = 0.01 versus RSH; P = 0.02 versus RSA). At 72 h, the lesions induced by the ΔsagA, ΔhasAB mutant were similar in size to those induced by the CsrRS+ parent strain. These observations suggest that neither the capsule nor SLS alone accounts for the incremental virulence of the ΔcsrRS mutant, and both factors are required to induce the large, ulcerating lesions characteristic of progressive necrotizing skin infection and a lethal outcome.
To understand the contribution of SpeB to the progress of skin infection, we tested a series of ΔcsrRS mutants that also have mutations in sagA or speB or both. Table 2 and Fig. 2 summarize the results of three separate experiments that have been combined for clarity. In all three experiments, infection with the ΔcsrRS mutant was uniformly lethal, but none of the 17 animals infected with the SLS− strain succumbed to infection (Table 2). Mortality was reduced but not eliminated after infection with the SpeB− mutant. To understand better how SLS and SpeB interact together in vivo, we induced murine infections by using inocula of comparable numbers of bacteria but with reduced numbers capable of synthesizing one or both of these factors. For example, in the inoculum containing RSA+RSB, the total inoculum size (in CFU) was the same as that used to test single mutants. However, only half of the bacteria in these inocula produce SLS, whereas the other half produces only SpeB. This approach allowed us to test quantitative and complementary effects of the two toxins. We found that lethality of the RSB+RSA combination was 58%, significantly less than after infection with the ΔcsrRS parent strain and significantly greater than after infection with the SLS− mutant alone (P = 0.0002; Table 2). The combination was not significantly more lethal than the SpeB− strain administered alone (P = 0.11), but there was a trend in that direction. Since only half of the bacteria in this combined inoculum produce each toxin, we also compared RSB+RSA combination with mixed inocula that completely lack one of the two toxins and have only half of the bacteria producing the other, i.e., RSB+RSAB and RSA+RSAB. Although the numbers are small, neither of these groups experienced significant mortality relative to RSB+RSA, suggesting that even though SLS is most correlated with lethality, the effects of the two toxins are at least additive with respect to a fatal outcome of infection. In addition, SLS appears to be required for lethality in this model since none of the 27 animals that received streptococcal inocula devoid of SLS died (i.e., in groups receiving RSA, RSA+RSAB, and RSAB). We conclude that the presence of SLS is required for a fatal outcome of infection in this model; the presence of SpeB may enhance this lethality, but it is not sufficient to produce this outcome at the inocula used.
TABLE 2.
Lethality of murine skin infection by various ΔcsrRS GAS strains or strain combinations
| Strain | Toxin phenotype
|
n | No. dead at 48 h (%) |
P (Fisher exact test)
|
||
|---|---|---|---|---|---|---|
| SLS | SpeB | vs strain RS | vs RSA+RSB | |||
| RS | + | + | 12 | 12 (100) | 0.012 | |
| RSA | − | + | 17 | 0 | <10−9 | 0.0002 |
| RSB | + | − | 17 | 5 (29) | 0.0003 | 0.11 |
| RSA+RSB | 1/2 | 1/2 | 19 | 11 (58) | 0.012 | |
| RSA+RSAB | − | 1/2 | 6 | 0 | 0.00005 | 0.02 |
| RSB+RSAB | 1/2 | − | 6 | 1 (17) | 0.0007 | 0.16 |
| RSAB | − | − | 4 | 0 | 0.0006 | 0.09 |
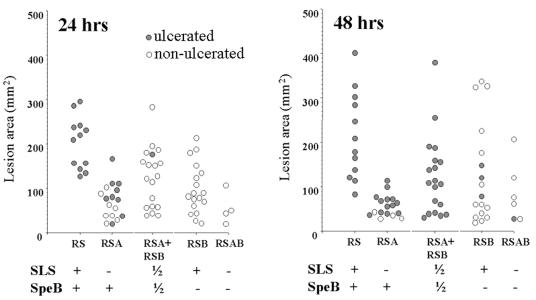
FIG. 2.
Areas of skin lesions resulting from subcutaneous inoculation of a S. pyogenes csrRS mutant or derivatives carrying additional mutations in either speB or sagA or both. Strains tested are designated by letter codes indicating which mutated genes they possess (i.e., RS, ΔcsrRS; A, ΔsagA; B, speB::ΩKm-2).
The extent and character of the lesions was also differentially affected by the presence of these two toxins (Fig. 2). The ΔcsrRS mutant (abbreviated RS) yielded lesions averaging 214 ± 99 mm2 in size 48 h after inoculation. Elimination of either exotoxin resulted in a reduction of the mean lesion areas. Those induced by the SpeB− strain (RSB) were modestly smaller (mean = 127 ± 112 mm2 [P = 0.04, Student t test]). However, those induced by the SLS− strain (RSA) were dramatically smaller (mean = 57 ± 24 mm2; P = 8 × 10−7). In fact, the mean size of the lesions induced by RSA was not significantly different than that of the lesions induced by a strain lacking both toxins (RSAB; mean = 49 ± 36 mm2). The combination of RSB+RSA produced lesions that are comparable in size to those induced by the SpeB− mutant (mean areas, 124 ± 88 mm2 versus 127 ± 112 mm2, respectively). These findings show that the total area of the skin lesion depends mostly on the presence of SLS and to a much lesser degree on the presence of SpeB.
A distinctive difference between the SLS− and SpeB− mutants was also noted with respect to the dermonecrosis and appearance of the lesions (Fig. 2). All of the mice injected with the ΔcsrRS mutant developed dermonecrosis with sloughing of the skin and production of a deep skin ulcer by 24 h after inoculation as indicated by the filled circles in Fig. 2. In contrast, mice injected with the SpeB− mutant were significantly less likely to have any ulceration of the lesions, even at 48 h after inoculation (18% ulcerated; P = 0.00001 [Fisher exact test]). The absence of SLS had much less impact on the tendency of the lesions to ulcerate. Seventy-seven percent of animals injected with the SLS− strain had ulcerative lesions (P = 0.12 compared to the ΔcsrRS parent strain).
We also observed that all mice receiving the RSB+RSA combination developed ulcerated lesions by 48 h. Unlike the group that received the ΔcsrRS strain alone, these animals did not have ulcerated lesions by 24 h, suggesting that the quantity of SpeB produced in the lesion influences the progress of the lesion toward skin breakdown. After a different combined inoculum in which half of the bacteria produced SpeB but none produced SLS (i.e., RSA+RSAB), only half of the animals developed ulceration at 48 h (P = 0.009, compared to the RSA+RSB group). This finding suggests that ulceration associated with SpeB is facilitated by the presence of SLS. However, since 24 of the 27 mice that received only SpeB− bacteria in these experiments (groups RSB, RSB+RSAB, and RSAB) failed to ulcerate, we conclude that SpeB is the key element contributing to dermal sloughing in this model. SLS may contribute modestly but is not required for this outcome.
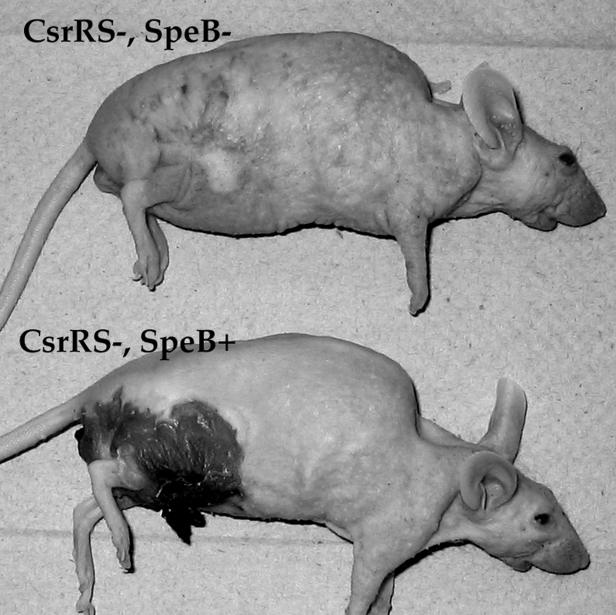
The lack of ulceration in the absence of SpeB meant that the lesions in animals inoculated with these mutants had a distinctive appearance. Instead of a large open wound, infections with SpeB− mutants produce an expanding area of pallor surrounded by a ring of erythema (Fig. 3). Histologic preparations of these lesions (not shown here) suggest that both SpeB+ and SpeB− bacteria grow and spread in the subdermal area of the skin. The epidermal sloughing that occurs with SpeB+ strains is not accompanied by the contiguous spread of large numbers of bacteria to the skin surface and through the open ulcers. In infection with SpeB− strains, bacteria also remain in the subdermal area, but the epidermis remains intact. These findings suggest that the epidermal sloughing associated with SpeB+ strains likely results from the action of a diffusible toxin, either SpeB itself or a another toxin that must be activated by the proteolytic activity of SpeB.
FIG. 3.
Appearance of murine skin lesions at 48 h after inoculation with a csrRS mutant or a csrRS speB::ΩKm-2 derivative.
DISCUSSION
We investigated the relative contributions of three important and well-described virulence factors regulated by CsrRS to the production of murine skin infection. We found that hyaluronic acid and SLS have additive effects both with respect to the size of lesions and the tendency of the lesions to progress to dermonecrosis. Deletion of both factors by site-directed mutagenesis results in greater attenuation of virulence than deletion of either factor independently. This relationship suggests that the contributions of these two factors to the progress of skin infection are distinct and do not overlap. By infecting animals with mutants lacking SLS and SpeB (cysteine proteinase), we also established that these two exotoxins have different roles in skin infection. Whereas SLS facilitates the spread of the infection subcutaneously and is required for a lethal outcome of infection in this model, SpeB contributes significantly less to these particular parameters but appears to be essential for the breakdown of the epidermis and ulceration over the infected tissues.
In contemplating the significance of these findings, it is important to recognize that they were made in the context of a particular model of streptococcal virulence, i.e., subcutaneous infection with a CsrR− GAS mutant in the hairless mouse. The use of a CsrR mutant in our virulence model permits us to induce lethal lesions in mice by using subcutaneous inocula in the ∼106 CFU range. Superficially, this system may seem artificial; however, we and others have learned that spontaneous CsrRS mutants emerge spontaneously when larger inocula of wild-type bacteria are used to induce murine skin infections (8, 30). This observation suggests that altered expression of CsrRS-regulated genes in vivo provides a selective advantage in mice. Indeed, we have observed evidence of synergy between CsrR− mutants and wild-type bacteria within the same subcutaneous lesion, producing larger and more lethal skin infections (8). Although CsrR− and CsrS− mutants also occur in patients with streptococcal infection, the frequency of isolation is significantly less than in mice (16). This may reflect a sampling bias, or more likely, it may imply that mice are more likely to enrich for spontaneous mutants either because they are less sensitive to certain CsrRS-regulated virulence factors or because they lack the signals for depression of genes controlled by CsrRS. In either case, inoculation of the ΔcsrRS mutant in place of the wild-type parent strain produces a productive infection with levels of virulence gene expression that generate fatal necrotizing skin infection.
Our work shows that this virulence is not attributable to any single factor. Early studies suggested that several streptococcal exoproteins were controlled by CsrRS (9, 12, 13, 23). More recent microarray data show that the list of regulated proteins is very large and likely includes several factors with a necessary role in dermonecrotic infection (11). To investigate the roles of these factors, we think it reasonable to use an animal model infection system in which they are produced in sufficient abundance in vivo to have demonstrable effects.
The hairless (hr/hr) mice used in these studies were selected for these studies because their baldness facilitates the measurement and characterization of wounds. Animals with this genotype also possess a quantitative deficiency of T lymphocytes; specifically, the ratio between Ly1 and Ly123 lymphocytes is reversed in the spleens of 3- to 3.5-month-old mice, without affecting the total lymphocytes counts (31). These animals have a defect in the proliferative response to alloantigens but no defect in mitogenic responses or lymphocyte cytotoxicity (25). Based on the age of the mice used in our study, their GAS-naive status, and the rapidity of skin lesion development, it seems unlikely that this quantitative defect influences the course of infection in our model.
Given these caveats concerning the model system, what conclusions can we draw from the current findings? The finding that hyaluronic acid capsule and SLS have additive effects on virulence is consistent with the distinct functions of these virulence factors. The capsule is important as an antiphagocytic factor and as an adherence factor that binds streptococci to cellular CD44 (6, 7). In contrast, SLS is a potent surface expressed cytotoxin that lyses host cells, including polymorphonuclear leukocytes, after insertion into their membrane. Based on the additive effect of these factors on virulence, one assumes that both functions are required for full expression of virulence.
With respect to the exotoxins, it has been suggested that several streptococcal proteins may synergize with one another and with host-derived substances to create tissue damage (10). Comparing the relative contributions of SLS and SpeB to lesion development, we found some evidence for potential synergy in that the presence of the two led to lesions that were larger and more necrotic than those produced by strains expressing only one or the other toxin. However, each toxin also has distinct effects that are clearly independent of the other; SLS promotes subcutaneous spread of the infection, and SpeB mediates the disruption of epidermis overlying the areas of subdermal infection. SpeB has a variety of mammalian substrates for its proteolytic activity, including interleukin-1β precursor, vitronectin, fibronectin, laminin, urokinase plasminogen receptor, and kininogens (14, 18, 20, 36). In addition, this molecule induces mast cell degranulation (35) and activates the 66-kDa human endothelial cell matrix metalloprotease and has therefore been associated with endothelial cell damage (5). The potential degradation of a large variety of streptococcal exoproteins and M protein by SpeB may also influence virulence (19, 22). However, studies of SpeB mutants from different laboratories have resulted in divergent conclusions regarding the role of this factor in pathogenesis (2, 3, 21, 24, 32, 34). Most relevant to our findings was the observation of Svennsson et al. that SpeB was associated with detachment of granular keratinocytes and release of the stratum corneum from underlying epidermis in explants of human foreskin to SCID mice (hu-skin-SCID) (33, 34). These authors hypothesize that SpeB may function as tryptic enzymes do in the degradation of intercellular desmosomes. If this hypothesis is correct, then the ulceration of lesions by SpeB+ strains may reflect this activity arising from the subdermal infection, and the spreading pallor/erythema seen with SpeB− and CsrS− strains may reflect the inability of other streptococcal exoproteins to substitute for this function.
Editor: D. L. Burns
REFERENCES
- 1.Alouf, J. E., and C. Loridan. 1988. Production, purification, and assay of streptolysin S. Methods Enzymol. 165:59-64. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh, C. D., H. B. Warren, V. J. Carey, and M. R. Wessels. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 102:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashbaugh, C. D., and M. R. Wessels. 2001. Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group A streptococcal infection. Infect. Immun. 69:6683-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betschel, S. D., S. M. Borgia, N. L. Barg, D. E. Low, and J. C. De Azavedo. 1998. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns, E. H., Jr., A. M. Marceil, and J. M. Musser. 1996. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect. Immun. 64:4744-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cywes, C., I. Stamenkovic, and M. R. Wessels. 2000. CD44 as a receptor for colonization of the pharynx by group A streptococcus. J. Clin. Investig. 106:995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cywes, C., and M. R. Wessels. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648-652. [DOI] [PubMed] [Google Scholar]
- 8.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043-1054. [DOI] [PubMed] [Google Scholar]
- 9.Federle, M., K. McIver, and J. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 181:3649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsburg, I., P. Ward, and J. Varani. 1999. Can we learn from the pathogenetic strategies of group A hemolytic streptococci how tissues are injured and organs fail in post-infectious and inflammatory sequelae? FEMS Immunol. Med. Microbiol. 25:325-338. [DOI] [PubMed] [Google Scholar]
- 11.Graham, M. R., L. M. Smoot, C. A. Lux Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A streptococci by a two-compenent gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 99:13855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR/CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath, A., A. Miller, V. J. DiRita, and C. N. Engleberg. 2001. Identification of a major, CsrRS-regulated secreted protein of group A streptococcus. Microb. Pathog. 31:81-89. [DOI] [PubMed] [Google Scholar]
- 14.Herwald, H., M. Collin, W. Muller-Esterl, and L. Bjorck. 1996. Streptococcal cysteine proteinase releases kinins: a virulence mechanism. J. Exp. Med. 184:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 16.Hoe, N. P., J. Vuopio-Varkila, M. Vaara, D. Grigsby, D. De Lorenzo, Y. X. Fu, S. J. Dou, X. Pan, K. Nakashima, and J. M. Musser. 2001. Distribution of streptococcal inhibitor of complement variants in pharyngitis and invasive isolates in an epidemic of serotype M1 group A streptococcus infection. J. Infect. Dis. 183:633-639. [DOI] [PubMed] [Google Scholar]
- 17.Humar, D., V. Datta, D. J. Bast, B. Beall, J. C. De Azavedo, and V. Nizet. 2002. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet 359:124-129. [DOI] [PubMed] [Google Scholar]
- 18.Hytonen, J., S. Haataja, D. Gerlach, A. Podbielski, and J. Finne. 2001. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 39:512-519. [DOI] [PubMed] [Google Scholar]
- 19.Kansal, R. G., M. A., D. E. Low, A. Norrby-Teglund, and M. Kotb. 2000. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 68:6362-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapur, V., S. Topouzis, M. W. Majesky, L. L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 21.Kuo, C. F., J. J. Wu, K. Y. Lin, P. J. Tsai, S. C. Lee, Y. T. Jin, H. Y. Lei, and Y. S. Lin. 1998. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei, B., S. Mackie, S. Lukomski, and J. M. Musser. 2000. Identification and immunogenicity of group A streptococcus culture supernatant proteins. Infect. Immun. 68:6807-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol. Microbiol. 30:209-219. [DOI] [PubMed] [Google Scholar]
- 24.Lukomski, S., S. Sreevatsan, C. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrissey, P. J., D. R. Parkinson, R. S. Schwartz, and S. D. Waksal. 1980. Immunologic abnormalities in HRS/J. mice. I. Specific deficit in T lymphocyte helper function in a mutant mouse. J. Immunol. 125:1558-1562. [PubMed] [Google Scholar]
- 26.Moses, A. E., M. R. Wessels, K. Zalcman, S. Alberti, S. Natanson-Yaron, T. Menes, and E. Hanski. 1997. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A streptococcus. Infect. Immun. 65:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musser, J., S. Kanjilal, U. Shah, D. Musher, N. Barg, K. Nelson, R. K. Selander, K. Johnson, P. Schlievert, J. Henrichsen, D. Gerlach, R. Rakita, A. Tanna, B. Cookson, and J. Huang. 1993. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (scarlet fever toxin). J. Infect. Dis. 167:337-346. [DOI] [PubMed] [Google Scholar]
- 28.Nizet, V., B. Beall, D. J. Bast, V. Datta, L. Kilburn, D. E. Low, and J. C. De Azavedo. 2000. Genetic locus for streptolysin S production by group A streptococcus. Infect. Immun. 68:4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Casal, J., J. A. Price, E. Maguin, and J. R. Scott. 1993. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol. Microbiol. 8:809-819. [DOI] [PubMed] [Google Scholar]
- 30.Ravins, M., J. Jaffe, E. Hanski, I. Shetzigovski, S. Natanson-Yaron, and A. E. Moses. 2000. Characterization of a mouse-passaged, highly encapsulated variant of group A streptococcus in in vitro and in vivo studies. J. Infect. Dis. 182:1702-1711. [DOI] [PubMed] [Google Scholar]
- 31.Reske-Kunz, A. B., M. P. Scheid, and E. A. Boyse. 1979. Disproportion in T-cell subpopulations in immunodeficient mutant hr/hr mice. J. Exp. Med. 149:228-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saouda, M., W. Wu, P. Conran, and M. D. Boyle. 2001. Streptococcal pyrogenic exotoxin B enhances tissue damage initiated by other Streptococcus pyogenes products. J. Infect. Dis. 184:723-731. [DOI] [PubMed] [Google Scholar]
- 33.Scaramuzzino, D. A., J. M. McNiff, and D. E. Bessen. 2000. Humanized in vivo model for streptococcal impetigo. Infect. Immun. 68:2880-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson, M. D., D. A. Scaramuzzino, U. Sjobring, A. Olsen, C. Frank, and D. E. Bessen. 2000. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol. Microbiol. 38:242-253. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, Y., Y. Todome, H. Ohkuni, S. Sakurada, T. Ishikawa, T. Yutsudo, V. A. Fischetti, and J. B. Zabriskie. 2002. Cysteine protease activity and histamine release from the human mast cell line HMC-1 stimulated by recombinant streptococcal pyrogenic exotoxin B/streptococcal cysteine protease. Infect. Immun. 70:3944-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf, B. B., C. A. Gibson, V. Kapur, M. Hussaini, J. M. Musser, and S. L. Gonias. 1994. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J. Biol. Chem. 269:30682-30687. [PubMed] [Google Scholar]