Abstract
Serologic parameters of kala-azar were evaluated by Western blot analysis. Sera from kala-azar patients with confirmed diagnoses were screened for immunoglobulin G (IgG) and IgG subclass-specific reactivity against Leishmania donovani membrane antigen (LAg). Heterogenous LAg-specific IgG reactivity with numerous proteins with molecular masses ranging from 18 to 190 kDa was observed. Though the individual band patterns were varied, seven polypeptides of approximately 31, 34, 51, 63, 72, 91, and 120 kDa were immunoreactive with all the sera tested from kala-azar patients. The band patterns of the immunoblots of sera from patients after treatment and clinical cure with sodium antimony gluconate revealed a decrease in the frequency of the bands. Still, recognition of the 63- and 120-kDa bands was 100%, and the 55- and 91-kDa fractions were recognized in 93% of the sera from cured individuals. Among the IgG subclasses, IgG1 reacted with the greatest number of polypeptides. The 63-kDa protein was again detected by all of the IgG subclasses of all the sera tested. Other fractions recognized by the subclasses of more than 70% of the serum samples included those of 47, 51, 55, and 78 kDa. Following treatment, 63- and 51-kDa bands were the most reactive with the IgG subclasses. LAg-associated cross-reaction with other reference human antisera revealed a mild reactivity of the 63-kDa polypeptide with some of the serum samples from leprosy, malaria, typhoid, tuberculosis, and healthy controls. Western blot analysis of LAg entrapped in liposomes, strong vaccine candidates against experimental visceral leishmaniasis, revealed a more restricted band pattern. The 63-kDa fraction revealed by all pre- and posttreatment sera showed almost negligible levels of cross-reaction with sera from patients with other diseases or from healthy controls. These observations provide insight into induced immunity during kala-azar infection for future application.
Protozoan parasites of the genus Leishmania cause a spectrum of diseases in humans. Cutaneous leishmaniasis is caused by a wide range of species, including Leishmania major and Leishmania tropica in the Old World and Leishmania mexicana, Leishmania braziliensis, and Leishmania amazonensis in the New World. These species cause a form of leishmaniasis characterized by skin lesions rich in parasites, which are usually localized and heal spontaneously. Active visceral leishmaniasis (VL) caused by members of the Leishmania donovani complex, including L. donovani donovani, L. donovani infantum, and L. donovani chagasi, is characterized by fever, cachexia, hepatosplenomegaly, and blood cytopenia and is usually fatal without specific chemotherapy. L. donovani infections in humans, however, do not always result in VL. In areas were VL is endemic, a significant population of individuals has a self-resolving infection detectable only by the development of specific antibodies and/or a T-cell response to leishmanial antigens (18, 35, 56). Furthermore, patients who have recovered from kala-azar are usually immune to reinfection (25, 40). The resistance to disease in these individuals is therefore met by an appropriate host response, possibly through Leishmania antigens, for the stimulation of protective responses in immune hosts. However, the nature of the parasite antigens involved in eliciting this immunity is not known.
The development of vaccines is the essential aim of studies of leishmaniasis. Current research to understand the varied aspects of regulation in the immune system is focused on the immunopathology of leishmanial infections. Extensive studies of experimental infections of L. major demonstrate the differential activation of CD4+-T-helper-cell subsets with the predominance of Th1 (interleukin-2 [IL-2] and gamma interferon [IFN-γ]) in resistant C3H and C57BL/6 mice and an exclusive Th2-dominant response (IL-4 and IL-5) in susceptible BALB/c mice (2, 26, 27, 47). The immunology of VL, even in the murine system, is not well defined. Studies of mice infected with L. donovani show no evidence of the production of CD4+-Th2-cell-restricted cytokines with the progression of infection. On the contrary, Th1- and Th2-cell-associated cytokines have been reported for experimental VL (32, 43). In human infections with L. donovani, a marked depression in the T-cell response is characterized by the absence of IL-2 and IFN-γ production in vitro (13, 16, 56). Studies of tissue cytokine mRNA expression demonstrate a role for IL-10 in downregulating T-cell immune responses as well as an association between increased IL-10 production and the pathology seen in L. donovani infections (22, 34). However, active VL is also correlated with enhanced induction of lesional IFN-γ and IL-2 together with IL-10 and/or IL-4 and elevated levels of IFN-γ, IL-4, IL-10, and immunoglobulin E (IgE) in serum (31, 34, 61, 65). After successful treatment, the presence of IFN-γ, IL-4, and IL-10 in vitro and in vivo suggests the coexistence of Th1 and Th2 in kala-azar patients as well as in cured individuals (11, 15, 33, 34). Thus, despite the impressive recent advances in understanding the cell-mediated immune responses of these infections, it is still difficult to delineate the responses leading either to visceral disease or to protective immunity.
Kala-azar is also characterized by strong antibody titers. Cytokines elaborated by activated T cells induce the switching of B lymphocytes to several IgG isotypes and are thus obligatory for some humoral responses. IFN-γ, the Th1 cytokine, upregulates the isotypes IgG2a and IgG3 in mice and probably IgG1 and IgG3 in humans. IL-4 and IL-5, Th2 cytokines, stimulate the production of high levels of IgM, IgE, and IgG isotypes such as IgG1 in mice and IgG4 in humans (1, 52). Analysis of Leishmania antigen-specific Ig isotypes and IgG subclasses in VL patient sera revealed elevated levels of IgG, IgM, IgE, and IgG subclasses during disease (7, 10, 17, 55). Drug resistance was associated with a reduction in IgG2 and IgG3 antibodies, with no significant change in the titers of IgG, IgM, IgA, IgE, and IgG4. A marked elevation, however, of IgG1 antibodies was observed in all these patients (8). A successful cure corresponded with a decline, most significantly, in the levels of IgE, IgG4, and IgG1 (8, 10, 17). The differential patterns of Ig isotypes observed during disease, drug resistance, and cure were specific for antigens of L. donovani. The L. donovani promastigote membrane antigens (LAg) used in our laboratory (8) also demonstrate a strong vaccine potentiality alone, and in association with liposomes, for experimental VL (3, 5). The purpose of this study was to dissect the humoral immune responses to the components of LAg in kala-azar patients by Western blot analysis. The recognition of the antigens by IgG and its subclasses before and after chemotherapy provides insight into the immunity induced by this pathogen. Analysis of LAg in liposomes with pre- and posttreatment IgG antibodies demonstrates the diagnostic and prognostic potentiality of this preparation for kala-azar.
MATERIALS AND METHODS
Study subjects.
The subjects of the longitudinal study of the present investigation consisted of 15 VL patients, 7 to 48 years old, from Bihar and West Bengal (Eastern India), the main areas of endemicity. This group, comprised of 5 women and 10 men, was admitted to the School of Tropical Medicine, Calcutta, India. A diagnosis of VL was based upon a set of collective elements: the presence of parasites in bone marrow and/or spleen aspirates, epidemiological history, and response to specific treatment. Each of the 15 patients included in the study was sampled for serum twice, on day 0, i.e., before initiation of therapy, and 50 days after successful treatment with 20 injections of 20 mg of sodium antimony gluconate (SAG) per kg of body weight. The 15 individuals included as controls were from Calcutta, a region where VL is not endemic, with no previous history of kala-azar. This group consisted of three malaria patients, three typhoid patients, three tuberculosis patients, three leprosy patients, and three healthy controls from the Indian Institute of Chemical Biology (IICB). The endemic diseases were confirmed bacteriologically in the cases of typhoid, tuberculosis, and leprosy and parasitologically in the case of malaria, and sera were collected before treatment. Sera were obtained by venipuncture from patients and controls. Blood was allowed to coagulate at room temperature and then centrifuged at 1,400 × g for 10 min. All sera were stored at −20°C until use. The study was approved by the Ethical Committee on Human Subjects, IICB. Informed consent was obtained from all patients for blood sampling.
Preparation of L. donovani antigen.
L. donovani antigen AG83, originally isolated from an Indian kala-azar patient, was cultured in vitro for antigen preparation as described earlier (3). Briefly, stationary-phase promastigotes, harvested after the third or fourth passage, were washed four times in cold phosphate-buffered saline, pH 7.2 (PBS), and resuspended at a concentration of 1.0 g of cell pellet in 50 ml of cold 5 mM Tris-HCl buffer, pH 7.6. The suspension was vortexed six times for 2 min each, with a 10-min interval of cooling on ice between each vortexing. The parasite suspension was then centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet thus obtained (42) was resuspended in the same Tris buffer and sonicated three times for 1 min each at 4°C in an ultrasonicator. The suspension was finally centrifuged at 5,190 × g for 30 min, and the supernatant containing the LAg was harvested and stored at −70°C until use. The amount of protein obtained from a 1.0-g cell pellet, as assayed by the method of Lowry et al. (39), was approximately 16 mg.
Electroelution of proteins with 63- and 72-kDa bands by SDS-PAGE.
Proteins with molecular masses of 63 and 72 kDa were eluted from leishmanial antigens (LAg) of L. donovani promastigotes subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) as described earlier (4). Proteins with molecular masses of 63 (gp63) and 72 kDa were localized in gels, stained with Coomassie blue, and eluted by electrophoresis in running buffer (0.025 M Tris, 0.192 M glycine, 1% SDS) at 10 mA for 5 h. After elution, the proteins were dialyzed, lyophilized, and resuspended in PBS.
Preparation of liposomes.
Positively charged liposomes were prepared with egg lecithin, cholesterol, and stearylamine at a molar ratio of 7:2:2 as reported previously (3). The thin, dry film consisting of the lipid mixture was dispersed in PBS for the preparation of empty liposomes. Vesicles with entrapped leishmanial antigen were prepared by the dispersion of the lipid film in PBS containing 1 mg of LAg per ml. The mixture was vortexed, and the suspension was sonicated for 30 s in an ultrasonicator. Liposomes with entrapped antigen were separated from the excess free antigen by three successive washings in PBS with centrifugation (105,000 × g for 60 min at 4°C). The protein content entrapped in liposomes was estimated by using bovine serum albumin as the standard in the presence of 1% SDS and appropriate blanks (39). The average amount of LAg associated per milligram of egg lecithin was 35 μg. Similarly, electroleluted 63- and 72-kDa polypeptides were entrapped in positively charged liposomes. The lipid film was dispersed in 1 ml of PBS containing 350 μg of protein, and 4.5 μg of the 63-kDa polypeptide and 3.5 μg of the 72-kDa polypeptide were associated with 1 mg of egg lecithin.
Protein electrophoresis and immunoblot analysis.
The leishmanial antigens were subjected to SDS-10% PAGE under standard conditions (36) in a Mini-Protean system (Bio-Rad Laboratories, Richmond, Calif.). Proteins free in PBS or entrapped in liposomes (6 μg per lane) separated on the gel were silver stained (64).
For immunoblot analysis, the resolved proteins were electrophoretically transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), and the assays were performed according to the method of Rolland-Burger et al. (51), with slight modifications. The nitrocellulose strips were blocked overnight in 100 mM Tris-buffered saline, pH 7.6 (TBS), containing 0.1% Tween 20 (T-20), washed once with 0.05% T-20 in TBS (washing buffer), and incubated for 1 h with human sera. All sera, including those from acute- and convalescent-phase kala-azar patients as well as sera from positive controls (patients with malaria, tuberculosis, typhoid, and leprosy) and negative controls (healthy personnel of the Institute who were not from areas of endemicity), were diluted 1:100 in washing buffer. For reactivity with electroeluted polypeptides, 1 μg of liposomal antigen per lane was subjected to SDS-10% PAGE, transferred to nitrocellulose membranes, and probed with kala-azar patient sera as described above. The blots were then washed three times for 20 min each and incubated again for 1 h with a 1:1,000 dilution of peroxidase-conjugated anti-human IgG (Sigma Immunochemicals, St. Louis, Mo.), followed by three washes as described above. For IgG subclass reactivity, the incubation of the membranes with human sera at a 1:100 dilution was followed by a 1-h reaction with anti-human IgG1, IgG2, IgG3, and IgG4 monoclonal antibodies (1:300 dilution; Sigma Immunochemicals). After being washed, the blots were reacted again for 1 h with a 1:500 dilution of peroxidase-conjugated goat anti-mouse IgG (Sigma Immunochemicals) and washed as before except for the last wash, which was done without T-20. Enzymatic activity was revealed with 15 mg of 3,3′-diaminobenzidine tetrahydrochloride (Sigma Immunochemicals) in 30 ml of TBS containing 15 μl of 30% H2O2. The efficacy of transfer of leishmanial proteins was regularly checked and confirmed by concurrent gel staining with Coomassie blue or silver and nitrocellulose membrane staining with 0.1% Ponceau S in 1% acetic acid. Following staining, the parts of the membrane containing molecular mass standards were marked with ink.
RESULTS
Study subjects.
The clinical and laboratory features of kala-azar patients studied herein are summarized in Table 1. The characteristic symptom of fever was observed in all patients on admission. Interestingly, three out of five patients from West Bengal also exhibited bilateral epitrochlear lymphoadenopathy. All patients responded to SAG therapy, and no parasites were found in the splenic aspirates at the end of treatment.
TABLE 1.
Clinical and laboratory features of kala-azar patients
| Characteristic | Study group
|
|
|---|---|---|
| Pretreatment | Posttreatment | |
| No. of patients | 15 | 15 |
| Age (yr) | 22.53 ± 3.16 | |
| Weight (kg) | 34.80 ± 2.48 | 36.60 ± 2.48 |
| Duration of illness (mo) | 3.83 ± 0.30 | |
| Karnofsky scorea | 76.53 ± 1.33 | 83.66 ± 1.06 |
| Splenic size (cm) | 10.72 ± 1.06 | 6.57 ± 0.99 |
| Splenic aspirate scoreb | 4.07 ± 0.18 | Negative |
| Leukocyte count (103/mm3) | 3.48 ± 0.15 | 4.11 ± 0.15 |
| Hemoglobin concn (g/dl) | 5.30 ± 0.29 | 7.30 ± 0.21 |
| Platelet count (103/mm3) | 0.71 ± 0.02 | 0.75 ± 0.03 |
| Liver function test | ||
| Total protein (g/dl) | 8.51 ± 0.30 | 8.28 ± 0.30 |
| Albumin (g/dl) | 3.17 ± 0.12 | 3.59 ± 0.13 |
| Globulin (g/dl) | 5.37 ± 0.32 | 4.66 ± 0.36 |
The Karnofsky performance scale is as follows: 100, normal, no complaints, no evidence of disease; 90, able to carry on normal activity, minor symptoms of disease; 80, normal activity with effort, some symptoms of disease; 70, cares for self, unable to carry on normal activity or active work.
5, > 10 to 100 parasites/field; 4, >1 to 10 parasites/field; 3, >1 to 10 parasites/10 fields; 2, >1 to 10 parasites/100 fields; 1, >1 to 10 parasites/1,000 fields; 0, no parasites or >1 to 10 parasites/>1,000 fields.
Antigen profile of LAg and LAg-liposome.
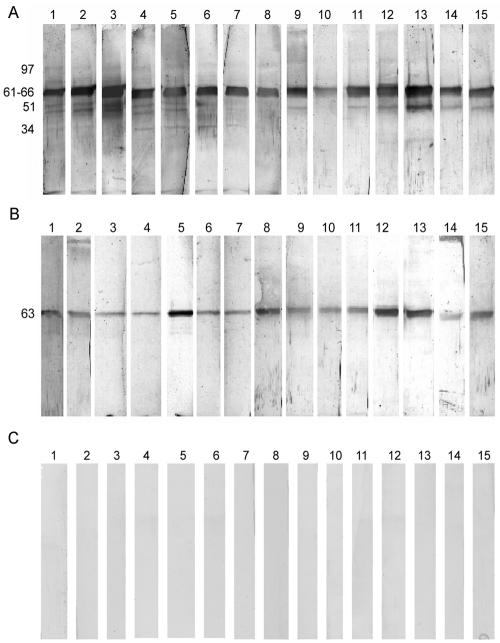
LAg, alone or in association with positively charged liposomes, was fractioned by SDS-10% PAGE (Fig. 1). Free LAg (lane 1) comprised approximately 35 polypeptides ranging in molecular mass from 18 to 190 kDa, with distinct polypeptides at 18, 22, 27, 31, 34, 39, 43, 45, 47, 51, 55, 63, 72, 78, 91, 97, 110, 120, and 145 kDa. LAg entrapped in positively charged liposomes (lane 2) comprised approximately 17 polypeptides in the range of 18 to 145 kDa. Although a large number of polypeptides of LAg were incorporated in the liposomes, a mechanism of selective entrapment for some polypeptides was observed. Furthermore, comparison of a similar concentration of protein (6 μg) in free LAg and LAg entrapped in liposomes revealed that although a number of polypeptides stained with almost equal intensities in LAg, a duplex of 61 to 66 kDa stained most intensely in liposome-associated LAg (lane 2), demonstrating a preferential entrapment of this polypeptide by the liposome.
FIG. 1.
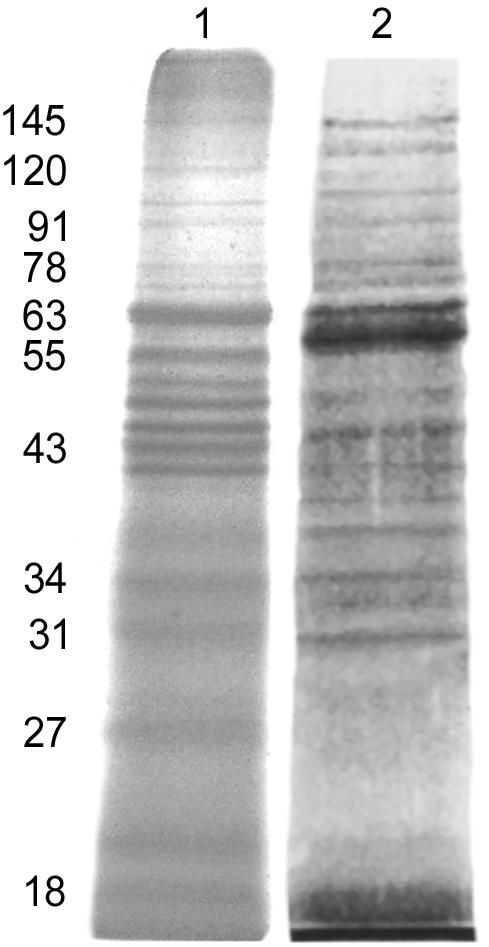
Silver-stained SDS-PAGE gel of Leishmania antigens and liposomes. Lanes: 1, LAg; 2, LAg-liposome. The positions of prominent bands are shown on the left at apparent molecular masses in kilodaltons.
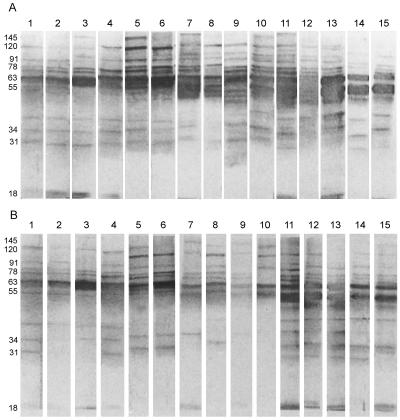
IgG-specific reactivity of LAg with VL-infected sera, before and after chemotherapy.
The sera from 15 VL-infected patients were tested for IgG reactivity to SDS-PAGE-separated polypeptides of LAg blotted onto nitrocellulose. The response of IgG, the predominant Ig class in VL-infected patient sera (7, 20), to L. donovani LAg polypeptides revealed specific recognition of polypeptides ranging from 18 to 190 kDa (Fig. 2). Binding patterns exhibited variability depending on individual serum reactivity. Despite heterogenous reactivity to LAg, seven polypeptides corresponding to relative molecular masses of 31, 34, 51, 63, 72, 91, and 120 kDa were reactive with all of the 15 sera from kala-azar patients prior to treatment. In addition, major bands of 55, 78, and 145 kDa (93%); 36 and 39 kDa (80%); 97 kDa (73%); 18 and 47 kDa (60%); and 20 and 43 kDa (40%) were recognized most frequently (Fig. 2A). With successful treatment with SAG, decreases in the frequency of recognition and in the intensities of bands were observed. Some fractions, such as those with bands at 36, 43, 47, 51, 97, and 145 kDa, showed strong reactivity with a large number of VL-infected-patient sera and reacted with a smaller number of sera after treatment (Fig. 2B). However, fractions such as those with bands of 55 and 91 kDa (93%); 34, 72, and 78 kDa (87%); and 31 kDa (73%) were still reactive with posttreatment sera with high frequencies, and two bands of 63 and 120 kDa were observed in all sera after cure.
FIG. 2.
Western blot analysis of kala-azar serum IgG reactivity with SDS-PAGE-separated (6-μg) membrane proteins of LAg before (A) and after (B) SAG treatment. Each lane represents one VL patient, and longitudinal samples have identical lane numbers. The apparent molecular masses of prominent bands in kilodaltons are marked on the left.
Antibody-type reactivity.
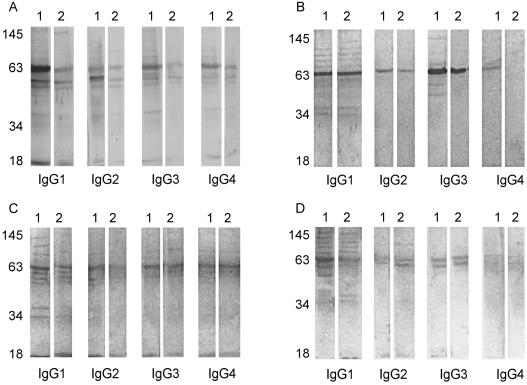
Western blot analysis of kala-azar serum reactivity against L. donovani antigen indicates a strong IgG reaction against the different polypeptides of LAg, before and after chemotherapy (Fig. 2). To determine which human subclasses of IgG were responsible for these interactions, Western blot assays specific for IgG1, IgG2, IgG3, and IgG4 were performed with seven sera from kala-azar patients in our longitudinal study, and data from four patients are presented in Fig. 3 (lanes 1, before treatment; lanes 2, following successful cure with SAG). All subclasses of IgG were reactive with the polypeptides of LAg, demonstrating multiband patterns of various frequencies. The most prominent reaction was observed with IgG1 (71 to 100% of the sera recognizing polypeptides of 18, 34, 39, 43, 47, 51, 55, 63, 72, 78, 91, and 120 kDa), followed by IgG3 (39, 47, 51, 55, 63, 78, and 91 kDa), IgG2 (34, 47, 51, 55, 63, 72, 78, and 91 kDa), and IgG4 (39, 47, 51, 55, 63, 72, 78, and 91 kDa). Immunoblot banding patterns with anti-IgG1 closely resembled those produced with anti-IgG, suggesting that IgG1 is the dominant IgG subclass induced during disease. Despite the reported fall in the IgG subclass antibody titers following treatment (8, 17), there was persistent recognition of the polypeptides of LAg through all of the subclasses. However, while the reactivity of IgG2, IgG3, and IgG4 was limited to two to four bands of 47, 51, 55, and 63 kDa, IgG1 demonstrated a pattern of reduced intensity that was almost identical to that prior to treatment.
FIG. 3.
Immunoblot profiles of LAg-specific IgG subclass reactivities of four (A to D) kala-azar patients before and after SAG treatment. Lanes 1, pretreatment sera; lanes 2, posttreatment sera. The positions of prominent bands at apparent molecular masses in kilodaltons are shown on the left.
Serologic reactivity with normal sera and other pathogens.
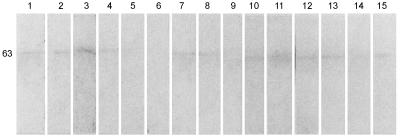
In order to assess the degree of cross-reactivity of the antigens of L. donovani with normal sera and sera from patients with other tropical diseases coendemic with kala-azar, LAg blots were probed with various control sera (Fig. 4). While most of the polypeptides of LAg did not show reactivity with control sera, samples used in this assay demonstrated cross-reaction of low intensity with the 63-kDa protein in 73% of the sera.
FIG. 4.
Western blot analysis of IgGs of various sera for reactivity to LAg. Lanes 1 to 3, sera from malaria patients; lanes 4 to 6, sera from typhoid patients; lanes 7 to 9, sera from tuberculosis patients; lanes 10 to 12, sera from leprosy patients; lanes 13 to 15, sera from healthy subjects. The position of the major band (in kilodaltons) is indicated on the left.
Immunoblot analysis of the LAg-liposome for IgG.
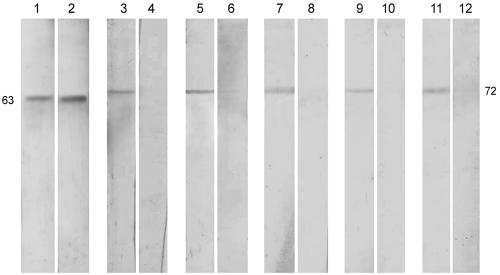
All the sera from 15 VL patients, before and after SAG treatment, and 15 control sera were further studied for IgG reaction with L. donovani antigens entrapped in liposomes. Pretreatment sera recognized 11 polypeptides of LAg in liposomes, corresponding to relative molecular masses of 31, 34, 47, 51, 63, 72, 78, 95, 97, 110, and 145 kDa (Fig. 5A). The patterns of response varied in the different sera tested. However, maximum reactivity was observed for the 51- and 63-kDa polypeptides, which were recognized by all sera, followed by the 47-kDa (87% of sera); 72-, 78-, and 97-kDa (73%); and 34-kDa (60%) polypeptides. Posttreatment sera reacted with only three polypeptides (47, 63, and 97 kDa) (Fig. 5B), with 100% recognition for the 63-kDa polypeptide. No cross-reactivity with any polypeptide of LAg in liposome was observed with positive-control (subjects with malaria, typhoid, tuberculosis, and leprosy) as well as with negative-control (healthy subjects) sera (Fig. 5C), demonstrating the 100% specificity of this assay. Recognition of polypeptides such as those of 51 kDa (100% of sera) and 72 and 78 kDa (73% of sera) by a high proportion of pretreatment sera and the loss of reactivity of these antigens by all posttreatment sera tested suggest that these polypeptides in liposomes can help discriminate sera positive for disease not only from control but also from posttreatment sera from cured individuals. Polypeptides with masses of 63 and 72 kDa were electroeluted by SDS-PAGE and entrapped in liposomes. Whereas the 63-kDa protein showed reactivity with both infected sera and sera from cured individuals (data from results with one of the five sera are shown), reaction with the 72-kDa protein was restricted to disease sera only (Fig. 6).
FIG. 5.
Western blot analysis of the serum IgG reactivities of 15 sera from kala-azar patients in our longitudinal study, with LAg being incorporated into liposomes following separation by SDS-PAGE. (A) Reactivity with acute-phase sera; (B) corresponding reactivity after SAG treatment; (C) cross-reactivity with serum from patients with malaria (lanes 1 to 3), typhoid (lanes 4 to 6), tuberculosis (lanes 7 to 9), and leprosy (lanes 10 to 12) and healthy controls (lanes 13 to 15). The major bands (in kilodaltons) are marked on the left.
FIG. 6.
Western blot analysis of liposomal 63- and 72-kDa antigens. Polypeptides of 63 kDa (lanes 1 and 2) and 72 kDa (lanes 3 to 12), purified by electroelution, were incorporated into liposomes and probed with kala-azar patient sera before (odd-numbered lanes) and after (even-numbered lanes) SAG treatment.
DISCUSSION
It is well established that infection with L. donovani and its control are mediated through the activities of Th1- and Th2-cell-associated cytokines. Successful treatment of VL patients generally results in resistance to reinfection, suggesting a redirection of polarized responses in the host. However, protection has often been found to be associated with both Th1 and Th2 responses (12, 22, 28, 34, 46, 61, 65). Moreover, determining the Th1 or Th2 nature of human immune responses is often problematic. On the other hand, analysis of the relative abundances of pathogen-specific antibodies reveals a distinct pattern of Ig isotypes and IgG subclasses during disease, resistance to treatment, and cure (7, 8, 17, 24). While the types of Igs produced, including different subtypes and subclasses, are regulated by the cytokine environment, elicitation of these immunoglobulins is Leishmania antigen specific. Hence, the identification of antigens involved in inducing these responses is of critical importance.
A mixture of LAg, potential vaccine candidates against experimental VL, shows strong reactivity with Indian kala-azar patient sera (7, 8). Attempts to identify the components of LAg recognized by the VL patient sera in immunoblot experiments showed variations in the patterns of antigen recognition by the different sera from infected patients. However, the specificity of IgG antibody for seven polypeptides of LAg, including the 63-kDa polypeptide, observed in all patients demonstrates the consistency of the banding pattern within the clinical manifestations. The 63- to 65-kDa polypeptide, or gp63 (4), recognized prominently by human VL sera (9, 37, 57), has been proposed as a candidate antigen in the immunodiagnosis of leishmaniasis (45, 49, 59). Apart from gp63, antigens specifically recognized by VL patient sera have been identified as gp70-2 and dp72 (29). Reactivity with crude parasite antigen demonstrated that >90% of the patients with VL produced antibodies to these proteins. Although the identity of the 72-kDa protein observed in our studies has not been proved to be dp72, its molecular mass and the extent of its immunogenicity point to a similarity with dp72. It is of interest that while the 72-kDa polypeptide of LAg reacted with 100% of the Indian kala-azar patient sera tested, this level of sensitivity could not be attained even with the pure dp72 (>93%). The other polypeptides of LAg recognized by all VL patient sera include those of 31, 34, 51, 91, and 120 kDa. While strict comparisons between our results and those reported in the literature are difficult due to the use of different strains and antigens, the immunodominant 31- and 34-kDa bands of LAg characterized in the present study appear to correspond to the p32, and 32- to 35-kDa, membrane-associated antigens and whole-parasite extracts of L. donovani infantum and L. donovani chagasi reported by other investigators (21, 48, 62). Further, the reactivity of the 120-kDa polypeptide in our study finds similarity with the 119-kDa polypeptide from L. donovani chagasi and L. donovani infantum reacting with all VL patient sera (19, 51).
The differences between the pre- and posttreatment sera were a decrease in the reactivity and intensity of most of the polypeptides and the disappearance of a few bands. A number of polypeptides, however, persisted in a large number of cured individuals. Two polypeptides, with molecular masses of 63 and 120 kDa, which were recognized by all pretreatment sera, were also reactive with all posttreatment sera. The reactivity of the 63-kDa band with sera from cured individuals is contradictory to what was observed with native gp63, purified from a Kenyan L. donovani isolate, which could distinguish between ongoing and previous infection with L. donovani (45). The antibody positivity may be due partly to the Western blot technique used in our studies, which was more sensitive than the enzyme-linked immunosorbent assay used by Okongo-Odera et al. (45). A similar lack of change of specific band patterns of pre- and posttreatment sera as determined by Western blot analysis has been reported for the sera of individual humans infected with L. donovani chagasi (48) and L. donovani infantum (41). The presence of Leishmania antigen-specific IgG antibodies, probably for years after clinical and parasitological “cure,” indicates the persistence of a subclinical infection in these individuals. While this state may cause a reemergence of active disease under conditions of immune suppression (6), complete elimination of Leishmania parasites in an immunocompetent individual may result in the loss of parasite-specific antibodies and susceptibility to reinfection (63).
In this study, we further characterized the humoral immunological response to the components of LAg by analyzing the distribution of IgG subclass antibodies in the sera of VL patients. As the four antibody subclasses revealed differential patterns in the sera of kala-azar patients after chemotherapy (8, 17), analysis of the antigens with respect to their patterns of reactivity to the different IgG subclasses may be further indicative of disease activity and pathogenicity. Western blot analysis in the present study indicates that with pretreatment sera, reaction to LAg as well as to total IgG appears to be maximally related to the IgG1 isotype of IgG, although significant reaction with IgG3 followed by IgG2 and IgG4 was also observed. The IgG antibody titers present in the sera of VL patients were predominantly of the IgG1 subclass (8, 24), and the high frequency of IgG1 with the L. donovani antigens suggests a dominance of IgG1 recognition. A number of investigators, however, have reported no correlation between antibody titer by radioimmunoassay, enzyme-linked immunosorbent assay, or indirect immunofluorescence and the intensity of the reaction or the number of bands recognized by Western blotting (30, 41, 50). In accordance, a persistence of reactivity of IgG subclasses determined by Western blot analysis was observed with posttreatment sera despite the reported fall in their titers after cure (8, 17), and the band pattern, especially of IgG1, was almost identical to that of pretreatment sera. IgG1 and IgG3 are the predominant IgG subclasses involved in functions such as attachment to Fc receptors on cell membranes (opsonization; primarily IgG1), mediation of antibody-dependent cellular cytotoxicity, and complement activation (primarily IgG3). The significance of the relative abundances of these Leishmania-specific antibodies during disease, and their persistence after cure, is unclear since immunity to intracellular pathogens such as Leishmania is controlled by cell-mediated effector mechanisms, with humoral immunity apparently having little or no role. A review of the literature, however, reveals considerable evidence that antibody can mediate protection against many viral and nonviral intracellular pathogens (14, 38). The recent observation that specific antibodies and Fc receptors are crucial for the development of an enhanced and effective Th1 response during Chlamydia reinfections (44) suggests a similar participation of Leishmania-specific IgG1 antibodies in Fc receptor-mediated enhancement of the Th1 response for faster and higher immune effectors for rapid clearing of reinfections. The possible role of antibody-mediated protection against Leishmania, though yet to be ascertained, signifies the importance of identification of antigens that may elicit protective antibodies. The predominant recognition of the 63-kDa band by all posttreatment kala-azar sera tested through most of the IgG subclasses points to a potential role of gp63 in protection against this disease. Attempts to analyze L. donovani antigens through reactivity with IgG subclasses have been reported by other researchers (23, 58). However, since those studies used a soluble promastigote extract as the antigen for the Western blot technique, it is difficult to compare our results with the results published by those groups.
Serologically, cross-reactivity between Leishmania species and other pathogens has been reported for whole-cell lysates (19, 60). Analysis of components of LAg that were reactive to antisera from patients with malaria, typhoid, tuberculosis, and leprosy and to sera from healthy controls demonstrated varied cross-reactivities in every case, including a mild reaction to normal human sera with the 63-kDa polypeptide.
Investigation of the antigenic structure of LAg of L. donovani is of fundamental importance for devising methodologies that will allow for more specific immunodiagnosis and prognosis for the development of successful vaccines. Leishmanial antigens have been studied extensively in experimental models for the development of vaccines. However, antigens protective in the murine host may not elicit immune responses in humans (54), emphasizing the importance of testing leishmanial antigens with human cells and sera. LAg, in association with liposomes, have been found to be strong vaccine candidates against experimental VL (3, 5). Since maximum protection was observed with LAg in positively charged liposomes (3), this antigen preparation was investigated for reactivity with sera from pre- and posttreatment kala-azar patients. Although a large number of polypeptides of LAg were associated with the liposomes, the preferential entrapment of some proteins by these liposomes, including proteins of 63 kDa, has been observed (Fig. 1 and 4). The reaction with the infected-patient sera revealed this difference, and the pattern of bands recognized with LAg-liposomes was limited compared to that with LAg alone (Table 2). The absence of cross-reactions between the sera from individuals with any other disease and the polypeptides in liposomes demonstrates that antibody reaction with LAg in liposomes is highly specific. This finding was again evident when LAg-liposomes were probed with sera obtained after treatment. Only the antibodies with high specificities appeared to react with the polypeptides (43, 63, and 97 kDa) in LAg-liposomes. The appearance of a 63-kDa band in all sera tested suggests the persistence of high-affinity anti-gp63 antibodies in cured kala-azar patients. Since gp63 is the major glycoprotein involved in the uptake of the parasites by macrophages (53), the persistence of anti-gp63 antibodies in all kala-azar patients after cure suggests that these antibodies may have an important role in life-long immunity generally observed in successfully cured individuals. The loss of reactivity of proteins such as those of 51, 72, and 78 kDa, which were recognized in high frequencies by patient sera, after treatment may help discriminate between active and cured disease. The specific reactivity of the electroeluted 72-kDa polypeptide in liposomes with infected kala-azar patient sera suggests the potential sensitivity and specificity of these polypeptides in liposomes for diagnosis, and their lack of reactivity after successful cure may be a powerful tool for the management of kala-azar.
TABLE 2.
Frequency of recognition of LAg-liposomes by acute- and convalescent-phase sera from VL patients
| Mass of antigen (kDa) | % Recognition
|
|
|---|---|---|
| Infected patients | Cured patients | |
| 145 | 6.7 | |
| 110 | 13.3 | |
| 97 | 73.3 | 13.3 |
| 95 | 40.0 | |
| 78 | 73.3 | |
| 72 | 73.3 | |
| 63 | 100.0 | 100.0 |
| 51 | 100.0 | |
| 47 | 86.7 | 20.0 |
| 34 | 60.0 | |
| 31 | 20.0 | |
Continued progress in the immune characterization of VL is critical in our efforts to decipher the complex nature of L. donovani infections for application in diagnosis, prognosis, and vaccine development.
Acknowledgments
We gratefully acknowledge the patients of the School of Tropical Medicine, Calcutta, India, who participated to this study. We express our thanks to the volunteer blood donors of IICB, Calcutta. We thank K. Kabir (Repromed Diagnostic, Calcutta) for providing access to blood samples of malaria, typhoid, and tuberculosis. Thanks are also due to Sreepurna Malakar for assistance in performing the present work.
This work was supported through grants from the CSIR, DST, ICMR, Government of India. R.R. is a research fellow supported by CSIR. We thank Samir Bhattacharya, director, IICB, Calcutta, for supporting this work.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Afonso, L. C. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trincheri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 3.Afrin, F., and N. Ali. 1997. Adjuvanicity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect. Immun. 65:2371-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afrin, F., R. Rajesh, K. Anam, M. Gopinath, S. Pal, and N. Ali. 2002. Characterization of Leishmania donovani antigens encapsulated in liposomes that induce protective immunity in BALB/c mice. Infect. Immun. 70:6697-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali, N., and F. Afrin. 1997. Protection of mice against visceral leishmaniasis by immunization with promastigote antigen incorporated in liposomes. J. Parasitol. 83:70-75. [PubMed] [Google Scholar]
- 6.Alvar, J., C. Cañavate, B. Gutiérrez-Solar, M. Jiménez, F. Laguna, R. Lopez-Vélez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, P. N. Gupta, S. K. Saha, and N. Ali. 1999. Immunoglobulin subclass distribution and diagnostic value of Leishmania donovani antigen-specific immunoglobulin G3 in Indian kala-azar patients. Clin. Diagn. Lab. Immunol. 6:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, S. K. Saha, and N. Ali. 1999. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infect. Immun. 67:6663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora, S. K., and S. Sehgal. 1989. Identification of major antigens of Leishmania donovani using kala-azar sera. Med. Microbiol. Immunol. 178:81-88. [DOI] [PubMed] [Google Scholar]
- 10.Atta, A. M., A. D'Oliviera, Jr., J. Correa, M. L. B. Atta, R. P. Almeida, and E. M. Carvalho. 1998. Anti-leishmanial IgE antibodies: a marker of active disease in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 59:426-430. [DOI] [PubMed] [Google Scholar]
- 11.Bahrenscheer, J., M. Kemp, J. A. L. Kurtzhals, G. S. Gachihi, A. Kharazmi, and T. G. Theander. 1995. Interferon-γ and interleukin-4 production by human T cells recognizing Leishmania donovani antigens separated by SDS-PAGE. APMIS 103:131-139. [PubMed] [Google Scholar]
- 12.Carvalho, E. M., O. Bacellar, C. Brownell, T. Regis, R. L. Coffman, and S. G. Reed. 1994. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J. Immunol. 152:5949-5956. [PubMed] [Google Scholar]
- 13.Carvalho, E. M., R. Badaro, S. G. Reed, T. C. Jones, and W. D. Johnson, Jr. 1985. Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J. Clin. Investig. 76:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 6:102-106. [DOI] [PubMed] [Google Scholar]
- 15.Cenini, P., N. Berhe, A. Hailu, K. McGinnes, and D. Frommel. 1993. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J. Infect. Dis. 168:986-993. [DOI] [PubMed] [Google Scholar]
- 16.Cillari, E., F. Y. Liew, P. L. Campo, S. Milano, S. Mansueto, and A. Salerno. 1988. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J. Immunol. 140:2721-2726. [PubMed] [Google Scholar]
- 17.da Matta, V. R., S. Hoshino-Shimizu, R. Dietze, and C. E. P. Corbett. 2000. Detection of specific antibody isotypes and subtypes before and after treatment of American visceral leishmaniasis. J. Clin. Lab. Anal. 14:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desjeux, P. 1992. Human leishmaniasis: epidemiology and public health aspects. World Health Stat. Q. 45:267-275. [PubMed] [Google Scholar]
- 19.Dos Santos, J. I., M. G. Morgado, and B. Galvao-Castro. 1987. Human visceral leishmaniasis: analysis of the specificity of humoral immune response to polypeptides of Leishmania donovani chagasi. Am. J. Trop. Med. Hyg. 37:263-270. [DOI] [PubMed] [Google Scholar]
- 20.El Assad, A. M. S., S. A. Younis, M. Siddig, J. Grayson, E. Petersen, and H. W. Ghalib. 1994. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin. Exp. Immunol. 95:294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans, T. G., E. C. Krug, M. E. Wilson, A. W. Vasconcelos, J. E. de Alencar, and R. D. Pearson. 1989. Evaluation of antibody responses in American visceral leishmaniasis by ELISA and immunoblot. Mem. Inst. Oswaldo Cruz 84:157-166. [DOI] [PubMed] [Google Scholar]
- 22.Ghalib, H. W., M. R. Piuvezam, Y. A. Skeeiky, M. Siddig, F. A. Hashim, A. M. el-Hassan, D. M. Russo, and S. G. Reed. 1993. Interleukin-10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Investig. 92:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh, A. K., S. Dasgupta, and A. C. Ghose. 1995. Immunoglobulin G subclass-specific antileishmanial antibody responses in Indian kala-azar and post-kala-azar dermal leishmaniasis. Clin. Diagn. Lab. Immunol. 2:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hailu, A., J. N. Menon, N. Berhe, L. Gedamu, T. H. Hassard, P. A. Kager, J. Olobo, and P. A. Bretscher. 2001. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J. Infect. Dis. 184:112-115. [DOI] [PubMed] [Google Scholar]
- 25.Haldar, J. P., S. Ghose, K. C. Saha, and A. C. Ghose. 1983. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect. Immun. 42:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holaday, B. J., M. M. Pompeu, S. Jeronimo, M. J. Texeira, A. A. de Sousa, A. W. Vasconcelos, R. D. Pearson, J. S. Abrams, and R. M. Locksley. 1993. Potential role for interleukin-10 in the immunosupression associated with kala-azar. J. Clin. Investig. 92:2626-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaffe, C. L., and M. Zallis. 1988. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol. Biochem. Parasitol. 27:53-63. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe, C. L., R. Shor, H. Trau, and J. H. Passwell. 1990. Parasite antigens recognized by patients with cutaneous leishmaniasis. Clin. Exp. Immunol. 80:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karp, C. L., S. H. el-Safi, T. A. Wynn, M. M. H. Satti, A. M. Kordofani, F. A. Hashim, M. Hag-Ali, F. A. Neva, T. B. Nutman, and D. L. Sacks. 1993. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J. Clin. Investig. 91:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye, P. M., A. J. Curry, and J. M. Blackwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine induced rate of cure in murine visceral leishmaniasis. J. Immunol. 146:2763-2770. [PubMed] [Google Scholar]
- 33.Kemp, M., J. A. L. Kurtzhals, K. Bendtzen, L. K. Poulsen, M. B. Hansen, D. K. Koech, A. Kharazmi, and T. G. Theander. 1993. Leishmania donovani reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect. Immun. 61:1069-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenny, R. T., D. L. Sacks, A. A. Gam, H. W. Murray, and S. Sundar. 1998. Splenic cytokine responses in Indian kala-azar before and after treatment. J. Infect. Dis. 177:815-819. [DOI] [PubMed] [Google Scholar]
- 35.Kurtzhals, J. A. L., A. S. Hey, T. G. Theander, E. Odera, C. B. V. Christensen, J. I. Githure, D. K. Koech, K. U. Schaefer, E. Handman, and A. Kharazmi. 1992. Cellular and humoral immune responses in a population from the Baringo district, Kenya, to Leishmania promastigote lipophosphoglycan. Am. J. Trop. Med. Hyg. 46:480-488. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Lepay, D. A., N. Nogueira, and Z. Cohn. 1983. Surface antigens of Leishmania donovani promastigotes. J. Exp. Med. 157:1562-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, J. S., and G. M. Winslow. 2003. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect. Immun. 71:4229-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 40.Manson-Bahr, P. E. C. 1961. Immunity in kala-azar. Trans. R. Soc. Trop. Med. Hyg. 55:550-555. [DOI] [PubMed] [Google Scholar]
- 41.Mary, C., D. Lamouroux, S. Dunan, and M. Quilici. 1992. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiological purposes. Am. J. Trop. Med. Hyg. 47:764-771. [DOI] [PubMed] [Google Scholar]
- 42.Mazumder, S., T. Mukerjee, J. Ghosh, M. Ray, and A. Bhaduri. 1992. Allosteric modulation of Leishmania donovani plasma membrane Ca(2+)-ATPase by endogenous calmodulin. J. Biol. Chem. 267:18440-18446. [PubMed] [Google Scholar]
- 43.Miralles, G. D., M. Y. Stoeckle, D. F. McDermott, F. D. Finkelman, and H. W. Murray. 1994. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 62:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, T. C., O. Ekworommadu, F. O. Eko, L. MacMillan, K. Ramey, G. A. Ananaba, J. W. Patrickson, P. R. Nagappan, D. Lyn, C. M. Black, and J. U. Igietseme. 2003. Fc receptor mediated antibody regulation of T cell immunity against intracellular pathogens. J. Infect. Dis. 188:617-624. [DOI] [PubMed] [Google Scholar]
- 45.Okongo-Odera, E. A., J. A. L. Kurtzhals, A. S. Hey, and A. Kharazmi. 1993. Measurement of serum antibodies against native Leishmania gp63 distinguishes between ongoing and previous L. donovani infection. APMIS 101:642-646. [DOI] [PubMed] [Google Scholar]
- 46.Raziuddin, S., R. E. Abdalla, E. H. el-Awad, and M. al-Janadi. 1994. Immunoregulatory and proinflammatory cytokine production in visceral and cutaneous leishmaniasis. J. Infect. Dis. 170:1037-1040. [DOI] [PubMed] [Google Scholar]
- 47.Reed, S. G., and P. Scott. 1993. T-cell and cytokine response in leishmaniasis. Curr. Opin. Immunol. 5:524-531. [DOI] [PubMed] [Google Scholar]
- 48.Reed, S. G., R. Badaro, and R. M. C. Lloyd. 1987. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J. Immunol. 138:1596-1601. [PubMed] [Google Scholar]
- 49.Reed, S. G., W. G. Shreffler, J. M. Burns, Jr., J. M. Scott, M. G. Orge, H. W. Ghalib, M. Siddig, and R. Badaro. 1990. An improved serodiagnosis procedure for visceral leishmaniasis. Am. J. Trop. Med. Hyg. 43:632-639. [DOI] [PubMed] [Google Scholar]
- 50.Rolland, L., V. Zilberfarb, A. Furtado, and M. Gentilini. 1994. Identification of a 94-kilodalton antigen on canine visceral leishmaniasis. Parasite Immunol. 16:599-608. [DOI] [PubMed] [Google Scholar]
- 51.Rolland-Burger, L., X. Rolland, C. W. Grieve, and L. Monjour. 1991. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J. Clin. Microbiol. 29:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothman, P., and R. L. Coffman. 1996. Immunoglobulin heavy chain class-switching, p. 19.1-19.4. In L. A. Herzenberg (ed.), Weir's handbook of experimental immunology, 5th ed. Blackwell Scientific Publications, Oxford, United Kingdom.
- 53.Russell, D. G., and H. Wilhelm. 1986. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J. Immunol. 136:2613-2620. [PubMed] [Google Scholar]
- 54.Russo, D. M., A. Jardim, E. M. Carvalho, P. R. Sleath, R. J. Armitage, R. W. Olafson, and S. G. Reed. 1993. Mapping human T cell epitopes in Leishmania gp63. J. Immunol. 150:932-939. [PubMed] [Google Scholar]
- 55.Ryan, J. R., A. M. Smithyman, G.-H. Rajasekariah, L. Hochberg, J. M. Stiteler, and S. K. Martin. 2002. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J. Clin. Microbiol. 40:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacks, D. L., S. L. Lal, S. N. Shrivastava, J. Blackwell, and F. A. Neva. 1987. An analysis of T cell responsiveness in Indian kala-azar. J. Immunol. 138:908-913. [PubMed] [Google Scholar]
- 57.Salotra, P., A. Raina, and V. Ramesh. 1999. Western blot analysis of humoral immune response to Leishmania donovani antigens in patients with post kala-azar dermal leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 93:98-101. [DOI] [PubMed] [Google Scholar]
- 58.Shiddo, S. A., G. Huldt, L.-A. Nilsson, O. Quchterlony, and R. Thorstensson. 1996. Visceral leishmaniasis in Somalia. Significance of IgG subclasses and of IgE response. Immunol. Lett. 50:87-93. [DOI] [PubMed] [Google Scholar]
- 59.Shreffler, W. G., J. M. Burns, Jr., R. Badaro, H. W. Ghalib, L. L. Button, W. R. McMaster, and S. G. Reed. 1993. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J. Infect. Dis. 167:426-430. [DOI] [PubMed] [Google Scholar]
- 60.Smrkovski, L. L., and C. L. Larson. 1977. Antigenic cross-reactivity between Mycobacterium bovis (BCG) and Leishmania donovani. Infect. Immun. 18:561-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundar, S., S. G. Reed, S. Sharma, A. Mehrotra, and H. W. Murray. 1997. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 56:522-525. [DOI] [PubMed] [Google Scholar]
- 62.Tebourski, F., A. El Gaied, H. Louzir, R. Ben Ismail, R. Kammoun, and K. Dellagi. 1994. Identification of an immunodominant 32-kilodalton membrane protein of Leishmania donovani infantum promastigotes suitable for specific diagnosis of Mediterranean visceral leishmaniasis. J. Clin. Microbiol. 32:2474-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uzonna, J. E., G. Wei, D. Yurkowski, and P. Bretscher. 2001. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167:6967-6974. [DOI] [PubMed] [Google Scholar]
- 64.Wray, W., T. Boulikas, V. P. Wray, and R. Hancock. 1981. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 118:197-203. [DOI] [PubMed] [Google Scholar]
- 65.Zwingenberger, K., G. Harms, C. Pedrosa, S. Omena, B. Sandkamp, and S. K. Neifer. 1990. Determinants of the immune response in visceral leishmaniasis: evidence for the predominance of endogenous interleukin 4 over interferon gamma production. Clin. Immunol. Immunopathol. 57:242-249. [DOI] [PubMed] [Google Scholar]