Abstract
A nonpathogenic mutant of Colletotrichum magna (path-1) was previously shown to protect watermelon (Citrullus lanatus) and cucumber (Cucumis sativus) seedlings from anthracnose disease elicited by wild-type C. magna. Disease protection was observed in stems of path-1-colonized cucurbits but not in cotyledons, indicating that path-1 conferred tissue-specific and/or localized protection. Plant biochemical indicators of a localized and systemic (peroxidase, phenylalanine ammonia-lyase, lignin, and salicylic acid) “plant-defense” response were investigated in anthracnose-resistant and -susceptible cultivars of cucurbit seedlings exposed to four treatments: (1) water (control), (2) path-1 conidia, (3) wild-type conidia, and (4) challenge conditions (inoculation into path-1 conidia for 48 h and then exposure to wild-type conidia). Collectively, these analyses indicated that disease protection in path-1-colonized plants was correlated with the ability of these plants to mount a defense response more rapidly and to equal or greater levels than plants exposed to wild-type C. magna alone. Watermelon plants colonized with path-1 were also protected against disease caused by Colletotrichum orbiculare and Fusarium oxysporum. A model based on the kinetics of plant-defense activation is presented to explain the mechanism of path-1-conferred disease protection.
The mechanism by which fungi from the genus Colletotrichum cause disease (anthracnose) is governed by a series of events that begins with the adhesion of fungal spores to host surface tissue, followed by spore germination, appressoria formation, and penetration into the first subcuticular cell (Bailey et al., 1992). If a compatible interaction ensues, the pathogen will exhibit necrotrophic characteristics and quickly disseminate through host tissues, ultimately resulting in necrotic lesions and, hence, plant disease. In an incompatible interaction, a rapid, localized collapse of tissue surrounding the initial infection zone occurs, resulting in disease resistance. The resistant reaction, designated the hypersensitive response, is believed to be genetically programmed and confers disease resistance due to the recognition and interaction of biochemical components from both the pathogen and host (Flor, 1971; Keen, 1982, 1986, 1990; Klement, 1982; Kuc, 1990; Alfano and Collmer, 1996; Hammond-Kosack and Jones, 1996; Jackson and Taylor, 1996; Knogge, 1996). In compatible interactions, widespread plant cell death may occur because of the delayed response of the plant to the presence of the pathogen and the ability of the pathogen to overcome the plant host-response system (Darvill and Albersheim, 1984; Davis et al., 1986; Ebel, 1986; Bailey et al., 1992; Jackson and Taylor, 1996).
Susceptibility or resistance to disease by Colletotrichum sp. or other plant pathogens appears to follow a common theme involving the temporal and spatial expression of plant-defense components activated by a number of fungal and/or plant metabolites (Anderson, 1978; DeWit et al., 1985; Ebel, 1986; Hamdan and Dixon, 1986; Kombrink and Hahlbrock, 1986; Anderson, 1988; Cuypers et al., 1988; Dixon and Lamb, 1990; Bailey et al., 1992; Nicholson and Hammerschmidt, 1992; Kamoun et al., 1993; Knogge, 1996). Kuc and Strobel (1992) implied that susceptible cultivars may be manipulated to resist pathogen attack by altering the timing and magnitude of the defense response.
An additional dimension to incompatible interactions involves the extent and spatial distribution of the plant-defense response resulting in a rapid, localized, and/or systemic form of protection. A localized response occurs around the site of pathogen ingress and protection is afforded to the plant cells in the surrounding area. Often, a small, necrotic lesion will be formed as a result of the hypersensitive response.
Key components postulated to play important roles in localized resistance include increased activity in peroxidase, deposition of lignin, and PAL activity. Peroxidase is involved in cross-linking extensin molecules and in the polymerization of hydroxycinnamyl alcohols to form lignin (Hammerschmidt et al., 1982; Irving and Kuc, 1990; Dalisay and Kuc, 1995a, 1995b). Increased lignin deposition is believed to play a role in barricading the pathogen from invading the plant through physical exclusion (Hammerschmidt and Kuc, 1982a, 1982b; Hammerschmidt et al., 1984; Nicholson and Hammerschmidt, 1992; Hammond-Kosack and Jones, 1996). PAL is responsible for the conversion of Phe to trans-cinnamic acid, a key intermediate in the pathway for production of lignin and SA. Depending on the plant species PAL may play a role in either localized resistance or SAR (Hammond-Kosack and Jones, 1996; Ryals, et al., 1996). It is believed that PAL activity is correlated with the synthesis of phenols in response to pathogen infection (Nicholson and Hammerschmidt, 1992).
Several factors are postulated to be involved in the SAR pathway, including SA, benzoic acid, and jasmonic acid. Upon conversion of Phe into trans-cinnamic acid via PAL, trans-cinnamic acid is then converted through either the oxidative or nonoxidative pathway into benzoic acid. The conversion of benzoic acid into SA is catalyzed by benzoic acid 3-hydroxylase, which is believed to be the rate-determining step in SA synthesis. SA has been recognized for some time as a response to pathogen infection and, at present, is the only plant-derived substance shown to induce SAR (Ryals et al., 1996).
Recently, a nonpathogenic fungal mutant (path-1) of Colletotrichum magna, the causal agent of anthracnose in cucurbits, was isolated (Freeman and Rodriguez, 1992). path-1 was no longer capable of eliciting disease symptoms but retained the ability to adhere, infect, and disseminate through plant tissue (Freeman and Rodriguez, 1993). Cucurbit plants colonized by the path-1 mutant showed no disease symptoms when exposed to lethal concentrations of the wild-type (L2.5) C. magna or Fusarium oxysporum f. sp. niveum (Freeman and Rodriguez, 1993). Data presented in this study indicate that a hypersensitive response did not occur in plants colonized with path-1 and that protection was localized and tissue specific. Biochemical analyses (peroxidase, PAL, SA, and lignin deposition) indicated that resistance to disease correlated with the ability of path-1-colonized plants to respond more quickly to the pathogen. We propose a working model to explain the basis of path-1 protection and how this system may be useful for biological control of fungal disease.
MATERIALS AND METHODS
Fungal Isolates and Plant Cultivars
The pathogenic wild-type isolate (L2.5) of Colletotrichum magna (Jenkins and Winstead, 1964) was obtained from S. Brown and O.C. Yoder (Cornell University, Ithaca, NY). The nonpathogenic mutant (path-1) of C. magna was isolated following UV mutagenesis of isolate L2.5 (Freeman and Rodriguez, 1992). Colletotrichum orbiculare (isolate 254) was obtained from the Colletotrichum Culture Collection (D. TeBeest, University of Arkansas, Fayetteville). Fusarium oxysporum isolate f. sp. niveum was obtained from C. Kistler (Florida State University, Tallahassee). Fungi were cultured in either liquid or solid modified Mather's medium (Tu, 1985) as previously described (Rodriguez and Owen, 1992). Anthracnose-susceptible cultivars of watermelon (Citrullus lanatus cv Sugar Baby) and cucumber (Cucumis sativus cv Marketmore) and resistant cultivars of watermelon (cv Jubilee) and cucumber (cv Pepino) were from Petoseed (Woodland, CA). All plant assays were carried out in growth chambers operated at 95% RH and 12-h light regimes at 22°C.
Experimental Treatments and Design
Plant bioassays were performed independently a minimum of three times for each cucurbit variety, treatment, and time tested. Each sampling consisted of 30 cucurbit seedlings. Similar results were obtained in each replicate. One representative replicate is presented for the bioassay and all results presented were taken from the same replicate sample.
Plant bioassays and biochemical analysis were conducted on cucurbit plants exposed to four treatments: treatment 1, water (control); treatment 2, mutant (path-1) conidia; treatment 3, wild-type (L2.5) conidia; and treatment 4, challenge conditions (inoculation into path-1 conidia for 48 h followed by exposure to lethal concentrations of a virulent wild-type pathogen). All assays were conducted with a minimum of 30 plant seedlings of equal size and age and were repeated a minimum of three times.
There were three separate challenge treatments: path-1-colonized plants exposed to 1.0 to 2.0 × 106 conidia mL−1 C. magna isolate L2.5 (challenge A), C. orbiculare isolate 254 (challenge B), and F. oxysporum isolate f. sp. niveum (challenge C). Although it is not mentioned in each instance, all studies conducted contained an additional control (designated treatment 7 in Table I). Cucurbit plants were exposed to water for 48 h and then exposed to lethal concentrations of C. magna isolate L2.5, C. orbiculare isolate 254, or F. oxysporum isolate f. sp. niveum as the treatment 7 control for challenge A, B, and C, respectively. This additional control was included to ensure that wild-type concentrations were lethal and produced 100% mortality in all susceptible cucurbit plants tested. In addition, treatment 7 was included as a control to ensure that age effects in cucurbit seedlings was not a contributing factor to the decrease in mortality observed in challenge treatments.
Table I.
Mortality and colonization in anthracnose-susceptible cucurbit cultivars
| Treatment | Root/Stem
|
Cotyledon
|
||
|---|---|---|---|---|
| Mortality | Colonization by mutant | Mortality | Colonization by mutant | |
| % | ||||
| 1 Water control | 0 | 0 | 0 | 0 |
| 2 Path-1 mutants | 0 | 100 | 0 | 0 |
| 3 L2.5 wild type | 100 | 0 | 100 | 0 |
| 4 Challenge A | 0 | 100 | 100 | 0 |
| Challenge B | 0–20 | 100 | 100 | 0 |
| Challenge C | 0–30 | 100 | 100 | 0 |
| (−) Controla | 100 | 0 | 100 | 0 |
The data presented represent the mortality (assessed at d 5) and colonization (assessed at d 3) of 30 anthracnose-susceptible watermelon (cv Sugar Baby) and cucumber (cv Marketmore) seedlings exposed to various treatments. The assays were repeated a minimum of three times. The only variations between experiments occurred with challenge conditions B and C.
(−) Control, Exposed to water for 48 h and then to challenges A, B, and C.
Inoculation Procedures
Conidial suspensions of C. magna, C. orbiculare, and F. oxysporum isolates were obtained using standard procedures (Freeman and Rodriguez, 1992). Inoculation procedures were optimized to maximize susceptible and resistant plant reactions (data not shown). Utilizing conidial concentrations of 1.0 to 2.0 × 106 conidia mL−1, cvs Sugar Baby and Marketmore were 100% susceptible to anthracnose elicited by L2.5. Conversely, cvs Jubilee and Pepino were 100% resistant to anthracnose when exposed to L2.5. Root and lower stem inoculation and incubation techniques were identical to those previously described (Freeman and Rodriguez, 1992). The cotyledons were inoculated by briefly submerging them in spore suspensions ranging from 1.0 to 2.0 × 106 conidia mL−1 followed by incubation in chambers with 95% RH.
Recovering Fungi from Plant Tissue
Colonization of plants by fungal isolates was determined by surface-sterilization of plants and outgrowth of fungi on Mather's medium and was expressed as the percentage of plants colonized. Plants were submerged in 2.0% (v/v) sodium hypochlorite for 20 to 30 min with moderate agitation and then thoroughly rinsed with 10 to 20 volumes of sterile distilled water. Plants were cut, using an aseptic technique, into sections representing the lower, middle, and upper stem sections, the roots, and the cotyledons. The sections were plated onto Mather's medium supplemented with 100 μg mL−1 ampicillin and incubated at room temperature under cool fluorescent lights for 5 to 7 d. Identification of fungi was verified after conidiation by microscopic analysis.
Fungal Inhibition Assays
In vitro inhibition assays were performed by inoculating both mycelial plugs and spore suspensions (1.0 to 3.0 × 106 spores mL−1) of path-1 and L2.5 on Mather's medium (Freeman and Rodriguez, 1992) with a separation distance of 1.5 cm. Inoculated replicate plates were incubated at 25°C in the dark and under cool fluorescent lights. Growth inhibition was assessed by the occurrence of clear zones at the interface between colonies.
To determine whether path-1 was producing growth inhibitors in vivo, approximately 100 mg from each of the 10 path-1-colonized plant stems was ground with a tissue homogenizer (Tekmar, Cincinnati, OH) and resuspended in an equivalent volume of a 0.01 m Tris-HCl buffer (pH 7.0). Growth inhibition was measured by mixing ratios of plant extract to water of 1:1 to 1:100 (v/v), spotting 50 μL on Mather's medium, and inoculating with single mycelial plugs of L2.5 adjacent to the plant extract.
Peroxidase and PAL Activities
Peroxidase and PAL assays were performed independently a minimum of three times for each cucurbit variety, treatment, and time tested. Similar trends in enzyme activities were observed between replicate samples. One representative replicate is presented for peroxidase and PAL activities and all biochemical results presented were taken from the same replicate samples.
Time-course studies during a 2-week span indicated that high levels of peroxidase and PAL activities were detected within the first 4 d (data not shown). All subsequent studies (with the exception of lignin deposition) presented in this paper were conducted during a period of 4 d.
The stems of 30 cucurbit seedlings of similar size and age were ground in liquid nitrogen, and 100 mg of ground tissue was placed into 1 mL of 0.01 m sodium phosphate buffer (pH 6.0). After the sample was centrifuged (10,000g for 5 min at 4°C), 5 to 200 μL of the supernatant was used to quantify protein and determine peroxidase and PAL activities. Protein content was quantified using BCA protein assay reagents (Pierce). Peroxidase activity was determined with 0.25% (v/v) guaiacol and 0.3% (v/v) H2O2 in 1 mL of 0.01 m sodium phosphate buffer (pH 6.0) (Hammerschmidt et al., 1982). The reaction was initiated by the addition of 5 μL of the supernatant extract to 995 μL of the reaction mixture. Activity was measured as a change in the A470 and expressed as the change in absorbance per minute per microgram of protein.
The reaction mixture for PAL activity consisted of 6 μm l-Phe, 0.5 m Tris-HCl buffer (pH 8.0), and 200 μL of plant extract. After 60 min at 37°C, the reaction was terminated by the addition of 0.05 mL of 5 n HCl. PAL activity was assessed by measuring the amount of cinnamic acid produced at 290 nm and is expressed as micrograms of cinnamic acid per microgram of protein, similar to the procedure described by Beaudoin-Eagan and Thorpe (1985).
Lignin Deposition
Lignin-deposition assays were performed independently a minimum of three times for each cucurbit variety, treatment, and time tested. A minimum of 30 cucurbit stems was assayed for each treatment and time tested. Similar trends in lignin deposition were observed between replicate samples. One representative replicate is presented for lignin deposition and all biochemical results presented were taken from the same replicate sample.
The pg-HCl test denoting lignin deposition was performed using a modification of the “phloroglucinol” method described by Gurr (1965). Watermelon seedlings were decolorized in 70% (v/v) ethanol for 24 to 48 h, washed with distilled water, and exposed to 1% (w/v) phloroglucinol (Sigma) for 1 to 2 h. The seedlings were then exposed to 6 m HCl until a red color developed, which denoted lignin deposition. The level of lignin deposition was qualitatively measured using water-inoculated plants as the negative background control and ranged from absent, basal, low, moderately high, to high.
SA Accumulation
SA assays were performed independently a minimum of three times for each cucurbit variety, treatment, and time tested. Ten cucurbit stems were assayed for each treatment and time tested. The same results were observed between replicate samples.
Stem sections of 10 cucurbit seedlings of similar size and age were ground in liquid nitrogen and resuspended in 5 mL of water. After the sample was centrifuged (5000g for 10 min at 4°C), an equal volume of acetone and chloroform was mixed with the supernatant and centrifuged (5000g for 10 min at 4°C), and the organic phase was passed through anhydrous sodium sulfate to remove any residual water. The organic phase was evaporated and resuspended in 0.5 mL of acetone, and 0.5 to 25 μL was spotted on TLC silica-gel plates (Whatman). TLC plates were chromatographed in a mixture of toluene:ethyl acetate:glacial acetic acid (80:10:10, v/v). The TLC plates were dried, sprayed with 20% (w/v) aluminum chloride in 95% (v/v) ethanol, and baked at 95°C for 10 min, and SA was visualized under long-wave UV. SA standard (1–100 ng, Sigma) was spotted onto the TLC plates as a positive control (Dr. N. Keller, personal communication).
RESULTS
Colonization and Protection of Cucurbit Roots and Stems
Previously, we demonstrated that the nonpathogenic mutant of C. magna (path-1) was capable of colonizing stem tissue and protecting watermelon seedlings from disease caused by wild-type C. magna (isolate L2.5) and by the wilt pathogen F. oxysporum f. sp. niveum (Freeman and Rodriguez, 1993). Although path-1 was capable of protecting plants against these pathogens, it was not known whether protection was afforded toward aggressive foliar pathogens. To test this, path-1-colonized plants were challenged with lethal conidial concentrations of C. orbiculare, an aggressive foliar pathogen of cucurbits. The data from several plant inoculation and challenge experiments indicated that path-1 was capable of protecting plants against virulent C. orbiculare (Table I).
To determine whether the protection to the various pathogens was systemic, the roots and stems of seedlings were colonized with path-1 for 48 h, and then the cotyledons were inoculated with lethal concentrations of C. magna L2.5 (challenge A), C. orbiculare 254 (challenge B), or F. oxysporum f. sp. niveum (challenge C; Table I). In all of these experiments, 100% plant mortality was observed. In surface-sterilization experiments path-1 was present in the root and stem sections but absent in the cotyledons of all cucurbit plants tested (Table I). These data indicated that path-1 induced a localized form of plant protection.
To determine whether path-1 protection was plant-tissue specific, cotyledons were colonized with path-1 for 48 h, and then inoculated with lethal concentrations of C. magna L2.5 (challenge A), C. orbiculare 254 (challenge B), or F. oxysporum f. sp. niveum (challenge C). Visible signs of necrosis and plant death were absent in cotyledons exposed to path-1. In contrast, 100% plant mortality was observed in cotyledons exposed to challenge A, B, and C treatments. In surface-sterilization experiments path-1 was absent in the cotyledons of all cucurbit plants tested (Table I). These data indicated that path-1 was able to colonize the roots and stems but not the cotyledons of cucurbits and, therefore, affords tissue-specific plant protection from disease.
To determine whether L2.5 was able to disseminate through host tissue colonized by path-1, genetically marked strains of L2.5 resistant to either benlate (wtB) or chlorate (wtC) were derived (Freeman and Rodriguez, 1993). The marked strains were not recovered from path-1-colonized plant stems after 1 week of exposure. However, both wtB and wtC were recovered from plants that had not been colonized with path-1. Plants inoculated with either wtB or wtC expressed pathological characteristics similar to plants inoculated with L2.5 (100% mortality, data not shown).
Fungal-Inhibition Assays
We were interested in determining whether path-1 was directly inhibiting the growth of L2.5 in vitro and/or in plant tissues. The in vitro assays involved screening for inhibition of L2.5 by path-1 after co-inoculation of these fungi on Mather's medium. Inhibition in plant tissues was determined by extracting total fluids from plants colonized with path-1 and performing liquid-inhibition assays with L2.5. Neither of these assays revealed any inhibition of L2.5 (data not shown), indicating that path-1 was not producing fungal inhibitors.
Lignin Deposition
The anthracnose-susceptible watermelon cv Sugar Baby and cucumber cv Marketmore and the anthracnose-resistant watermelon cv Jubilee and cucumber cv Pepino were assessed for lignin deposition during a 5-d period. Plants were exposed to treatments 1 through 4 (see Methods). Pg-HCl caused intense staining in plant tissue of both susceptible and resistant cultivars exposed to L2.5 (Table II). The highest levels of lignin deposition occurred in resistant cultivars exposed to L2.5 and in challenge-A treatments for susceptible cultivars. When path-1-colonized plants were challenged with L2.5 (at d 2), lignin deposition increased rapidly within a 24-h period to levels that either nearly paralleled or exceeded those of resistant and susceptible plants inoculated for 72 h with L2.5, respectively (Tables II and III). No detectable pg-HCl staining was observed in the stems of water-exposed plants and little to no staining was detected in plants exposed to path-1 in either resistant or susceptible cultivars. Although the timing of lignin deposition was similar, the kinetics of challenge-A conditions were different in resistant versus susceptible cultivars.
Table II.
Lignin deposition in cucurbits
| Treatment | 1 d | 2 d | 3 d | 4 d | 5 d |
|---|---|---|---|---|---|
| cv Sugar Baby (susceptible) | |||||
| Treatment 1 | − | − | − | − | − |
| Treatment 2 | − | − | − | + | + |
| Treatment 3 | − | + | ++ | +++ | ++++ |
| Challenge A | − | − | +++ | ++++ | +++++ |
| cv Jubilee (resistant) | |||||
| Treatment 1 | − | − | − | − | − |
| Treatment 2 | − | − | + | + | + |
| Treatment 3 | + | ++ | +++ | ++++ | +++++ |
| Challenge A | − | − | ++ | +++ | ++++ |
| cv Marketmore (susceptible) | |||||
| Treatment 1 | − | − | − | − | − |
| Treatment 2 | − | − | + | + | + |
| Treatment 3 | − | + | ++ | +++ | ++++ |
| cv Challenge A | − | − | +++ | ++++ | +++++ |
| cv Pepino (resistant) | |||||
| Treatment 1 | − | − | − | − | − |
| Treatment 2 | − | − | + | + | + |
| Treatment 3 | − | + | +++ | ++++ | +++++ |
| Challenge A | − | − | ++ | +++ | ++++ |
The data presented represent the average levels of lignin deposition from 30 pooled plant stems of equal size and age (variations between plant stems were negligible). Lignin deposition was measured on a qualitative basis and ranged from absent (−), basal (+), low (++), moderate (+++), moderately high (++++), to high (+++++) levels.
Table III.
Enzyme activities and lignin deposition after 24, 48, 72, and 96 h of exposure to C. magna wild-type L2.5 in susceptible cucurbit plant varieties
| Variety | Peroxidase Activity
|
PAL
Activity
|
Lignin Deposition
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | |
| A min−1 ug−1 protein | A min−1 protein | |||||||||||
| Sugar Baby (−)a | 2.76 | 3.46 | 4.62 | 5.34 | 2.27 | 2.90 | 2.50 | 2.6 | − | + | ++ | +++ |
| Sugar Baby (+)b | 5.77 | 6.30 | NDc | ND | 2.50 | 3.70 | ND | ND | +++ | ++++ | *d | * |
| Marketmore (−) | 0.63 | 1.31 | 1.9 | 2.1 | 0.02 | 0.25 | 0.11 | 0.17 | − | + | ++ | +++ |
| Marketmore (+) | 1.8 | 2.34 | ND | ND | 0.27 | 0.34 | ND | ND | +++ | ++++ | * | * |
Peroxidase activity, PAL activity, and lignin deposition on anthracnose-susceptible cultivars after exposure to the wild-type L2.5 isolate. The data presented were obtained from 30 cucurbit seedling stems of similar size and age and were processed as required for each assay. Peroxidase activity was monitored as a change in A470. PAL activity was monitored at 290 nm. Lignin deposition was measured on a qualitative basis and ranged from absent (−), basal (+), low (++), moderate (+++), to moderately high (++++) levels.
–, Not colonized with the path-1 mutant.
+, Colonized with the path-1 mutant; path-1 colonized mutants were assessed for 48 h prior to exposure to the wild type (data not shown) and for an additional 48 h post challenge.
ND, No data.
Statistical analysis (ANOVA) revealed statistically significant differences (P < 0.05) in the treatments among time points in all assays for both cultivars, with the exception of PAL activity (P > 0.05) in the Marketmore cucumber variety.
Statistical analysis of the data using a two-factor ANOVA without replication revealed statistically significant differences among the treatments and time points in cvs Sugar Baby (treatments: F3,12 = 6.073, P = 0.009; time points: F4,12 = 4.095, P = 0.026), Marketmore (treatments: F3,12 = 5.776, P = 0.011; time points: F4,12 = 4.254, P = 0.023), Jubilee (treatments: F3,12 = 11.543, P = 0.001; time points: F4,12 = 4.826, P = 0.015), and Pepino (treatments: F3,12 = 6.738, P = 0.00; time points: F4,12 = 4.673, P = 0.017; Zar, 1984). Collectively, these data indicate that in challenge-A treatments lignin deposition increased rapidly within a 24-h period post challenge to levels that paralleled and/or exceeded those of plants inoculated with L2.5 alone (Tables II and III).
Peroxidase Activity
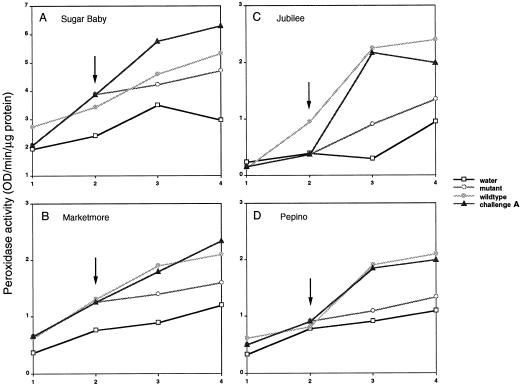
Peroxidase activity was measured in cucurbit plant stems exposed to four different treatments (see Methods). A summary of peroxidase activity in anthracnose-susceptible watermelon cv Sugar Baby and cucumber cv Marketmore and anthracnose-resistant watermelon cv Jubilee and cucumber cv Pepino during a 4-d period postinoculation is presented in Figure 1.
Figure 1.
Peroxidase activity in plant stems of anthracnose-susceptible (A and B) and anthracnose-resistant (D and C) watermelon (A and C) and cucumber (B and D) cultivars were monitored for 4 d in plants exposed to the four different treatments. The arrows on the graphs indicate the time at which path-1-colonized plants were exposed to the wild type. Each point represents 30 plants that were combined for the analysis. Peroxidase activity was monitored as a change in A470 and is reported as absorbance per minute per microgram of protein. Statistical analysis (ANOVA) revealed significant differences (P < 0.05) between the treatments among time points for all varieties. Susceptible and resistant plants in treatments 1, 2, and 4 were healthy throughout the experiment and identical in appearance. Susceptible plants in treatment 3 expressed disease symptoms on d 3 and were dead on d 5. Resistant plants did not show disease symptoms with any of the treatments.
In susceptible cultivars, a higher level of peroxidase activity was observed in plants exposed to path-1 compared with the water control plants, and the highest peroxidase activities were observed in challenge-A treatment plants. In addition, the kinetics of peroxidase activity in susceptible plants exposed to challenge-A treatments was different from plants inoculated with L2.5 alone. During the period before challenge inoculation (d 1 and 2), plants exposed to L2.5 expressed peroxidase activities that were slightly lower (Fig. 1A) or paralleled (Fig. 1B) the activities observed in path-1-exposed plants. When path-1-colonized plants were challenged with L2.5 (at d 2), peroxidase activity increased rapidly within a 24-h period to levels that paralleled and then exceeded those of plants inoculated for 72 h with L2.5 (Fig. 1; Table III).
In resistant cultivars plant mortality was not observed in any of the treatments, and the highest level of peroxidase activity occurred in plants exposed to the virulent wild type (L2.5). Peroxidase activities in mutant (path-1) exposed plants were only slightly higher than the water control plants. The activity of peroxidase in challenge-A treatment plants was slightly lower or nearly paralleled the activities observed in L2.5-exposed plants over time (Fig. 1, C and D). When path-1-colonized plants were challenged with L2.5 (at d 2), peroxidase activity increased rapidly within a 24-h period to levels that nearly paralleled but did not exceed those of plants inoculated for 72 h with L2.5 (Fig. 1). Although the timing of peroxidase expression was similar, the kinetics of challenge-A conditions were different in resistant versus susceptible cultivars.
Statistical analysis of the data using a two-factor ANOVA without replication revealed statistical differences among the treatments and among time points sampled in cvs Sugar Baby (treatments: F3,9 = 6.114, P = 0.015; time points: F3,9 = 14.933, P = 0.0010), Marketmore (treatments: F3,9 = 10.181, P = 0.003; time points: F3,9 = 26.323, P = 0.000), Jubilee (treatments: F3,9 = 3.530, P = 0.062; time points: F3,9 = 9.310, P = 0.004), and Pepino (treatments: F3,9 = 4.474, P = 0.035; time points: F3,9 = 14.943, P = 0.001; Zar, 1984). Collectively, these data indicate that the rapid accumulation of peroxidase activity within a 24-h period in anthracnose-susceptible Sugar Baby and Marketmore varieties exposed to challenge-A treatments was statistically significant.
PAL Activity
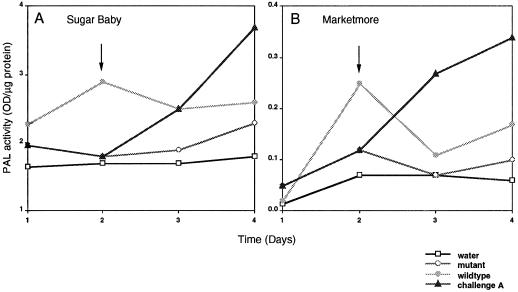
PAL activity was determined in anthracnose-susceptible watermelon cv Sugar Baby and cucumber cv Marketmore and anthracnose-resistant watermelon cv Jubilee and cucumber cv Pepino exposed to treatments 1 to 4 during a 4-d period (see Methods). Initial studies indicated that the majority of PAL activity was localized in the lower stem sections of cucurbits (data not included). Plants of similar size and age were used, and the lower halves of stems from 30 plants were pooled and processed in the same manner as described for the peroxidase activity studies. In susceptible cultivars, a trend was observed for PAL activity (Fig. 2; Table III) that was similar to that observed for peroxidase activity (Fig. 1; Table III). Prior to d 3, the highest level of PAL activity was observed as a transient response in plants exposed to L2.5, with significantly lower levels of PAL activity occurring in path-1- and water-inoculated plants. Within 24 h of exposure to the wild-type L2.5 isolate (challenge A), a rapid increase in PAL activity was noted in the path-1-colonized plant and was found to be statistically significant. PAL activity increased to levels that exceeded those of plants exposed to L2.5, alone (Fig. 2; Table III). A two-factor ANOVA without replication revealed statistically significant differences between the treatments of watermelon cv Sugar Baby (F3,9=4.536, P = 0.034) and cucumber cv Marketmore (F3,9=3.032, P = 0.086; Zar, 1984).
Figure 2.
PAL activity in lower stem sections of anthracnose-susceptible watermelon (A) and cucumber (B) cultivars were monitored for 4 d in plants exposed to the four different treatments. The arrows indicate the time at which path-1-colonized plants were exposed to the wild type. PAL activity was monitored at 290 nm and reported as the absorbance per microgram of protein. Statistical analysis (ANOVA) revealed significant differences in the treatments among time points for both cultivars (P < 0.05). Plants in treatments 1, 2, and 4 were healthy throughout the experiment and identical in appearance. Plants in treatment 3 expressed disease symptoms on d 3 and were dead on d 5.
In resistant cultivars PAL activity in plants exposed to the different treatments was not observed to be significantly different from plants exposed to the water treatment (data not shown).
Detection of SA Accumulation
SA was not detected in anthracnose-susceptible watermelon cv Sugar Baby or cucumber cv Marketmore or in anthracnose-resistant watermelon cv Jubilee or cucumber cv Pepino exposed to treatments 1 to 4 during a 5-d period post inoculation (data not shown). These results corroborated the plant colonization and protection assay results (Table I) showing that cotyledons of path-1-colonized plants were not protected from disease under challenge A conditions, indicating localized and not systemic protection.
DISCUSSION
The genetic and biochemical factors that allow Colletotrichum spp. to cause plant disease are unknown. A variety of factors (toxins, cutinases, chitinases, cellulases, and phytoalexin demethylases) have been implicated as causal components of fungal plant disease; however, the exact mechanism by which fungi cause disease is largely unknown (Ciuffetti et al., 1983; Kolattukudy, 1985; Dickman and Patil, 1986; Johal and Briggs; 1992). The path-1 mutant of C. magna provides a useful tool for investigating the basis of disease expression, plant host response, and protection against fungal disease. To understand how path-1 was able to grow through host tissue without causing necrosis and protect plants against virulent pathogens, two strategies were followed: (a) inoculation and colonization assays and (b) biochemical analysis of cellular pathways correlated with plant defense responses. In each study plants were tested with each of the four treatments.
Inoculation studies confirmed that plant death was not observed in path-1-exposed plants and 100% mortality occurred in L2.5-exposed susceptible cultivars (Table I). Furthermore, 100% of the plants exposed to path-1 were colonized in the roots and stems but not in the cotyledons. To determine whether protection from disease was systemic or localized and/or tissue specific, the cotyledons or roots and stems of path-1-colonized plants were exposed to lethal spore concentrations of C. magna, C. orbiculare, or F. oxysporum (challenge A, B, or C, respectively). The mortality in challenged cotyledons was found to be 100%, whereas root and stem challenge ranged from 0% to 30% (Table I). These results collectively indicated that path-1-colonized plants were afforded tissue-specific, localized plant protection. Induced systemic resistance has been reported in certain cucurbit hosts by prior inoculation with specific concentrations of pathogenic, hypovirulent, and nonpathogenic isolates (Dean and Kuc, 1987; Kuc, 1990). However, fungus-induced systemic resistance is accompanied by some degree of necrosis in plant tissue, a phenomenon that was not observed with path-1 (Keen, 1982, 1990). Therefore, we are designating this unique phenomenon of path-1-conferred resistance as “endophyte-associated resistance.”
Colonization studies indicated that under challenge conditions L2.5 was not capable of surviving or being recovered in path-1-colonized plants, and that path-1 did not produce chemicals in vitro or in vivo that inhibited the growth of L2.5. Since Colletotrichum and Fusarium spp. have different mechanisms of pathogenesis, it is unlikely that path-1 confers protection by physically occluding these pathogens (Bailey et al., 1992; Alabouvette et al., 1993; Larkin et al., 1993). Therefore, we conclude that the basis of path-1-induced protection involves biochemical interactions between path-1 and the host plant.
Biochemical analyses indicated that, in general, path-1-exposed plants showed null to low levels of PAL, peroxidase, or lignin activity, indicating little or no induction of these indicators of the plant-defense response. In contrast, with the exception of PAL activity in resistant cultivars, plants exposed to L2.5 displayed a significant plant-defense response. PAL activity was not detected at significant levels in resistant cultivars exposed to any of the treatments. These results corroborate findings from earlier studies conducted on anthracnose-resistant cucurbits that suggested that plant resistance was correlated with elevated levels of lignin deposition and peroxidase activity (Hammerschmidt and Kuc, 1982a, 1982b; Hammerschmidt et al., 1982). Collectively, these results suggest that PAL does not play as significant a role as peroxidase activity or lignin deposition in the cucurbit defense response. Statistical analysis of PAL activity using a two-factor ANOVA indicated that significant differences between the treatments occurred in anthracnose-susceptible watermelon cv Sugar Baby (P = 0.034 (Fig. 2). No significant differences were observed in anthracnose-resistant watermelon cv Jubilee and cucumber cv Pepino (data not shown). These results suggest that the usefulness of PAL activity as an indicator of the plant defense response may be cultivar and/or species specific.
Within 24 h after exposure to virulent pathogens, both susceptible and resistant path-1-colonized plants exhibited a rapid accumulation of peroxidase and lignin that paralleled and/or exceeded those of plants exposed only to L2.5 for 72 h (Fig. 1; Tables II and III). These results indicated that path-1-colonized plants were able to mount a rapid defense response that inevitably resulted in protection from pathogen ingress and, hence, protection from disease.
The absence of detectable levels of SA (data not shown) in any of the cultivars corroborated the results from our inoculation studies (Table I), which indicated that path-1-induced disease protection was not systemic; it was localized and correlated with the physical presence of path-1 in plant tissues. In addition, the fact that path-1 did not colonize or protect cotyledons may indicate some level of tissue specificity.
The mechanism(s) that allows path-1 to protect plants against disease appears to involve an interaction between the mutant and the plant defense system. It has been suggested in several host-pathogen systems that the most critical component of host resistance involves the timing and activation of the defense response following recognition of the pathogen (Madamanchi and Kuc, 1991; Kuc and Strobel, 1992). In view of the data presented here, we propose a working model to explain the protection of plants by path-1. This model, designated endophyte-associated resistance, is based on the fact that path-1 either activates plant defenses to very low levels or primes the defense system without activation. When path-1-colonized plants are challenged with virulent fungi, a strong defense response is activated in less than 24 h. Activation of the defense system may be the result of a “threshold response” similar to action potentials in nerve fibers (Stevens, 1979). For nerve fibers to “fire” and transmit signals, they must receive a stimulus of proper strength and duration. The stimulus induces a depolymerization of nerve cell membranes that establishes an electrochemical gradient. If the electrochemical gradient reaches a certain threshold potential, the nerve fiber will fire. If the duration or strength of the stimulus is insufficient to achieve the threshold potential, the nerve fiber will be excited but will not fire. Perhaps plants do not activate defense systems until the concentration of fungal metabolites reaches a threshold to activate the system. path-1 may expose the plant to concentrations of fungal metabolites slightly below threshold levels, thus preventing defense system activation. Under challenge conditions, path-1-colonized plants are exposed to concentrations of fungal metabolites from the virulent fungus that, through an additive effect, potentiate the system, and threshold concentrations necessary for defense system activation are achieved. Disease-resistant cultivars may simply have lower threshold potentials to specific fungal metabolites than do susceptible cultivars.
The development of biological control agents for protecting plants against fungal root diseases is complicated by the fact that the organisms of interest must compete in the soil environment (Deacon, 1988). In addition, it is assumed that fungal biological control is based on the production of antifungal chemicals, mycoparasitism, or physical occlusion, all of which are sensitive to environmental factors (Scher and Baker, 1982; Alabouvette et al., 1993; Deacon and Berry, 1993; Larkin et al., 1993; Nielsen and Sorensen, 1997). path-1 avoids the potential biocontrol problems by colonizing plant tissues and stimulating the plant to protect itself in a multigenic manner. As a result, it may be very difficult for a virulent pathogen to overcome this type of resistance, which allows for the development of long-term control strategies. Finally, we have conducted in vivo experiments for the last 6 years (data not shown) that indicate that the path-1 nonpathogenic phenotype is stable and reversion to a pathogenic phenotype is unlikely because of the pleitrophic nature of the path-1 mutant (Freeman and Rodriguez, 1993). Collectively, our studies indicate that the path-1 mutant may result in the development of a novel, long-term biocontrol strategy for plant protection.
ACKNOWLEDGMENTS
We would like to thank Judy Ranson, Dr. Jennifer Smith, Dr. Richard Stange, and Dr. Ray Hammerschmidt for helpful suggestions. We would also like to thank Dr. Jennifer Lorang for editorial assistance.
Abbreviations:
- ANOVA
analysis of variance
- PAL
Phe ammonia-lyase
- SA
salicylic acid
- SAR
systemic acquired resistance
Footnotes
This research was supported in part by a postdoctoral fellowship grant from the U.S.-Israel Agricultural Research and Development Fund (BARD) awarded to S.F. Partial support was also provided by a joint National Science Foundation, Department of Energy-U.S. Department of Agriculture grant (to R.J.R. as co-private investigator) and by a BARD grant awarded to R.J.R. and S.F.
LITERATURE CITED
- Alabouvette C, Lemanceau P, Steinberg C. Recent advances in the biological control of Fusarium wilts. Pestic Sci. 1993;37:365–373. [Google Scholar]
- Alfano JR, Collmer A. Bacterial pathogens in plants: life up against the wall. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ. Extracellular enzymes produced by Colletotrichum lindemuthianum and Helminthosporium maydis during growth on isolated bean and corn walls. Phytopathology. 1978;68:1585–1589. [Google Scholar]
- Anderson AJ (1988) Elicitors, the hypersensitive response, and phytoalexins. In NT Keen, T Kosuge, LL Walling, eds, Physiology and Biochemistry of Plant-Microbial Interactions. American Society of Plant Physiologists, Rockville, MD, pp 103–110
- Bailey JA, O'Connell RJ, Pring RJ, Nash C (1992) Infection strategies of Colletotrichum species. In JA Bailey, MJ Jeger, eds, Colletotrichum: Biology, Pathology and Control. CAB International, Wallingford, CT, pp 88–120
- Beaudoin-Eagan LD, Thorpe TA. Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985;78:438–441. doi: 10.1104/pp.78.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffetti LM, Pope M, Dunkle LD, Daly JM, Knoche H. Isolation and structure of an inactive product derived from the host-specific toxin produced by Helminthosporium carbonum. Biochemistry. 1983;22:3507–3510. [Google Scholar]
- Cuypers B, Schmelzer E, Hahlbrock K. In situ localization of rapidly accumulated phenylalanine ammonia-lyase mRNA around penetration sites of Phytophthora infestans in potato leaves. Mol Plant-Microbe Interact. 1988;1:157–160. [Google Scholar]
- Dalisay RF, Kuc JA. Persistence of induced resistance and enhanced peroxidase and chitinase activities in cucumber plants. Physiol Mol Plant Pathol. 1995a;47:315–327. [Google Scholar]
- Dalisay RF, Kuc JA. Persistence of reduced penetration by Colletotrichum lagenarium into cucumber leaves with induced systemic resistance and its relation to enhanced peroxidase and chitinase activities. Physiol Mol Plant Pathol. 1995b;47:329–338. [Google Scholar]
- Darvill AG, Albersheim P. Phytoalexins and their elicitors—a defense against microbial infection in plants. Annu Rev Plant Physiol. 1984;35:243–275. [Google Scholar]
- Davis KR, Darvill AG, Albersheim P, Dell A. Several biotic and abiotic elicitors act synergistically in the induction of phytoalexin accumulation in soybean. Plant Mol Biol. 1986;6:23–32. doi: 10.1007/BF00021303. [DOI] [PubMed] [Google Scholar]
- Deacon JW. Biocontrol of soil-borne plant pathogens with introduced inocula. Philos Trans R Soc Lond-Biol Sci. 1988;318:249–264. [Google Scholar]
- Deacon JW, Berry LA. Biocontrol of soil-borne pathogens: concepts and their application. Pestic Sci. 1993;37:417–426. [Google Scholar]
- Dean RA, Kuc J. Rapid lignification in response to wounding and infection as a mechanism for induced systemic protection in cucumber. Physiol Mol Plant Pathol. 1987;31:69–81. [Google Scholar]
- DeWit PJGM, Hofman AE, Welthuis GCM, Kut JA. Isolation and characterization of an elicitor of necrosis isolated from intercellular fluids of compatible interactions of Cladosporium fulvum (syn. Fulvia fulva) and tomato. Plant Physiol. 1985;77:642–647. doi: 10.1104/pp.77.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman MB, Patil SS. Cutinase deficient mutants of Colletotrichum trifolii. Curr Genet. 1986;14:241–246. [Google Scholar]
- Dixon RA, Lamb CJ. Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol. 1990;41:339–367. [Google Scholar]
- Ebel J. Phytoalexin synthesis: the biochemical analysis of the induction process. Annu Rev Phytopathol. 1986;24:235–264. [Google Scholar]
- Flor HH. The current status of the gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275–296. [Google Scholar]
- Freeman S, Rodriguez RJ. A rapid, reliable bioassay for pathogenicity of Colletotrichum magna on cucurbits and its use in screening for nonpathogenic mutants. Plant Dis. 1992;76:901–905. [Google Scholar]
- Freeman S, Rodriguez RJ. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science. 1993;260:75–78. doi: 10.1126/science.260.5104.75. [DOI] [PubMed] [Google Scholar]
- Gurr E (1965) Rational Use of Dyes in Biology. Williams & Wilkins, Baltimore, MD, pp 422
- Hamdan MAMS, Dixon RA. Differential biochemical effects of elicitor preparations from Colletotrichum lindemuthianum. Physiol Mol Plant Pathol. 1986;28:329–344. [Google Scholar]
- Hammerschmidt R, Kuc J. Lignification as a mechanism for induced systemic resistance in cucumber. Physiol Plant Pathol. 1982a;20:61–71. [Google Scholar]
- Hammerschmidt R, Kuc JA. Lignification as a mechanism for induced systemic resistance in cucumber. Physiol Plant Pathol. 1982b;20:61–71. [Google Scholar]
- Hammerschmidt R, Lamport DTA, Muldoon EP. Cell wall hydroxyproline enhancement and lignin deposition as an early event in the resistance of cucumber to Cladosporium cucumerinum. Physiol Plant Pathol. 1984;24:43–47. [Google Scholar]
- Hammerschmidt R, Nuckles EM, Kuc J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol. 1982;20:73–82. [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependant plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Kuc JA. Local and systemic induction of peroxidase, chitinase and resistance in cucumber plants by K2HPO4. Physiol Mol Plant Pathol. 1990;37:355–366. [Google Scholar]
- Jackson AO, Taylor CB. Plant-microbe interactions: life and death at the interface. Plant Cell. 1996;8:1651–1668. doi: 10.1105/tpc.8.10.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SF, Jr, Winstead NN. Glomerella magna, cause of a new anthracnose of cucurbits. Phytopathology. 1964;54:452–454. [Google Scholar]
- Johal GS, Briggs SP. Reductase activity encoded by the HM1 disease resistance gene of maize. Science. 1992;258:985–987. doi: 10.1126/science.1359642. [DOI] [PubMed] [Google Scholar]
- Kamoun S, Young M, Glascock CB, Tyler BM. Extracellular protein elicitors from Phytophthora: host-specificity and induction of resistance to bacterial and fungal phytopathogens. Mol Plant-Microbe Interact. 1993;6:15–25. [Google Scholar]
- Keen NT. Specific recognition in gene-for-gene host-parasite systems. Adv Plant Pathol. 1982;1:35–82. [Google Scholar]
- Keen NT. Pathogenic strategies of fungi. In: Lugtenberg B, editor. Recognition in Microbe-Plant Symbiotic and Pathogenic Interactions. Berlin: Springer-Verlag; 1986. pp. 171–188. [Google Scholar]
- Keen NT. Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet. 1990;24:447–463. doi: 10.1146/annurev.ge.24.120190.002311. [DOI] [PubMed] [Google Scholar]
- Klement Z (1982) Hypersensitivity. In MS Mount, GH Lacy, eds, Phytopathogenic Prokaryotes, Ed 2. Academic Press, New York, pp 149–177
- Knogge W. Fungal infection in plants. Plant Cell. 1996;8:1711–1722. doi: 10.1105/tpc.8.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE. Enzymatic penetration of the plant cuticle by fungal pathogens. Annu Rev Phytopathol. 1985;23:223–250. [Google Scholar]
- Kombrink E, Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi. Plant Physiol. 1986;81:216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc J. A case for self defense in plants against disease. Phytoparasitica. 1990;18:3–8. [Google Scholar]
- Kuc J, Strobel NE (1992) Induced resistance using pathogens and nonpathogens. In ES Tjamos, ed, Biological Control of Plant Diseases. Plenum Press, New York, pp 295–303
- Larkin RP, Hopkins DL, Martin FN. Ecology of Fusarium oxysporum f. sp. niveum in soils suppressive and conducive to Fusarium wilt of watermelon. Phytopathology. 1993;83:1105–1116. [Google Scholar]
- Madamanchi NR, Kuc J (1991) Induced systemic resistance in plants. In GT Cole, HC Hoch, eds, The Fungal Spore and Disease Initiation in Plants and Animals. Plenum Press, New York, pp 347–362
- Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. [Google Scholar]
- Nielsen P, Sorensen J. Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol. 1997;22:183–192. [Google Scholar]
- Rodriguez RJ, Owen JL. Isolation of Glomerella musae [teleomorph of Colletotrichum musae (Berk and Curt.) Arx.] and segregation analysis of ascospore progeny. Exp Mycol. 1992;16:291–301. [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher FM, Baker R. Effect of Pseudomonas pudita and a synthetic iron chelator on induction of soil suppression to Fusarium wilt pathogens. Phytopathology. 1982;39:345–352. [Google Scholar]
- Stevens CF. The neuron. Sci Am. 1979;3:54–65. doi: 10.1038/scientificamerican0979-54. [DOI] [PubMed] [Google Scholar]
- Tu JC. An improved Mather's medium for growth, sporulation and germination of spores of Colletotrichum lindemuthianum. Microbiosis. 1985;44:87–93. [Google Scholar]
- Zar JH. Two-factor analysis of variance. In: Kurtz B, editor. Biostatistical Analysis, Ed 2. Englewood Cliffs, NJ: Prentice-Hall; 1984. pp. 206–235. [Google Scholar]