Abstract
Campylobacter jejuni continues to be a leading cause of bacterial enteritis in humans. However, because there are no readily available animal models to study the pathogenesis of C. jejuni-related diseases, the significance of potential virulence factors, such as cytolethal distending toxin (CDT), in vivo are poorly understood. Mice deficient in NF-κB subunits (p50−/− p65+/−) in a C57BL/129 background are particularly susceptible to colitis induced by another enterohepatic microaerobe, Helicobacter hepaticus, which, like C. jejuni, produces CDT. Wild-type C. jejuni 81-176 and an isogenic mutant lacking CDT activity (cdtB mutant) were inoculated into NF-κB-deficient (3X) and C57BL/129 mice. Wild-type C. jejuni colonized 29 and 50% of the C57BL/129 mice at 2 and 4 months postinfection (p.i.), respectively, whereas the C. jejuni cdtB mutant colonized 50% of the C57BL/129 mice at 2 p.i. but none of the mice at 4 months p.i. Although the C57BL/129 mice developed mild gastritis and typhlocolitis, they had robust immunoglobulin G (IgG) and Th1-promoted IgG2a humoral responses to both the wild-type strain and the C. jejuni cdtB mutant. In contrast, 75 to 100% of the 3X mice were colonized with both the wild type and the C. jejuni cdtB mutant at similar levels at all times examined. Wild-type C. jejuni caused moderately severe gastritis and proximal duodenitis in 3X mice that were more severe than the gastrointestinal lesions caused by the C. jejuni cdtB mutant. Persistent colonization of NF-κB-deficient mice with the wild type and the C. jejuni cdtB mutant was associated with significantly impaired IgG and IgG2a humoral responses (P < 0.001), which is consistent with an innate or adaptive immune system defect(s). These results suggest that the mechanism of clearance of C. jejuni is NF-κB dependent and that CDT may have proinflammatory activity in vivo, as well as a potential role in the ability of C. jejuni to escape immune surveillance. NF-κB-deficient mice should be a useful model to further study the role of CDT and other aspects of C. jejuni pathogenesis.
Because of the importance of Campylobacter jejuni as a primary enteric pathogen in humans, mice have been used in numerous in vivo experiments involving C. jejuni. However, with few exceptions, oral dosing of different strains of mice with a variety of C. jejuni strains has resulted in intestinal colonization and in some cases bacteremia, but there has been a lack of consistent development of gastroenteritis in the models to date (42).
NF-κB is a family of proteins that form homo- or heterodimer complexes that regulate transcription of proinflammatory genes (6). These NF-kB complexes are members of the Rel protein family, which includes p50, p65, cRel, Relb, and p52. Several mouse models lacking NF-κB family members have been developed. Mice lacking p65 subunits die during embryogenesis, whereas mice homozygously deficient for p50 (p50−/−) and also heterozygous for p65 (p50−/− p65+/−), referred to as 3X mice, are viable. Both p50−/− and p50−/− p65+/− mice developed spontaneous typhlocolitis when they were maintained as a virus antibody-free colony but were infected with Helicobacter spp. (6). Rederived Helicobacter-free mice with these genotypes that were subsequently experimentally infected with Helicobacter hepaticus for 6 weeks developed severe colitis with increased proinflammatory cytokine expression; this was particularly true for infected 3X mice and, to a lesser extent, for p50−/− mice. C57BL/129 mice and p65+/− mice were clinically unaffected. These data indicated that p50 and p65 subunits of NF-κB had an unexpected role in inhibiting the development of colitis (6). These observations augmented studies demonstrating that H. hepaticus could induce lower-bowel inflammation in a variety of immune dysregulated mice (3, 6, 7, 22).
A bacterial toxin that causes cell cycle arrest in the G2/M phase with progressive distension and death of Chinese hamster ovary cells, termed cytolethal distending toxin (CDT), was first described by Johnson and Lior in an enteropathogenic strain of Escherichia coli (17). Toxins belonging to the same group were later identified in several other diarrheagenic bacteria, including Campylobacter spp. (Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus) (16, 28), other pathogenic strains of E. coli (2, 26, 27, 33), Shigella spp. (24), and a variety of enterohepatic helicobacters, including H. hepaticus (4, 40). The genes encoding this toxin were identified as a cluster of three adjacent genes, cdtA, cdtB, and cdtC, which produce 28-, 30, and 20-kDa proteins, respectively (27, 33). To date, there has been only one description of the colonization efficacy or ability to induce disease in SCID Beige mice with a wild-type C. jejuni strain and isogenic counterparts lacking CDT activity (30).
We therefore designed a set of experiments to ascertain whether a pathogenic strain of C. jejuni (strain 81-176) was also capable of experimentally producing gastrointestinal disease in the 3X mouse model (1, 32). Furthermore, because a pilot experiment indicated that C. jejuni induced gastrointestinal lesions in 3X mice, we also determined in a subsequent experiment if an isogenic mutant of C. jejuni lacking CDT (cdtB mutant) could colonize wild-type and 3X mice and whether the C. jejuni cdtB mutant induced less pathology in the gastrointestinal tract than the wild-type C. jejuni strain induced.
MATERIALS AND METHODS
Animals and housing.
Specific-pathogen-free (free of antibodies to 11 murine viruses, endo- and ectoparasites, Citrobacter rodentium, Salmonella spp., and Helicobacter spp.), 4-week-old, NF-κB-deficient 3X mice and wild-type mice with the same mixed background (129 × C57BL/6) were obtained from a barrier-maintained breeding colony at the Massachusetts Institute of Technology. The mice were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care and were housed in polycarbonate microisolator cages and given food and water ad libitum.
Bacterial strains and culture conditions.
Wild-type C. jejuni strain 81-176, previously demonstrated to cause clinical disease in humans and nonhuman primates, was used (1, 32). An isogenic mutant of this strain lacking the functional B subunit of CDT (cdtB mutant) was also orally inoculated into mice. Species identification was based on routine biochemical characterization (including oxidase, catalase, and urease activity, hippurate, and indoxyacetate hydrolysis tests and sensitivity to nalidixic acid and cephalothin), and identities were confirmed by PCR by using species-specific primers.
The C. jejuni wild-type strain and the C. jejuni cdtB mutant were grown on blood agar at 37°C under microaerobic conditions. For experimental inoculation, bacteria were harvested after 48 h of growth and resuspended in Trypticase soy broth, and the optical density at 660 nm (OD660) was determined. Tenfold dilutions of the inoculum were plated onto blood agar plates, and the results showed that an OD660 of 1.0 was equivalent to ∼108 CFU of C. jejuni.
Construction of C. jejuni 81-176 cdtB mutants. (i) Cloning and insertional mutagenesis of C. jejuni cdt genes.
Two primers (forward primer 5′-ATTTGAAGATACTGATCCTT-3′ and reverse primer 5′-TTGCACAGCTGAAGTTGT-3′) were derived from the cdtA and cdtC genes of C. jejuni 81-176 (28). A 1.88-kb DNA fragment was amplified from C. jejuni 81-176 chromosomal DNA with Taq DNA polymerase as described previously (11). Subsequently, the PCR product was cloned into pCR 2.1-TOPO by using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.). Clones containing the cdt insert were identified by fluorescent DNA sequencing with an ABI 310 sequencer (PE Biosystems, Foster City, Calif.).
A single HincII recognition site, which is present in the C. jejuni cdtB gene and which encodes the catalytic subunit of the CDT, was used for insertional mutagenesis. Briefly, the plasmid DNA containing the cdt insert was digested with HincII, and this was followed by ligation with the HincII-digested cat cassette (chloramphenicol resistance) characterized originally from C. coli (37). After transformation, recombinants containing the cdtB knockout were selected by both ampicillin and chloramphenicol resistance on Luria-Bertani agar. The orientation of the cat cassette in cdtB was determined by DNA sequencing.
(ii) Creation of cdtB mutant by natural competence.
Isogenic mutants of C. jejuni 81-176 with defects in catB were created by a previously described procedure, with some modifications (38). Briefly, freshly grown C. jejuni cells were spotted heavily on sheep blood agar (Remel, Lenexa, Kans.), and this was followed by incubation for 6 h at 37°C under microaerobic conditions. Ten microliters of TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 7.5) containing 0.5 to 1 μg of plasmid DNA was dropped on bacterial spots. After overnight incubation, transformants (single colonies) were selected on brain heart infusion agar containing yeast extract supplemented with 25 μg of chloramphenicol per ml (11). A mutant with a defect in cdtB was verified by PCR amplification and DNA sequencing. The CDT activities of both parental strain C. jejuni 81-176 and the mutant were assayed by using HeLa cells and by flow cytometry (see below).
Experimental infection.
In a pilot experiment, 18 3X mice (7 males and 11 females) that were 6 weeks old were dosed with ∼108 CFU of C. jejuni suspended in 0.2 ml of brucella broth by oral gavage. The animals were evaluated at 10 and 15 weeks postinfection (p.i.). In a subsequent experiment, 16 3X mice that were 6 weeks old were dosed with the C. jejuni cdtB mutant, and an equal number of mice were dosed with the wild-type C. jejuni strain. Another 32 C57BL/129 mice were dosed with either the wild-type C. jejuni strain or the C. jejuni cdtB mutant. An additional 16 C57BL/129 mice and 16 3X mice were sham dosed with brucella broth and served as controls. At necropsy, these mice were analyzed at 2 and 4 months p.i.
Necropsy and histopathology.
In the pilot experiment, mice were euthanized with CO2 at 10 and 15 weeks p.i., and in the follow-up experiment, eight mice from each experimentally infected group plus four wild-type mice and four 3X control mice were euthanized at 2 and 4 months p.i. At necropsy, the gastric luminal contents were removed, and the mucosa was rinsed with saline. Linear strips extending from the gastric squamocolumnar junction through the proximal duodenum were collected along the lesser curvature, placed flat on a card, and fixed in 10% neutral-buffered formalin. The ileocecocolic junction containing ∼1 cm of attached ileum, cecum, and proximal colon was excised from the rest of the intestinal tract. Cecal and proximal colonic luminal contents were manually expressed, and the lumen was flushed with formalin. Distal colon, mesenteric lymph nodes, and thin sections of each liver lobe were also collected. Tissues were fixed in formalin overnight and embedded in paraffin, and 5-μm sections were cut and stained with hematoxylin and eosin (H&E) or with Warthin-Starry silver stain. Selected samples were also stained with periodic acid-Schiff stain (PAS)-Alcian blue (pH 2.5). Specific identities of bacteria in tissues were confirmed by immunohistochemistry by using a mouse monoclonal anti-C. jejuni antibody (Novocastra Laboratories, Newcastle-upon-Tyne, United Kingdom) and an ARK kit (DAKO, Carpinteria, Calif.).
Lesions were evaluated by a comparative pathologist blinded to the sample source and were graded semiquantitatively on a scale from 0 to 4 as described previously (8, 10). The stomach was graded for inflammation, hyperplasia, and dysplasia, while the duodenum, colon, and liver were assigned grades only for inflammation.
Statistical comparison of lesion severity.
Mean lesion scores were statistically compared for groups of wild-type and 3X mice with or without one of the two C. jejuni strains (a total of six groups). The Kolmogorov-Smirnov test demonstrated that the lesion score distribution was not Guassian within all groups; therefore, the Kruskal-Wallis (nonparametric one-way analysis of variance) test was used to compare all six cohorts at 2 and 4 month p.i. For direct comparisons of cohort pairs infected with either the wild type or the C. jejuni cdtB mutant at a given time, the Mann-Whitney test was used. The statistical analysis of C. jejuni colonization in different groups consisted of a paired Student's t test with equal variances. Statistical analyses were performed by using the Prism 3.0 software for the Macintosh (GraphPad, San Diego, Calif.).
Microbiological culture.
Fecal pellets were collected from both infected and control mice prior to euthanasia. Feces were homogenized in 0.5 ml of sterile phosphate-buffered saline (PBS), and 50 μl was plated on Trypticase soy agar with cefoperazone, vancomycin, and amphotericin B (TSA-CVA) plates (Remel). A portion of the corpus of the stomach was also cultured. For quantitative culture, gastric tissue was weighed and then homogenized by using glass tissue grinders, and the homogenate was diluted 100- and 1,000-fold in brucella broth containing fetal calf serum. One hundred microliters of each dilution was spread on CVA medium (Remel). The plates were incubated microaerobically at 37°C for 2 days. After verification by colony morphology, Gram staining, and urease and catalase reactions, the C. jejuni colonies were counted, and the number of CFU per gram of tissue was calculated. Comparisons between groups were based on the log concentrations of the bacteria.
Characterization of the genotypes of C. jejuni isolates from mice.
C. jejuni 81-176 and the C. jejuni cdtB mutant were isolated from the ceca of infected mice. Chromosomal DNA from bacterial cells was prepared by using a High Pure PCR template preparation kit according to the supplier's protocol (Roche Diagnostics Corporation, Indianapolis, Ind.). PCR amplification was performed as described above.
CDT assay. (i) Preparation of cell sonicates.
Bacteria were grown on blood agar plates (Remel) under microaerobic conditions, and sonicates were prepared as previously described (41). Sonicates were stored at −70°C until they were used.
(ii) Tissue culture assay for morphological changes.
One milliliter of HeLaS3 cells at a concentration of 103 cells/ml of Eagle minimal essential medium with Earle's salts (Sigma Chemical Company, St. Louis, Mo.) containing 10% fetal calf serum (Summit Technology, Ft. Collins, Colo.) was placed in each chamber of Lab-Tek 2 chamber slides (Nalge Nunc International, Naperville, Ill.). After incubation for 1 h at 37°C in the presence of 6% CO2, 10 μl of filter-sterilized sonicate was added to each chamber. The slides were incubated for 72 h and were then washed with PBS, stained with Diff-Quick (Dade International, Miami, Fla.), and observed microscopically.
(iii) Flow cytometry.
Tissue culture flasks (25 cm2) were seeded with 5 ml of Eagle minimal essential medium with Earle's salts containing 10% fetal calf serum and 1 × 105 HeLaS3 cells/ml. After 1 h of incubation at 37°C in the presence of 6% CO2, 100 μl of filter-sterilized sonicate was added to each flask, and the flasks were incubated for 72 h. Cells were detached and stained, and 1 × 104 cells were analyzed as previously described (4). DNA analysis was carried out by using a FACScan flow cytometer (Beckman Dickenson, Franklin Lakes, N.J.) with the Cell Quant software (Beckton Dickinson) for data acquisition. The ModFit program was used for data analysis.
Evaluation of serum antibody responses to C. jejuni.
Blood was collected at necropsy from CO2-euthanatized mice infected with the wild type or the C. jejuni cdtB mutant via cardiac puncture at 2 and 4 months p.i. Enzyme-linked immunosorbent assays (ELISAs) for serum immunoglobulin G (IgG) and IgG2a (Th1-promoted isotype in C57BL/129 mice) and IgG1 (Th2-promoted isotype) to C. jejuni were developed. Briefly, C. jejuni was harvested from plates, washed three times, and resuspended in sterile PBS. The suspension was sonicated on ice (Artek Sonic Dismembranator; Artek Systems, Framingdale, N.Y.) for four cycles consisting of 30 s on and 30 s off at a duty cycle of 50% and at a power setting of 60 W. After sonication, the mixture was examined by phase-contrast microscopy to confirm the absence of intact bacteria, and this was followed by determination of the protein concentration of the sonicate with a Sigma P5656 protein assay kit. Sonicate antigen from wild-type C. jejuni and the C. jejuni cdtB mutant were coated overnight at 4°C onto Immulon II plates (Thermo Labsystems, Franklin, Mass.) at a concentration of 1 μg/ml for serum IgG and at a concentration of 10 μg/ml for subclass isotyping of the IgG response. Plates were blocked with PBS-2% bovine serum albumin overnight at 4°C. Sera were diluted 1:100, and the biotinylated secondary antibodies included goat anti-mouse IgG (Southern Biotechnology Associates, Inc., Birmingham, Ala.) diluted 1:4,000. Monoclonal anti-mouse antibodies produced by clones G1-6.5 and R19-15 (Pharmingen, San Diego, Calif.) for detection of IgG1 and IgG2a, respectively, were used at a dilution of 1:1,000. Incubation with extravidin peroxidase (Sigma) at a dilution of 1:2,000 was followed by incubation with the 2,2′azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for color development. The OD405 was recorded with an ELISA plate reader (Dynatech MR7000; Dynatech Laboratories, Inc., Chantilly, Va.). Sera from all mice were first tested for serum IgG to sonicate prepared from both the wild-type strain and the C. jejuni cdtB mutant with a high degree of correlation in the ELISA optical density values obtained (r2 = 0.99 [data not shown]). Data were subsequently analyzed based on optical density values obtained from serum IgG, IgG1, and IgG2a responses to each experimental infection (C. jejuni wild type or C. jejuni cdtB mutant). Sera from uninfected controls were tested against wild-type C. jejuni sonicate antigens. Data were analyzed by analysis of variance.
RESULTS
Wild-type C. jejuni and the C. jejuni cdtB mutant persistently infect 3X mice but not C57BL/129 mice.
To ascertain the feasibility of wild-type C. jejuni colonization and development of gastrointestinal disease in 3X mice, a pilot study was undertaken. One mouse developed a rectal prolapse 6 weeks after experimental infection. Mice were colonized in both the colon and stomach at 10 weeks p.i., and bacteria which stained positive with the Warthin-Starry silver stain were present in the glandular crypts of inflamed stomachs of all the 3X mice analyzed at 15 weeks p.i. C. jejuni was not isolated from either the mesenteric lymph or the liver in mice analyzed 10 weeks p.i.
In the second experiment, at necropsy wild-type C. jejuni was cultured from both the stomachs and feces of six of eight (75%) of the 3X mice at 2 months p.i. and from both the stomachs and feces of seven of seven (100%) of the mice at 4 months p.i. (Table 1). In contrast, the cdtB mutant was cultured from seven of seven (100%) of the 3X mice at both 2 and 4 months p.i. C57BL/129 mice had lower colonization indices for both the wild-type C. jejuni strain and the cdtB mutant. At 2 months p.i., wild-type C. jejuni colonized two of seven (29%) of the C57BL/129 mice examined at necropsy, and four of eight (50%) of the C57BL/129 mice were colonized with the C. jejuni cdtB mutant. For the groups of mice examined at necropsy at 4 months p.i., none of the C57BL/129 mice were colonized with the mutant C. jejuni strain and only 50% were colonized with the wild-type C. jejuni strain.
TABLE 1.
Colonization indices for wild-type and cdtB mutant C. jejuni in NF-κB-deficient (3X) and C57BL/129 mice
| Mouse strain | No. colonized/no. tested
|
|||
|---|---|---|---|---|
| Wild-type C. jejuni
|
C. jejuni cdtB mutant
|
|||
| 2 mo p.i.a | 4 mo p.i.a | 2 mo p.i. | 4 mo p.i. | |
| 3X | 6/8 | 7/7b | 7/7b | 7/7b |
| C57BL/129 | 2/7b | 4/8 | 4/8 | 0/8 |
Mice were cultured for 2 and 4 months postinoculation and euthanized.
One mouse from the group was not included because of death unrelated to the experiment.
At 2 months p.i., wild-type C. jejuni was isolated from the liver of one male 3X mouse, whereas at 4 months p.i., the C. jejuni cdtB mutant was isolated from two male 3X mice and the wild-type C. jejuni strain was cultured from the liver of one female 3X mouse. None of the sham-dosed 3X or C57BL/129 mice were colonized with C. jejuni.
Quantitative culture.
At 4 months p.i. the gastric colonization densities of the wild-type C. jejuni strain and the cdtB mutant in 3X mice were similar. Wild-type C. jejuni colonized all 3X mice to the same extent as wild-type C. jejuni colonized the four of eight C57BL/129 mice still colonized at 4 months p.i. (Table 2). However, the C. jejuni cdtB mutant was eliminated from all the C57BL/129 mice at 4 months p.i. but persistently colonized all 3X mice at 4 months p.i.
TABLE 2.
Wild-type C. jejuni and the cdtB mutant in NF-κB-deficient (3X) and C57BL/129 mice at 4 months p.i.
| Group | CFU/mg of stomach (mean ± SD) | P < value log CFU |
|---|---|---|
| 3X mice, wild-type C. jejuni | 418 ± 703 | 0.3092a |
| 3X mice, C. jejuni cdtB mutant | 859 ± 1,675 | |
| C57BL/129 mice, wild-type C. jejuni | 544 ± 857 | 0.8922b |
Value for 3X mice infected with wild-type C. jejuni versus 3X mice infected with the C. jejuni cdtB mutant.
Value for 3X mice infected with wild-type C. jejuni versus C57BL/129 mice infected with wild-type C. jejuni.
Verification of isogenic mutant status of the C. jejuni cdtB mutant isolated from mice.
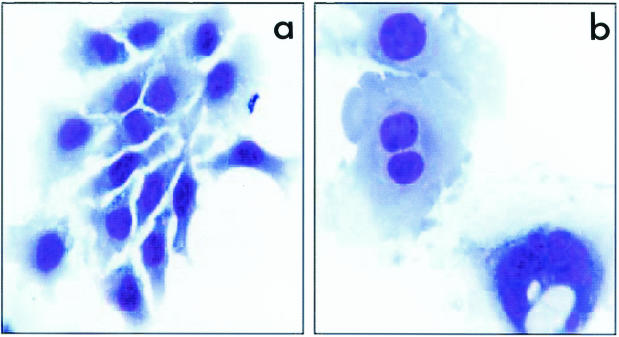
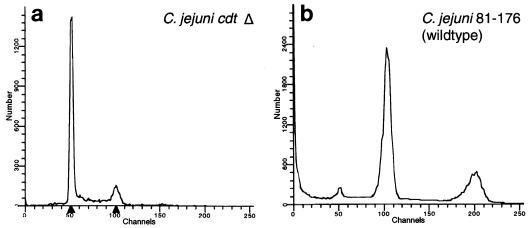
It has been reported that the bacterial toxin B subunit (CdtB) plays a crucial role in CDT activity (5, 21). We inactivated the cdtB gene in C. jejuni 81-176 by using the cat cassette. The transcriptional orientation of the cat cassette in the mutant was opposite that of the cdt operon. The cdtB mutant did not exhibit toxin activity, as demonstrated by the lack of cytopathic effects on HeLa cells and by the lack of cell cycle arrest, as demonstrated by flow cytometry (Fig. 1 and 2).
FIG. 1.
Geimsa-stained HeLa cells exposed to PBS or filter-sterilized supernatant fractions of cell sonicates of the C. jejuni cdtB mutant (a) and C. jejuni wild-type strain 81-176 (b). The C. jejuni cdtB mutant and the PBS control caused no morphological changes in cells. The wild-type C. jejuni strain produced cytoplasmic and nuclear distensions characteristic of CDT activity.
FIG. 2.
DNA content of HeLa cells as analyzed by flow cytometry. HeLa cells were exposed for 72 h to PBS or bacterial sonicates from the C. jejuni cdtB mutant (Δ) (a) or C. jejuni wild-type strain 81-176 (b). Compared to negative control cells, the C. jejuni cdtB mutant-treated cells showed no increase in cells with a 4N DNA content. In contrast, with the C. jejuni wild type 61% of the cells were in the G2/M phase.
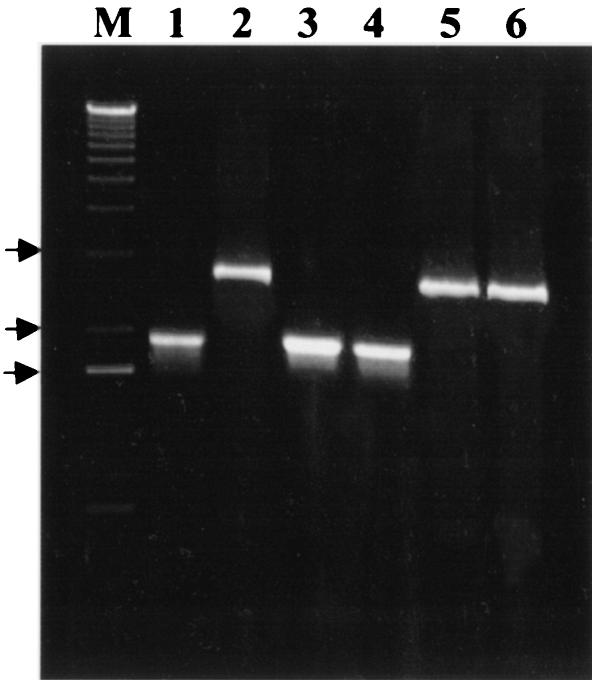
The genotype of the cdtB mutant was confirmed by PCR with the primers mentioned above. A 1.88-kb amplicon identical to that from the wild-type cdt plasmid template containing the insert (Fig. 3, lane 1) was produced from C. jejuni isolates from mice inoculated with the wild type (lanes 3 and 4), whereas a 2.7-kb amplicon identical to that from the mutated cdt plasmid template (lane 2) was generated from isolates from mice inoculated with the cdtB mutant (lanes 5 and 6).
FIG. 3.
Detection of the C. jejuni cdt operon with the primers described in the text. Lanes 1 and 2, recombinant plasmid DNA containing either the wild-type (lane 1) or the mutated (lane 2) cdt insert; lanes 3 and 4, isolates from mice infected with the C. jejuni wild type; lanes 5 and 6, isolates from mice infected with the C. jejuni cdtB mutant. Lane M contained a 1-kb plus molecular size ladder, and the arrows indicate the positions of 3, 2, and 1.6 kb (from top to bottom).
Histopathology.
The characteristics of gastrointestinal and hepatic lesions were generally similar in the 3X mouse groups in this study, and the lesions varied mainly in severity. In the first experiment, wild-type C. jejuni infection of 3X mice resulted in mild to moderately severe gastritis and proximal duodenitis at the time of necropsy 10 to 15 weeks p.i., with milder inflammatory lesions in the large intestine and liver.
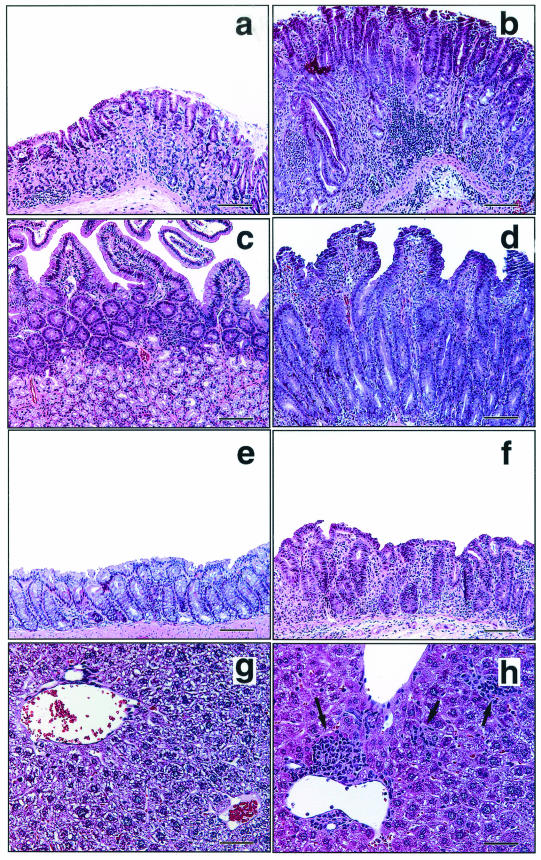
In the second experiment, infection with the C. jejuni cdtB mutant resulted in disease which was slightly less severe than the disease caused by wild-type C. jejuni at 2 months p.i. However, by 4 months p.i., 3X mice infected with the C. jejuni cdtB mutant exhibited less severe gastritis lesions despite being colonized, while mice infected with wild-type C. jejuni exhibited progressively worsening disease (Fig. 4). The C57BL/129 mice infected with either wild-type C. jejuni or the C. jejuni cdtB mutant developed mild disease at 2 months p.i., which was not present in the wild-type mice at 4 months p.i. (data not shown).
FIG.4.
Histopathology of 3X mice infected with the C. jejuni cdtB mutant or wild-type C. jejuni at 4 months p.i. There was little or no inflammation of the stomach (a), duodenum (c), colon (e), or liver (g) in mice infected with the C. jejuni cdtB mutant. In contrast, mice infected with wild-type C. jejuni exhibited marked chronic active gastritis with glandular atrophy, epithelial hyperplasia, and mild dysplasia (b), duodenitis with similar glandular and epithelial changes (d), moderate colitis with edema (f), and mild multifocal hepatic lobular and portal histologic activity (arrows) (h). The sections were stained with H&E. (a and b) Bars = 250 μm; (c to f) bars = 100 μm; (g and h) bars = 50 μm.
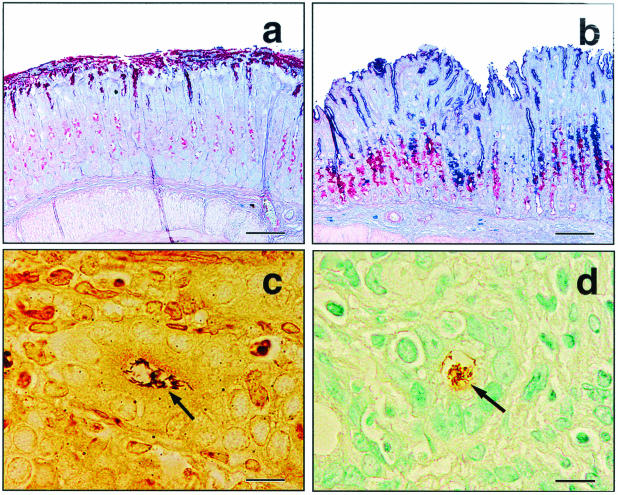
In the stomachs and proximal duodena of 3X mice infected with wild-type C. jejuni, inflammatory cells diffusely infiltrated the mucosa and submucosa, and the highest concentrations were found near the muscularis mucosae. Leukocytic infiltrates were comprised chiefly of lymphocytes and polymorphonuclear cells, and eosinophils made up a significant fraction of these cells. Inflammatory foci also contained some macrophages, mast cells, and plasma cells. With H&E staining bright 0.5- to 3-μm eosinophilic droplets in gastric surface epithelial cells, which were confined to the immediate cardiac region in uninfected mice, sometimes extended the full length of the corpus in infected mice with significant gastritis. In severe cases of gastritis and duodentitis there were submucosal edema and perivascular inflammation following linear perimyseal tracks through the tunica muscularis to the serosa, with extension into the adjacent mesentery. In the gastric corpus of infected mice there was expansion in the midglandular region of foamy or mucous neck-type cells, and there was a concomitant loss of parietal cells. Decreased numbers of chief cells invariably accompanied this change. Alcian blue-PAS staining demonstrated that there was upregulation of both endogenous neutral gastric mucins (PAS positive) and metaplastic acidic intestinal-type mucins (Alcian blue positive) (Fig. 4a and b). In most instances of gastritis there was little evidence of reactive epithelial hyperplasia. In some mice there was multifocal gastric dysplasia characterized by dilation of glands with variably attenuated or elongated epithelial lining cells with formation of crescentic glands and pits, sometimes with central plugs of degenerate and necrotic cells and debris (Fig. 4b). Rare glands exhibited epithelial cell piling up to three cells deep. Small (1 by 3 to 4 μm) spiral and comma-shaped C. jejuni cells were visualized on the surfaces and in glands of the stomach and the duodenum with Warthin-Starry stain and by immunohistochemistry (Fig. 5c and d).
FIG. 5.
Stomach tissue from 3X mice infected for 4 months with the C. jejuni cdtB mutant (a) or wild-type C. jejuni (b) stained for gastric-type (red) and intestinal-type (blue) mucins. Note the normal intense red staining of gastric-type (nonacidic) mucins on the surface with scattered weakly mucus-producing cells in the glandular zone of a mouse infected with the cdtB mutant (a). In contrast, a mouse infected with wild-type C. jejuni exhibited metaplasia to intestinal-type (acidic) mucins (dark blue) on the surface and in the upper glandular zone and increased production of gastric-type mucins (dark red) in a deeper glandular zone (b). Small comma-shaped and spiral argyrophilic bacteria were seen in gastric glands of C. jejuni-infected mice (c) but not uninfected mice (data not shown). Organisms were confirmed to be C. jejuni by immunohistochemistry (d). (a and b) Alcian blue-PAS stain. Bars = 100 μm. (c) Warthin-Starry stain. Bar = 10 μm. (d) Immunoperoxidase stain with 3,3-diaminobenzidine chromogen and methyl green counterstain. Bar = 10 μm.
Lesions in the large intestines and livers of both 3X and C57BL/129 mice were generally mild to moderate (Fig. 4). Typhlocolitis was characterized by small to moderate numbers of lymphocytes and polymorphonuclear cells in the lamina propria and/or submucosa, usually centered at the base of the cecum, with variable extensions into the proximal colon. Similar inflammatory populations formed microscopic periportal and random nodules in the livers of some infected mice. Small numbers of apoptotic or necrotic hepatocytes accompanied the inflammatory foci.
Statistical comparison of lesion severity.
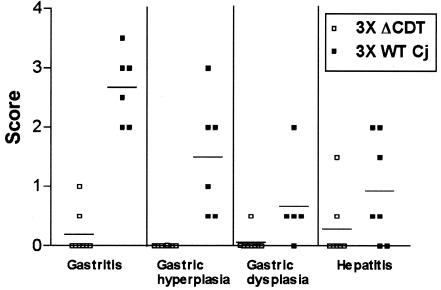
There were no significant differences in lesion severity between males and females within any cohort (data not shown); therefore, gender data were combined to establish group means (Fig. 6). As determined by the Kruskal-Wallis test, there were significant differences (P < 0.04) between groups for every gastric and hepatic lesion parameter assessed at both 2 and 4 month p.i. except for gastric dysplasia at 2 months p.i. (P = 0.09). Because of the mild and inconsistent presence of inflammation in the large bowel, there were no statistically significant differences in typhlocolitis between groups (data not shown). Although the mean gastritis scores in C57BL/129 mice infected with wild-type C. jejuni were relatively low, they remained statistically significant compared to the scores for mice infected with the C. jejuni cdtB mutant (P < 0.05). Lesion parameters other than gastritis were not statistically different for different groups of C57BL/129 mice infected with the wild type or the C. jejuni cdtB mutant. In 3X mice, the difference between the gastritis severity in mice infected with the wild-type strain and the gastritis severity in mice infected with the C. jejuni cdtB mutant approached, but did not achieve, statistical significance at 2 months p.i. (P = 0.07), but the difference was highly significant by 4 months p.i. (P = 0.001). Gastric hyperplasia and dysplasia were also significantly greater (P < 0.02) in 3X mice infected with wild-type C. jejuni than in 3X mice infected with the C. jejuni cdtB mutant at 4 months p.i. Proximal duodenitis scores could not be compared statistically due to insufficient tissue representation on a subset of slides, but lesions in this region generally mirrored inflammation severity in the stomach. For all of the gastric parameters assessed, the wild type but not the C. jejuni cdtB mutant induced significantly higher lesion scores in 3X mice than in C57BL/129 mice (P < 0.05).
FIG. 6.
Gastric lesions and hepatitis severity scores in 3X mice infected for 4 months with wild-type C. jejuni (3X WT Cj) or the C. jejuni cdtB mutant (3X ΔCDT).
Seroconversion to C. jejuni.
None of the sera from uninfected control C57BL/129 or 3X mice contained antibody reactive to C. jejuni antigens in the ELISA (data not shown). The C57BL/129 mice that were persistently colonized with the wild type or the C. jejuni cdtB mutant developed robust serum IgG responses that were similar in magnitude (P = 0.53) and much higher (P < 0.001) than the responses in 3X mice that were persistently colonized with either the wild type or the C. jejuni cdtB mutant (Table 3). Serum IgG levels were lower in C57BL/129 mice that had eliminated the infection by 2 months p.i. (P < 0.04). C57BL/129 mice that had eliminated the infection with wild-type C. jejuni by 4 months p.i. still had an elevated titer, suggesting that there was loss of colonization between 2 and 4 months p.i. The Th1-promoted IgG2a responses of C57BL/129 and 3X mice followed the same pattern; the responses were elevated in C57BL/129 mice that were persistently colonized, and there was a trend for lower antibody levels in mice that were dosed with the C. jejuni cdtB mutant (P = 0.17). Th2-promoted IgG1 antibody responses were detectable only in sera obtained at 2 months p.i. from C57BL/129 mice colonized with the C. jejuni cdtB mutant.
TABLE 3.
ELISA optical density values obtained from testing sera obtained from C57BL/129 and NF-κB-deficient (3X) mice at 2 and 4 months after infection with wild-type C. jejuni or the cdtB mutant
| Immuno- globulin | Colonized | OD (mean ± SE)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type C. jejuni
|
C. jejuni cdtB mutant
|
||||||||
| C57BL/129 mice
|
NF-κB-deficient mice
|
C57BL/129 mice
|
NF-κB-deficient mice
|
||||||
| 2 mo p.i. | 4 mo p.i. | 2 mo p.i. | 4 mo p.i. | 2 mo p.i. | 4 mo p.i. | 2 mo p.i. | 4 mo p.i. | ||
| IgG | + | 1.92 ± 0.17 | 2.11 ± 0.19 | 0.46 ± 0.17 | 0.17 ± 0.11 | 2.20 ± 0.12 | 0.17 ± 0.14 | 0.06 ± 0.02 | |
| − | 0.98 ± 0.31 | 2.26 ± 0.15 | 1.65 ± 0.22 | 0.84 ± 0.22 | 0.84 ± 0.22 | ||||
| IgG2a | + | 0.80 ± 0.29 | 0.50 ± 0.14 | 0.06 ± 0.04 | 0 ± 0 | 0.52 ± 0.10 | 0.02 ± 0.01 | 0.01 ± 0.01 | |
| − | 0.10 ± 0.09 | 0.49 ± 0.17 | 0.49 ± 0.16 | 0.05 ± 0.02 | |||||
| IgG1 | + | 0 ± 0 | 0.03 ± 0.03 | 0 ± 0 | 0 ± 0 | 0.41 ± 0.37 | 0.10 ± 0 | 0 ± 0 | |
| − | 0 ± 0 | 0.01 ± 0.01 | 0.01 ± 0 | 0.06 ± 0.03 | |||||
DISCUSSION
Partially purified preparations of Shigella dysenteriae CDT are capable of causing diarrhea with colonic erosions and reparative enterocyte hyperplasia in suckling mice (23). In the present study, the C. jejuni cdtB mutant failed to persistently colonize C57BL/129 mice but colonized the stomachs and lower bowels of all 3X mice at 2 and 4 months p.i. However, 3X mice colonized with the C. jejuni cdtB mutant had significantly less gastritis and hyperplasia at 4 months p.i. than 3X mice colonized with wild-type C. jejuni, which also colonized 100% of the 3X mice. Unlike the INS-GAS mouse model of gastric Helicobacter spp. infection, there was not a significant gender effect in 3X mice infected with C. jejuni (10). The data which show that there was attenuated inflammation in 3X mice infected with the C. jejuni cdtB mutant support in vitro studies reporting that C. jejuni CDT induces interleukin-8, a proinflammatory cytokine, from INT407 cells (13).
Also, the C. jejuni cdtB mutant was eliminated from all immunocompetent C57BL/129 mice, whereas in 3X mice both the C. jejuni cdtB mutant and the C. jejuni wild type persistently colonized the stomach and lower bowel. These results imply that CDT may play a role in the ability of C. jejuni to escape immune surveillance of the C57BL/129 mice but not immune surveillance of the 3X mice. Given that clonal expansion of lymphocytes is required for both humoral immunity and cell-mediated immunity, one proposed explanation for the mechanism of CDT activity in promoting escape of immune surveillance by bacteria expressing CDT is the potential ability of the toxin to cause cell cycle arrest of lymphocytes in the G2/M phase, as it does in epithelial cells (12). Also, C. jejuni outer membrane preparations have been shown to cause apoptosis of chicken lymphocytes (46).
Because infection of immunocompromised human patients with C. jejuni is associated with more severe clinical disease and prolonged carriage (31, 34, 45), investigators have used immunocompromised mice for C. jejuni infection studies. Athymic, germfree nude mice were efficiently colonized by C. jejuni when they were challenged orally (43, 44). Infected nude mice developed transient diarrhea and mild typhlitis and colitis when they were infected with a mouse-adapted C. jejuni strain. In contrast, euthymic, germfree mice having the same genetic background (BALB/c mice) did not develop diarrhea or lesions, although they were also consistently colonized. In another recent study, in which CB-17 scid mice were used, infection with fresh isolates of C. jejuni resulted in diarrhea in ∼10% of the mice. The affected mice had significant colitis but not gastritis (14). In a subsequent experiment performed with the same scid model, neither wild-type C. jejuni nor a C. jejuni cdtB mutant elicited inflammation in the intestines of infected mice 7 days p.i. (30). The authors, however, did indicate that wild-type C. jejuni was found more often than the isogenic cdtB mutant in the spleens, livers, and blood of mice 2 h postinoculation (30).
Studies with outbred ICR-scid mice, as well as wild-type ICR mice, challenged with C. fetus demonstrated that in both types of animals the gastric mucosa was consistently colonized (41). The stomachs of the infected ICR-scid mice exhibited progressive hyperplasia and a persistent active inflammatory response characterized by large numbers of infiltrating neutrophils and eosinophils. Similar to our findings, in wild-type mice the anatomic extent of colonization was more limited, and the degree of inflammation and epithelial alterations was significantly less than that observed in infected scid mice (41). Although Yrios and Balish (43, 44) reported chronic gastric colonization of germfree nude mice with C. fetus or C. jejuni, they did not observe gastritis.
Intracellular and extracellular hyaline eosinophilic granules and crystals in the superficial mucosa observed in some mice in this study match those described previously for C57BL and 129 mice with different targeted mutations and genetic backgrounds (39). This syndrome was termed gastric hyalinosis, and the material produced by the epithelial cells was identified as Ym2, a chitinase homologous to Ym1, which acts as an eosinophil chemoattractant and may have a direct antiparasitic function. Although CYP1A2 deletion was identified as a major risk factor for development of gastric hyalinosis, there was also evidence (based on silver staining of affected gastric tissue) of a potential gastric microbial infection in some affected animals (39). Ym1/Ym2 hyalinosis also occurs along other murine epithelial surfaces, including respiratory and biliary surfaces, suggesting a basic mucosal defense function analogous to intestinal defensins. The role of Ym1/Ym2 in murine gastric injury and inflammation clearly requires further investigation (39).
C. jejuni is occasionally associated with hepatitis in humans (43). It is therefore interesting that hepatitis was observed in this study in the mice 2 to 4 months after oral dosing with C. jejuni in combination with recovery of both the C. jejuni cdtB mutant and wild-type C. jejuni from livers of select 3X mice but not from livers of C57BL/129 mice. C. jejuni-associated hepatitis in the mouse also has been reported previously (18-20). Focal necrotic hepatitis, similar to what we observed, in the absence of diarrhea was noted in mice 1 to 2 months after oral dosing with selected strains of C. jejuni (18, 20). The liver lesions persisted, and by 4 months p.i. C. jejuni was still recoverable by culture, and inflammation in both the parenchyma and the portal triads was extensive (18, 20). Ten micrograms of a purified hepatotoxic factor isolated from selected strains (4 of 20 strains tested) of C. jejuni reproducibly caused acute hepatic necrosis in dd-Y mice when it was inoculated intravenously (18, 20). Interestingly, when the hepatotoxic factor was given intravenously at two times 14 days apart, a diffuse mononuclear inflammatory hepatitis developed which mimicked the chronic hepatopathy noted in mice orally infected with C. jejuni 10 months previously (19). The authors suggested that the increasing intensity of mononuclear inflammation was due to a persistent host response to the active moiety of the hepatotoxic factor, a possible consequence of an immunopathological tissue response (19). Although it is not known whether in these previous studies the hepatotoxic factor was CDT, the presence of CDT in C. jejuni in the present study did not statistically affect the ability of the two strains to colonize the liver, nor did it affect the severity of the hepatic lesions observed in both C57BL/129 and 3X mice. Like the marked effect that the mouse genotype has on H. hepaticus-associated hepatitis, the results may also be influenced by the genetic background of the mouse being studied (9, 15).
The IgG responses of the C57BL/129 mice infected with the wild type or the C. jejuni cdtB mutant reflect one aspect of a normal immune response that may have contributed to clearance of the infection. The C57BL/129 mice that had cleared the infection by 2 months p.i. had lower IgG levels than the mice that remained colonized. At 4 months p.i., C57BL/129 mice that were still colonized or that were free of wild-type C. jejuni had similar IgG levels, supporting the hypothesis that the infection is cleared from some mice between 2 and 4 months p.i. The isogenic C. jejuni cdtB mutant was cleared in all C57BL/129 mice by 4 months p.i. and was associated with a declining IgG response. The IgG2a response of the C57BL/129 mice, which indirectly reflected a Th1-associated proinflammatory response, was lower in mice that were not colonized and declined to background levels by 4 months p.i.; the IgG2a levels corresponded to clearance of the C. jejuni cdtB mutant in all C57BL/129 mice. The Th2-promoted IgG1 response did not develop in C57BL/129 mice infected with the wild-type C. jejuni, which may suggest that clearance of the infection was Th1 mediated. Infection with the C. jejuni cdtB mutant caused only a transient IgG1 response in wild-type mice at 2 months p.i., but this response reached background levels as the organism was cleared. Compared to C57BL/129 mice, 3X mice persistently colonized with wild-type C. jejuni and the C. jejuni cdtB mutant had significantly impaired IgG and IgG subclass humoral responses to C. jejuni antigens. This apparent lack of an adaptive antibody response by C. jejuni-infected 3X mice is consistent with previously reported defects in adaptive immunity involving mitogen and antigen-specific activation and function of mature B cells (29). The full spectrum of functional immune defects in p50−/− p65+/− lymphocytes of 3X mice is unknown, but the importance of NF-κB subunits that translocate to the nucleus and influence transcription of genes critical to innate immunity, lymphocyte function, and regulation of dendritic cells is being intensively studied (25). Although the NF-κB-deficient mice used in our study appear to have normal numbers of T and B cells systemically (6), these mice lack marginal zone B cells, and p50−/− B cells have a marked reduction in ability to undergo normal antibody isotype class switching (35).
Similar to the persistent C. jejuni colonization and gastritis noted in the 3X mice in this study, 3X mice infected with H. hepaticus develop chronic typhlocolitis (6). Interestingly, transmural perivasculitis with extension into the adjacent mesentery was a component of the severe inflammatory bowel lesions which Erdman et al. described and was identical to the transmural gastroduodenal lesions which we observed in some C. jejuni-infected 3X mice in the present study (6). In studies recently performed in our laboratories, the typhlocolitis in H. hepaticus-infected 3X mice appears to be in part a defect in innate immunity (36). When H. hepaticus-infected RAG-2−/− or 3X/RAG-2−/− mice were reconstituted with either wild-type or 3X splenocytes, the H. hepaticus-induced colitis in RAG-2−/− mice was reduced (36). Interestingly, in H. hepaticus-infected 3X/RAG2−/− mice repopulated with either wild-type or 3X splenocytes the severity of colitis persisted (36). Our data demonstrating the apparent inability to inhibit proinflammatory cytokine responses and the resultant gastritis in 3X mice infected with C. jejuni may be related to a defect in innate immunity, as well as adaptive immunity. However, studies involving reconstitution of splenocytes in 3X/Rag2−/− mice infected with C. jejuni are required to definitely address this hypothesis.
In summary, we demonstrated that a C. jejuni cdtB mutant is less efficient than the wild type in colonizing C57BL/129 mice but not 3X mice. It is interesting to speculate that CDT may target the cells of the immune system in the lamina propria that influence the host's ability to clear bacterial pathogens. Despite 100% colonization of 3X mice, the C. jejuni cdtB mutant produced significantly less gastritis than the wild-type C. jejuni produced, indicating that CDT has a proinflammatory effect, similar to the effect noted in vitro. In addition to studies of the role of NF-κB in modulating bacterial infections, this model system should prove to be useful in identifying potential C. jejuni virulence factors and in determining how these factors interact with the host.
Acknowledgments
This work was supported by NIH grants R01 AI50952 (to J.G.F.) and R01 CA67529 (to J.G.F.) and by a Crohn's and Colitis Foundation award (to B.H.H. and S.E.E.).
Editor: V. J. DiRita
REFERENCES
- 1.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 2.Bouzari, S., and A. Varghese. 1990. Cytolethal distending toxin (CLDT) production by enteropathogenic Escherichia coli (EPEC). FEMS Microbiol. Lett. 59:193-198. [DOI] [PubMed] [Google Scholar]
- 3.Cahill, R. J., C. J. Foltz, J. G. Fox, C. A. Dangler, F. Powrie, and D. B. Schauer. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect. Immun. 65:3126-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 5.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37:952-963. [DOI] [PubMed] [Google Scholar]
- 6.Erdman, S., J. G. Fox, C. A. Dangler, D. Feldman, and B. H. Horwitz. 2001. Typhlocolitis in NF-kappa B-deficient mice. J. Immunol. 166:1443-1447. [DOI] [PubMed] [Google Scholar]
- 7.Erdman, S. E., T. Poutahidis, M. Tomczak, A. B. Rogers, K. Cormier, B. Plank, B. H. Horwitz, and J. G. Fox. 2003. CD4(+) CD25(+) regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162:691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 9.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect. Immun. 64:1548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox, J. G., A. B. Rogers, M. Ihrig, N. S. Taylor, M. T. Whary, G. Dockray, A. Varro, and T. C. Wang. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 63:942-950. [PubMed] [Google Scholar]
- 11.Ge, Z., K. Hiratsuka, and D. E. Taylor. 1995. Nucleotide sequence and mutational analysis indicate that two Helicobacter pylori genes encode a P-type ATPase and a cation-binding protein associated with copper transport. Mol. Microbiol. 15:97-106. [DOI] [PubMed] [Google Scholar]
- 12.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgson, A. E., B. W. McBride, M. J. Hudson, G. Hall, and S. A. Leach. 1998. Experimental campylobacter infection and diarrhoea in immunodeficient mice. J. Med. Microbiol. 47:799-809. [DOI] [PubMed] [Google Scholar]
- 15.Ihrig, M., M. D. Schrenzel, and J. G. Fox. 1999. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am. J. Pathol. 155:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb. Pathog. 4:115-126. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, W. M., and H. Lior. 1987. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat labile (LB) enterotoxin. FEMS Microbiol. Lett. 3:19-23. [Google Scholar]
- 18.Kita, E., N. Katsui, K. Nishi, M. Emoto, Y. Yanagase, and S. Kashiba. 1986. Hepatic lesions in experimental Campylobacter jejuni infection of mice. J. Gen. Microbiol. 132:3095-3103. [DOI] [PubMed] [Google Scholar]
- 19.Kita, E., F. Nishikawa, N. Kamikaidou, A. Nakano, N. Katsui, and S. Kashiba. 1992. Mononuclear cell response in the liver of mice infected with hepatotoxigenic Campylobacter jejuni. J. Med. Microbiol. 37:326-331. [DOI] [PubMed] [Google Scholar]
- 20.Kita, E., D. Oku, A. Hamuro, F. Nishikawa, M. Emoto, Y. Yagyu, N. Katsui, and S. Kashiba. 1990. Hepatotoxic activity of Campylobacter jejuni. J. Med. Microbiol. 33:171-182. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 22.Maloy, K. J., L. Salaun, R. Cahill, G. Dougan, N. J. Saunders, and F. Powrie. 2003. CD4+ CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda, J., M. Fukumoto, Y. Takeda, and M. Nishibuchi. 1997. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect. Immun. 65:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda, J., H. Kurazono, and Y. Takeda. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167-172. [DOI] [PubMed] [Google Scholar]
- 25.Ouaaz, F., J. Arron, Y. Zheng, Y. Choi, and A. A. Beg. 2002. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 16:257-270. [DOI] [PubMed] [Google Scholar]
- 26.Peres, S. Y., O. Marches, F. Daigle, J. P. Nougayrede, F. Herault, C. Tasca, J. De Rycke, and E. Oswald. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095-1107. [DOI] [PubMed] [Google Scholar]
- 27.Pickett, C. L., D. L. Cottle, E. C. Pesci, and G. Bikah. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickett, C. L., E. C. Pesci, D. L. Cottle, G. Russell, A. N. Erdem, and H. Zeytin. 1996. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect. Immun. 64:2070-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohl, T., R. Gugasyan, R. J. Grumont, A. Strasser, D. Metcalf, D. Tarlinton, W. Sha, D. Baltimore, and S. Gerondakis. 2002. The combined absence of NF-kappa B1 and c-Rel reveals that overlapping roles for these transcription factors in the B cell lineage are restricted to the activation and function of mature cells. Proc. Natl. Acad. Sci. 99:4514-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purdy, D., C. M. Buswell, A. E. Hodgson, K. McAlpine, I. Henderson, and S. A. Leach. 2000. Characterisation of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J. Med. Microbiol. 49:473-479. [DOI] [PubMed] [Google Scholar]
- 31.Rao, G. G., and M. Fuller. 1992. A review of hospitalized patients with bacterial gastroenteritis. J. Hosp. Infect. 20:105-111. [DOI] [PubMed] [Google Scholar]
- 32.Russell, R. G., M. J. Blaser, J. I. Sarmiento, and J. Fox. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skirrow, M. B., and M. J. Blaser. 1995. Campylobacter, p. 825-848. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 35.Snapper, C. M., P. Zelazowski, F. R. Rosas, M. R. Kehry, M. Tian, D. Baltimore, and W. C. Sha. 1996. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 156:183-191. [PubMed] [Google Scholar]
- 36.Tomczak, M. F., S. E. Erdman, T. Poutahidis, A. B. Rogers, H. Holcombe, B. Plank, J. G. Fox, and B. H. Horwitz. 2003. NF-kappaB is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J. Immunol. 171:1484-1492. [DOI] [PubMed] [Google Scholar]
- 37.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Y., and D. E. Taylor. 1990. Natural transformation in Campylobacter species. J. Bacteriol. 172:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, J. M., M. Yoon, M. R. Anver, D. C. Haines, G. Kudo, F. J. Gonzalez, and S. Kimura. 2001. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am. J. Pathol. 158:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]
- 41.Young, V. B., C. A. Dangler, J. G. Fox, and D. B. Schauer. 2000. Chronic atrophic gastritis in SCID mice experimentally infected with Campylobacter fetus. Infect. Immun. 68:2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, V. B., D. B. Schauer, and J. G. Fox. 2000. Animal models of Campylobacter infection, p. 287-301. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 43.Yrios, J. W., and E. Balish. 1986. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect. Immun. 53:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yrios, J. W., and E. Balish. 1986. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect. Immun. 53:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and T. Miyatake. 1993. A bacterium lipopolysaccharide that elicits Guillain-Barre syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, J., R. J. Meinersmann, K. L. Hiett, and D. L. Evans. 1999. Apoptotic effect of outer-membrane proteins from Campylobacter jejuni on chicken lymphocytes. Curr. Microbiol. 38:244-249. [DOI] [PubMed] [Google Scholar]