Abstract
The success rate of titanium implants for dental and orthopedic applications depends on the ability of surrounding bone tissue to integrate with the surface of the device, and it remains far from ideal in patients with bone compromised by physiological factors. The electrical properties and electrical stimulation of bone have been shown to control its growth and healing and can enhance osseointegration. Bone cells are also sensitive to the chemical products generated during corrosion events, but less is known about how the electrical signals associated with corrosion might affect osseointegration. The metallic nature of the materials used for implant applications and the corrosive environments found in the human body, in combination with the continuous and cyclic loads to which these implants are exposed, may lead to corrosion and its corresponding electrochemical products. The abnormal electrical currents produced during corrosion can convert any metallic implant into an electrode, and the negative impact on the surrounding tissue due to these extreme signals could be an additional cause of poor performance and rejection of implants. Here, we review basic aspects of the electrical properties and electrical stimulation of bone, as well as fundamental concepts of aqueous corrosion and its electrical and clinical implications.
Keywords: biopotentials, electrical stimulation, corrosion, titanium, bone, osseointegration of dental and orthopedic implants
Biopotentials
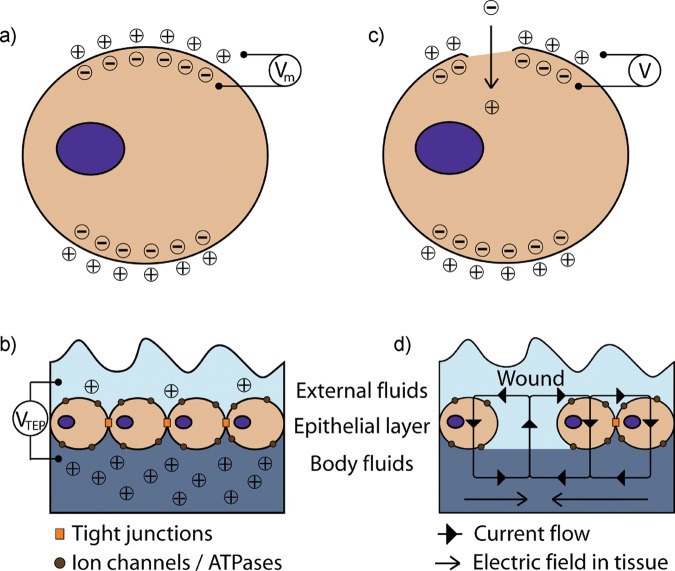
Exogenous electrical control of cell and tissue physiology has been studied since the late 1700s with the work of scientists such as Luigi Galvani, Alessandro Volta, Carlo Matteucci, and Emil Du-Bois Reymond, and the discovery of biopotentials and injury potentials (Black, 1986; Piccolino, 1997). Biopotentials are natural electrical properties that control normal growth and development of different types of cells and tissues (Ferrier et al., 1986; Levin et al., 2002) (Figs. 1a, 1b). Injury potentials are alterations to the normal potential patterns of intact tissue (Becker et al., 1977; Levin, 2007), characterized by stable, long-lasting direct current (DC) voltage potentials induced between injured and intact tissues that persist until the wound has healed. These potentials can span hundreds of microns and are generated by current or ions flowing through the injured tissue (Lokietek et al., 1974; McCaig et al., 2005) (Figs. 1c, 1d). Currents of 1-100 µA/cm2 have been measured in injured tissues (Lokietek et al., 1974; Borgens et al., 1980), and, assuming the resistivity of soft tissues to be 100 Ω·cm (Faes et al., 1999; McCaig et al., 2005), these currents create voltage differences of 10-100 mV/cm across hundreds of microns.
Figure 1.
Schematic shows: (a) electrical potential of a cell across an intact plasma membrane (Vm), and (b) the inward current flow, and its respective potential (V) after localized injury to the plasma membrane; (c) transepithelial electrical potential (VTEP) across an intact cell layer of the skin; and (d) short-circuit caused by a wound. Adapted from McCaig et al. (2005).
Recent findings underscore the importance of endogenous electrical potentials in cell signaling and gene expression. Endogenous electrical potentials, and specifically injury potentials, have been associated with epithelial cell migration and advancement of the wound-healing front through activation of Src and inositol-phospholipid signaling pathways in a rat corneal model (Zhao et al., 2006). Disruption of endogenous electric potentials affected the migration speed and direction of the wound-healing front. The same group also found that corneal epithelial cells from bovine eyes were sensitive to directional cues such as nanogrooves (i.e., contact guidance) and electric fields (i.e., electrotaxis) through the activation of small GTPases, rho and cdc42, respectively (Rajnicek et al., 2007). The study showed that electrotaxis seemed to be more potent than, but not completely dominant over, contact guidance by setting the electric fields orthogonally to the nanogrooves and measuring the distance traveled by the cells. Furthermore, a cell-membrane voltage sensor, Ciona intestinalis voltage-sensor-containing phosphatase (Ci-VSP), has been identified, which is activated by changes in membrane potential and can initiate signaling cascades (Murata et al., 2005; Iwasaki et al., 2008).
Electrical Signals in Bone
Both biopotentials and injury potentials are found in bone. Electrical properties and electrical stimulation of bone have been investigated since the 1950s, beginning with the piezoelectric nature of osseous tissue (Fukada and Yasuda, 1957). When forces were applied to sections of previously dried human and ox femurs, directly proportional voltages could be measured that were dependent on the collagen fiber alignment. This led to the idea that electrical signals could be related to the process of bone formation. Additional endogenous electrical properties of bone have been discovered since and are suggested to play a role in the feedback mechanism of bone remodeling and development (Guzelsu and Demiray, 1979; Rubinacci et al., 1988).
Biopotentials in bone are classified into two subgroups, due in part to the complexity of bone structure: strain-related potentials (SRP) and biopotentials. SRPs include the piezoelectric behavior (i.e., electric potential in response to applied forces) of bone due to the structure and dipolar charge of collagen, and streaming potentials associated with the flow of fluid and ions through porous bone. The subgroup of biopotentials in bone results from contribution of biological processes such as osteoblast membrane potential, extracellular matrix acidification and ion release caused by osteoclast bone resorption, and cell junctions of osteocytes. In vivo, these electrical signals work in concert to provide the correct environment for normal bone growth and development, but can be disrupted or altered by injury potentials after trauma and during healing.
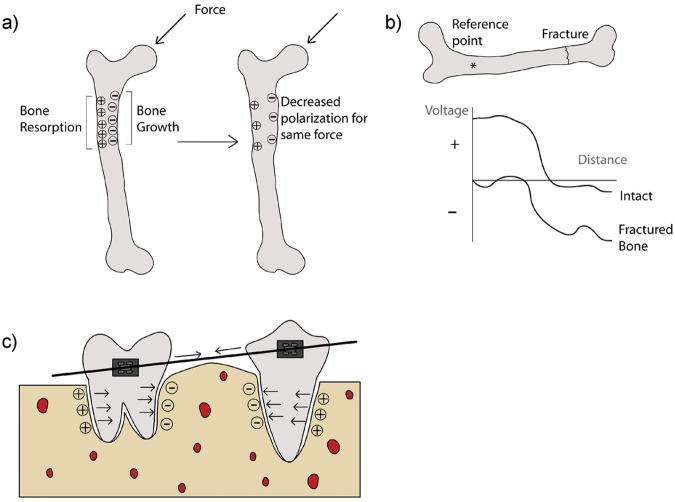
Mechanical forces have been shown to direct the process of bone remodeling (Burr et al., 2002; Hou et al., 2007). Accordingly, areas of bone under stress tend to grow, and those areas under no mechanical load tend to be resorbed (Duncan and Turner, 1995). This is believed to be a result of the physical stress alteration and biochemical activation of particular bone cells (Duncan and Turner, 1995). As a parallel event, however, areas of bone that are under mechanical load generate a more negative polarity than areas under smaller or no loads (Fukada and Yasuda, 1957; Bassett and Becker, 1962) (Fig. 2a). Thus, bone growth could also be attributed to negative polarity and bone resorption to positive polarity, suggesting that electrical signals work as a feedback mechanism for bone remodeling (Becker et al., 1977; Black, 1986).
Figure 2.
Schematic of: (a) polarization of bone under applied mechanical forces; (b) voltage vs. distance comparison between intact and one-hour post-fracture bones; and (c) simplified mechanical forces and respective polarization of bone and periodontal ligament under orthodontic treatment. Adapted from Black (1986).
The relationship between negative potentials and bone growth is seen during long-bone fracture-healing (Bassett et al., 1964; Becker et al., 1977; Black et al., 1984). In children, fractured long bones tend to overgrow with respect to their counterparts (Aitken et al., 1939; Wray and Goodman, 1961), and there is an increase in apoptosis in the growth plate (Gaber et al., 2009). Interestingly, both the healing site and growth plate tend to have a more negative potential compared with that of the nearby intact tissue (Friedenberg and Brighton, 1966; Black, 1986) (Fig. 2b). During development, the growth plate has a negative potential, while the growth plate of a mature individual tends to have a neutral voltage (Lai et al., 2000). Consequently, negative potentials in the growth plate after fracture may be related to bone overgrowth, since cortical bone healing and repair should not increase bone length. However, the negative potential found at the fracture site may coordinate bone healing and may directly influence the polarity of the growth plate.
The relationship among mechanical stimuli, electrical potentials, and bone remodeling can also be seen in orthodontic treatment of individuals with malocclusion. Tipping and translational mechanical forces applied during orthodontic treatment deform and remodel alveolar bone and periodontal tissue, resulting in tooth movement (Isaacson et al., 1993; Meikle, 2006). Quantitative techniques such as finite element analysis have been used to assess forces affecting tooth movement (McGuinness et al., 1991; Cobo et al., 1993). Although the experimental design and parameters measured varied between and among studies, the results indicate a direct relationship between the magnitude of the applied stress and the level of bone and periodontal ligament remodeling. Some studies have correlated excessive forces to orthodontically induced inflammatory root resorption (Brezniak and Wasserstein, 2002).
Despite innate differences between the origin of long bones and maxillofacial bone, forces applied on teeth and surrounding alveolar bone generate similar electrical potentials (Cochran et al., 1967; Zengo et al., 1973). The electrical polarization of these tissues has also been correlated to bone remodeling. Areas with high osteoblast activity and bone growth show negative polarization, while areas under resorption due to higher osteoclast levels show a positive or neutral polarization (Norton et al., 1984) (Fig. 2c). One suggested hypothesis states that these electrical potentials may provide a more direct measurement of the mechanical forces delivered by orthodontic devices and help provide a more personalized treatment (Norton, 1975).
Electrical Stimulation of Bone
The roles of these electrical signals in bone growth and development have prompted several research groups to study bone repair using methods to stimulate cells and tissues electrically in vitro (Wang et al., 1998; Kim et al., 2006) and in vivo (Brighton and Hunt, 1986; Shafer et al., 1995) with very successful outcomes. Some have associated bone growth enhancement after electrical stimulation with the production of osteoinductive factors such as bone morphogenetic proteins (BMPs) 2, 4, 5, and 6 (Kim et al., 2006; Wang et al., 2006), as well as with levels of intracellular and extracellular calcium (Wang et al., 1998; Aaron et al., 2004). However, differences in experimental design and the electrical parameters used, and the over-simplification of in vitro models that do not account for many aspects of in vivo conditions, have hindered the systematic investigation of the molecular pathways involved with cell responses. Additionally, it is not well-understood if electrical stimulation affects every type of bone (e.g., cortical, trabecular, membranous) similarly. Fundamental understanding of the molecular pathways involved in electrical stimulation is necessary to elucidate the role of electrical signals in the bone-implant interface, thus allowing for better system designs for personalized treatment.
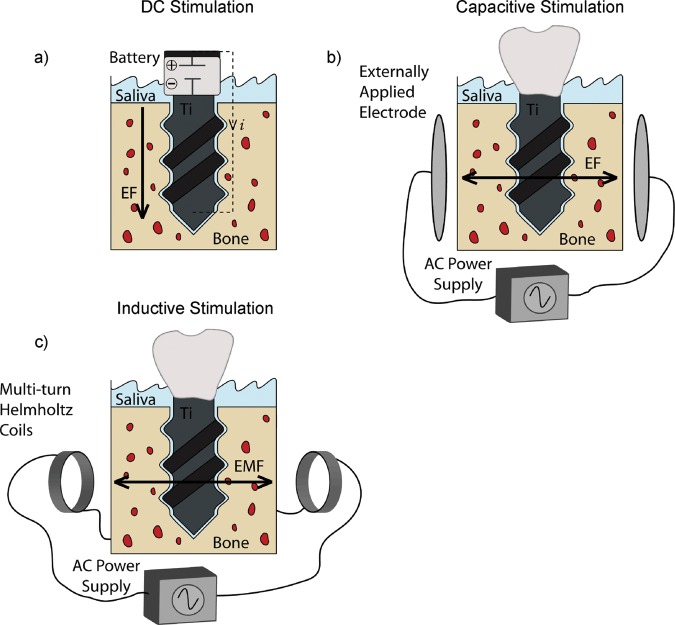
Electrical stimulation systems can be classified into 3 main groups, depending on the nature of the electrical signals being supplied: direct current stimulation, capacitive stimulation, and inductive stimulation.
Direct Current (DC) Stimulation
DC stimulation, or faradic stimulation, is an invasive method that applies a DC electric field to growing cells, either directly through the surface on which they are growing, or indirectly through the medium in which they are growing (Fig. 3a). Common parameters applied include fixed currents of 1-50 µA/cm2, which can affect osteoblast proliferation and expression of differentiation markers (Bodamyali et al., 1999; Kim et al., 2006). The majority of published studies of in vitro DC stimulation used electrodes submerged in the tissue culture medium, establishing a DC electric field and inducing electrochemical currents between the anode and the cathode (Curtze et al., 2004; Ercan and Webster, 2008). However, the products generated at the cathode and the anode that have enhancing or detrimental effects on cell response, respectively (Black et al., 1984; Bodamyali et al., 1999), may obscure the results of DC electrical stimulation.
Figure 3.
Schematics of different electrical stimulation systems. (a) DC electrical stimulation set-up consisting of a battery that generates an electric field (EF) directly through the implant device. The implant becomes the cathode, the anode is exposed to the oral cavity, and the surrounding tissue serves as a path to close the circuit and allow for the flow of current. (b) Capacitive stimulation set-up, consisting of 2 externally applied electrodes that generate an electric field (EF). (c) Inductive stimulation set-up, consisting of a pair of multi-turn Helmholtz coils connected in series to generate an electromagnetic field (EMF).
Titanium (Ti) implants can be used as cathodes for DC electrical stimulation (Dodge et al., 2007). One such device was developed to fit inside a dental implant healing abutment and supply electrical stimulation to canine mandibular bone (Song et al., 2009). Biphasic electrical stimulation increased bone formation and bone-to-implant contact when compared with control implants. Although Ti is one of the most-used materials for bone implants, to our knowledge there are no in vitro studies evaluating osteoblast response to electrical stimulation when grown directly on a Ti cathode. Ti substrates used in a typical configuration with submerged electrodes in the media showed increased osteoblast density by DAPI staining (Ercan and Webster, 2008, 2010).
Capacitive Stimulation
In capacitive stimulation, electrodes are applied externally to the skin above the area to be stimulated, inducing an electric field that can influence cell response (Fig. 3b). Common external stimulation systems use alternating current (AC) parameters that vary between 1 and 50 V at frequencies of 60-200,000 Hz, and effective electric field strengths from 0.1 to 5 V/m (Lorich et al., 1998; Shigino et al., 2001). Cells grown in vitro on tissue culture substrates are either stimulated through the media with an AC power supply or sandwiched between electrodes without media contact. Capacitive stimulation is advantageous because it is non-invasive, and it has been shown to have an effect both in vitro (Brighton et al., 2001; Wang et al., 2006) and in vivo (Brighton et al., 1985; Lirani-Galvao et al., 2009). However, the therapeutic results depend on patient compliance, and high voltages and frequencies applied may cause irritation (Black, 1986; Gan and Glazer, 2006). Since capacitive stimulation cannot be applied directly to the affected osseous tissue, and because of the complexities of measuring local current densities in the site of interest, it is difficult to predict the subsequent effects.
Inductive Stimulation
Inductive stimulation is a non-invasive method that uses a coil or pair of coils connected in series, with their axes perpendicular to the long bone, to generate pulsed electromagnetic fields (PEMFs) and small secondary electric fields (Black, 1986) (Fig. 3c). These magnetic fields and the induced electrical fields have been shown to influence cell response and gene expression (Bodamyali et al., 1998; Brighton et al., 2001; Schwartz et al., 2008).
In a series of studies performed in our laboratory, the effects of PEMFs on MG63 osteoblast-like cells were shown to reduce cell number, and increase osteoblast maturation, collagen synthesis, and local factor production, including transforming growth factor-β1 (TGF-β1) (Lohmann et al., 2000b). Cells from human hypertrophic and atrophic non-union tissues have been used to evaluate the effects of PEMFs on non-union fractures, commonly treated with electrical stimulation (Guerkov et al., 2001). Cells exposed to PEMFs increased TGF-β1, with no effect on cell proliferation or differentiation, suggesting that improvements in non-unions after PEMFs result from changes in local factor production near the affected area. Finally, mesenchymal stem cells (MSCs) have been used to evaluate the effects of PEMFs on progenitor cell differentiation, one of the first types of cells to arrive after implant placement (Schwartz et al., 2008, 2009). PEMF synergistically increased MSC osteogenesis when cells were cultured on calcium phosphate disks in the presence of the osteoinductive factor bone morphogenetic protein-2 (BMP-2), as determined by increased alkaline phosphatase, osteocalcin, and TGF-β1. These results suggest that electrical stimulation may also improve bone healing and osseointegration by increasing osteogenic differentiation of MSCs.
Like capacitive stimulation, inductive stimulation has no electrochemical effect on the tissue, because it is non-invasive. Clinically, one disadvantage is that therapy success depends on patient compliance (Gan and Glazer, 2006). Additionally, the non-localized application of inductive stimulation may affect multiple types of tissues surrounding the injury site.
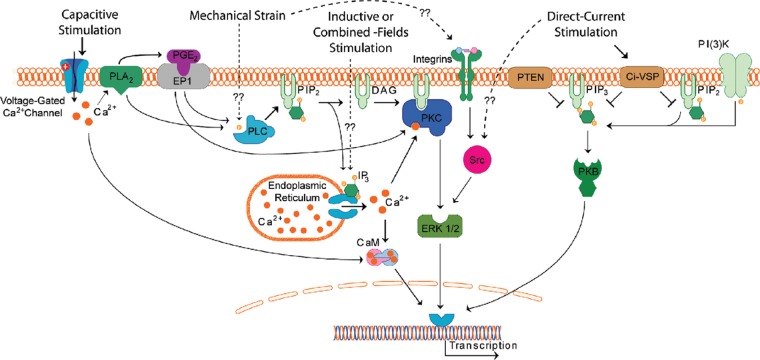
Taking into consideration what is known about mechanical stimulation and the different electrical stimulation systems, improvements in bone growth and repair can be achieved through different pathways, including integrin- and IP3-mediated pathways (Wang et al., 1993; Brighton et al., 1996) (Fig. 4).
Figure 4.
Schematic of possible signaling pathways used by mechanical and electrical stimulation systems.
Electrical Implications of Corrosion
Metals are used for dental and orthopedic implants because of their mechanical properties, such as weight-to-strength ratio and good biological performance. However, metallic devices are prone to corrosion, particularly in aqueous environments under extreme conditions. Corrosion resistance depends on temperature, pH, ion concentration, substrate size, and chemistry, but it is not inherent to the material itself as has been implied in many studies (Bhattarai et al., 2008). Ti is corrosion-resistant under controlled environments in the absence of load. In the human body, the physiological environment in combination with constant, cyclic implant loading can significantly enhance corrosion rates (Long and Rack, 1998; Papakyriacou et al., 2000; Brunette et al., 2001; Lewis et al., 2005). Extreme acidic conditions found during inflammation (Lassus et al., 1998), fretting between implant and bone (Gilbert et al., 2009), and galvanic corrosion between Ti implants and other metallic alloys used for common dental procedures (Grosgogeat et al., 1999) could greatly affect the mechanical stability and outcome of dental implants.
Basic Electrochemistry
The basic unit of electrochemistry is the electrochemical cell, which is composed of 2 electrodes (anode and cathode) and an aqueous electrolyte serving as a connecting path. Electrochemical reactions on the surface of an electrode can be oxidative (anodic), generating electrons and ions, or reductive (cathodic), consuming electrons and generating metal atoms or other molecules (Fig. 5). An electrode is defined by how reactive it is compared with the opposite electrode to which it is connected. In some situations, a single metal device can serve as both the anode and the cathode, and so a second electrode is not required to complete the circuit.
Figure 5.
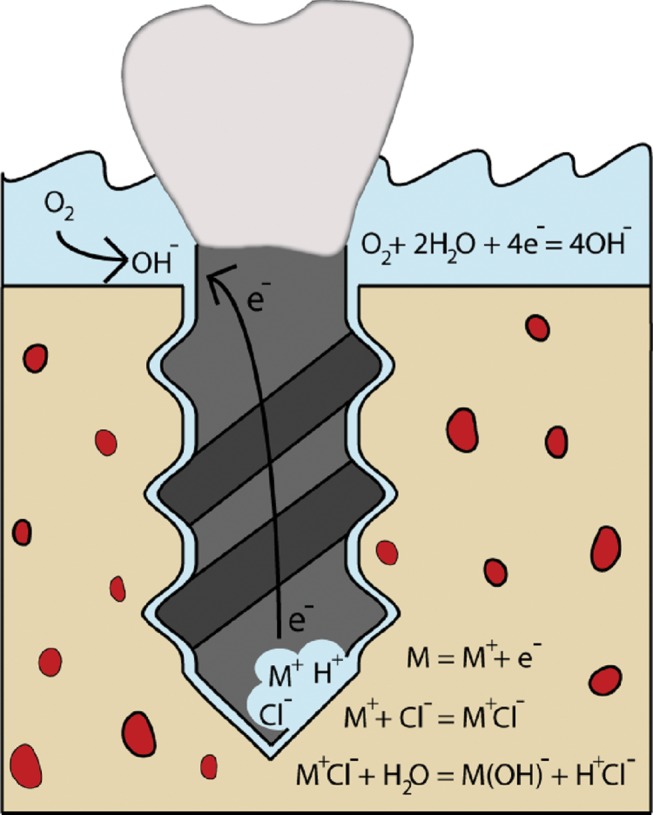
Schematic of initiation and mechanism of corrosion of a dental implant.
Metallic implants for bone applications submerged in ion-rich electrolytes in the body constitute a basic corrosion cell. Large currents can be induced by the flow of ions and electrons generated during electrochemical reactions occurring between the corroding metallic surface and the electrolyte. These currents are generally used to measure the corrosion rate of a metal, because they are directly related to the release of metal ions or, in other words, to the material’s degradation. Consequently, corrosion events result in the formation of small pits on the surface of the device that can amplify the corrosive environment around the implant and compromise its mechanical stability. This can lead to shortening of the implant’s lifetime and sudden failure (Papakyriacou et al., 2000; Teoh, 2000; Mudali et al., 2003) (Fig. 5). Products of the electrochemical reactions may have cytotoxic or even neoplastic effects on the tissue surrounding the implant, serving as an additional cause of rejection or aseptic loosening (Doran et al., 1998; Denaro et al., 2008b; McGuff et al., 2008). However, the electrical implications of corrosion on the surrounding tissue have not been extensively investigated (Denaro et al., 2008a).
Passivity of Titanium
Certain metals like Ti oxidize easily, forming a very thin, stable passive layer that is self-limiting and protects the surface of the metal from further oxidation. This behavior, passivity, gives Ti its high corrosion resistance under certain controlled conditions where, otherwise, it would undergo strong active corrosion. Metals can have stable passivity, where the oxide layer self-heals immediately after being ruptured, or unstable passivity, where the oxide layer is unable to heal after disruption and the bare metal is exposed to active corrosion. Both of these events depend on the oxidizing or reducing potential of the environment. The passive oxide layer formed on the surface of Ti may be responsible for its good biological performance, since it is less reactive than bare Ti. Additionally, it may mimic the ceramic nature of bone and allow biochemical bonding with the newly formed bone (Sul et al., 2005).
Most materials chosen for implant applications exhibit passivity properties and, thus, relatively low corrosion rates compared with those of other more reactive metals, such as zinc, magnesium, or vanadium, which undergo active corrosion even in relatively neutral pH. However, certain environmental conditions can breach the protective oxide layer formed on the surfaces of these passive materials and cause corrosion, affecting the mechanical integrity of the implant and the health of the surrounding tissue.
Work by our laboratory and many others has shown that implant surface properties such as roughness, chemistry, and energy directly influence tissue response by affecting protein adsorption and modulating cell proliferation and differentiation (Schwartz and Boyan, 1994; Kieswetter et al., 1996). Additionally, innovations in surface modification techniques have improved the biological performance of metallic implants (Wang et al., 2003; Buser et al., 2004). However, some modifications may diminish mechanical properties of the bulk material, resulting in surface micro-cracks, increased corrosion rates (Papakyriacou et al., 2000; Teoh, 2000; Hazar Yoruc and Kelesoglu, 2009), and, thus, increased corrosion currents and potentials that may affect surrounding cells and tissues.
Types of Corrosion
The most common types of corrosion found in metallic materials used for implant applications are galvanic, fretting, and pitting/crevice corrosion, as well as environmentally induced cracking (EIC).
Galvanic corrosion occurs with direct contact of two dissimilar metals in an electrolytic solution (Kaesche, 2003). The difference in electrochemical potential of the two metals promotes oxidation of the more reactive metal. This becomes the anode, which generates a flow of electrons and ions to the cathode. In one study, spine implant constructs consisting of pedicle screws, connectors, and rods that had mixed components made of stainless steel (SS) and titanium were investigated for signs of galvanic corrosion under dynamic loads (Serhan et al., 2004). The results showed no evidence of corrosion on surfaces of the implant that had not been in contact with other components, and only minor signs of corrosion at the interfaces between SS-Ti, Ti-Ti, and SS-SS, with the latter actually having the greatest amount of corrosion. Galvanic corrosion is not common in dental implant applications because of the presence of only one component, the dental screw, and the insulating nature of the protective passive layer that forms on the surface. Nevertheless, in some individuals the surrounding tissue could serve as a medium for electrical flow between metallic implants and other types of alloys used in dentistry for amalgams or orthodontic devices. Galvanic corrosion could also amplify the rates of corrosion initiated by other mechanisms described below (Reclaru and Meyer, 1994; Grosgogeat et al., 1999).
Fretting corrosion is caused by the repeated micro-motion or friction of a metal component against another material that causes mechanical wear and breaks up the passivating layer on the contact surface of the metallic device (Landolt, 2007). Fretting between dental implants and bone during implantation and due to cyclic loads imparted from chewing has been suggested as a cause of Ti corrosion and metal ion release (Denaro et al., 2008b; Gilbert et al., 2009). Fretting could also be an issue in total hip replacements, where it could generate wear debris and ions from friction between joint and socket (Long and Rack, 1998; Ingham and Fisher, 2000). The release of metal debris and ions has been linked to inhibition of cell differentiation, cytotoxicity, phagocytosis of Ti particles by macrophages and other cells, inflammation, and neoplasia (Sun et al., 1997; Doran et al., 1998; Lohmann et al., 2000a; Rahal et al., 2000). Recent studies have shown that fretting and oxide disruption at the surface of load-bearing implants can cause corrosion current densities to increase and generate open-circuit potentials in excess of -500 mV (Goldberg and Gilbert, 1997; Gilbert et al., 2009). Abnormal electrical signals may affect the response and stability of the adjacent tissue, and fretting corrosion may amplify other types of corrosion by rupturing the passivating film and exposing bare Ti.
Pitting corrosion occurs as a result of the spontaneous breakdown of the passive film on a flat and evenly exposed area (Evans, 1960; Landolt, 2007). Crevice corrosion is a localized corrosion due to a geometric confinement in the design of the device or from a previously corroded region on the surface. The common mechanism of propagation for both usually involves a differential aeration cell (Fig. 5). In this, the region undergoing active corrosion has restricted solution flow due to geometric confinement and initially depletes local oxygen concentration, generating high levels of metal ions and electrons that are consequently consumed by the surface exposed to high levels of oxygen. While pitting corrosion is not likely to occur on Ti surfaces, crevice corrosion has been found (Charles and Ness, 2006; Denaro et al., 2008b). In one study, corrosion currents from Ti alloy lumbar interbody fusions were directly related to lumbar pain and periprosthetic bone loss in patients (Denaro et al., 2008b).
EIC is the brittle mechanical failure of metallic devices under stress levels significantly lower than their ultimate tensile strength. This occurs in susceptible materials in corrosive environments and under continuous loading. The magnitudes of the forces that can cause EIC vary over a wide range and include forces that, under non-corrosive conditions, would be considered negligible. EIC is the most common cause of corrosion in implants for bone applications (Bundy et al., 1983; Lewis et al., 2005) and, because of its localized nature, may go unnoticed until catastrophic failure.
Clinical Relevance of Corrosion
Corrosion of metallic implants, a topic extensively discussed in orthopedic literature, may jeopardize the mechanical stability of the implant and the integrity of the surrounding tissue (Jacobs et al., 1998; Gilbert et al., 2009). Implant failure in the form of aseptic loosening, or osteolysis, may result from metal release in the form of wear debris or electrochemical products generated during corrosion events (Dorr et al., 1990; Jacobs et al., 2001, 2009). Metal ions such as Ti4+, Co2+, and Al3+ have been shown to decrease DNA synthesis, mitochondrial dehydrogenase activity, mineralization, and mRNA expression of alkaline phosphatase and osteocalcin in ROS 17/2.8 cells (Sun et al., 1997). Similarly, phagocytosis of Ti particles caused cytotoxicity in a concentration- dependent manner in neonatal rat calvarial osteoblasts (Pioletti et al., 1999) and MG63 cells (Lohmann et al., 2000a).
While implant loosening is less prominent in the dental literature, metal traces originating from dental implants have been found in blood, liver, lungs, and lymph nodes (Lugowski et al., 1991; Smith et al., 1997; Finet et al., 2000). These metal ions and wear debris may also contribute to aseptic loosening by promoting inflammatory complications that may result in macrophage activation, bone resorption, and, rarely, in the potential development of neoplasia (Poggio, 2007; McGuff et al., 2008). Recently, titanium dioxide (TiO2) was classified as possibly carcinogenic to human beings (i.e., group 2B) at the International Agency for Research on Cancer (IARC) (Baan et al., 2006). Animal studies in rodents provided sufficient evidence of the carcinogenic effects of TiO2, although epidemiological cohort studies in humans were inconclusive. Furthermore, the immediate and systemic cytotoxic and neoplastic effects of corrosion remain controversial because of conflicting studies that have found no effects of Ti ions or Ti particles on cells (Doran et al., 1998). Moreover, the nanograms of metal per gram of tissue found in vivo (Frisken et al., 2002; Hanawa, 2004) are difficult to compare with the micrograms and milligrams of metal per milliliters of solution used to create an effect in in vitro studies (Pioletti et al., 1999; Lohmann et al., 2000a).
The electrical implications of corrosion and its effect on the surrounding tissue may be an important key to this puzzle, but such effects still remain unclear. Corrosion events generate electrical currents due to electron transfer from ions in the solution to the metallic surface where reactions are occurring. These abnormal currents, and coupled electrical potentials, are directly related to the cyclic loads applied to the implant (Goldberg and Gilbert, 1997; Gilbert et al., 2009). In dental and orthopedic applications, cyclic loads are to be expected from the forces exerted after every bite or every step, respectively. Consequently, it is fair to suggest that cells and tissues in individuals with implants are exposed to abnormal electrical signals for extended periods of time. As described previously, bone cells are sensitive to electrical signals and, thus, could be strongly affected by these corrosion currents. Moreover, these abnormal electrical signals may provide an alternate explanation for the unresolved causes of inflammatory complications and eventual aseptic loosening.
With the growing popularity of treatments like early implant loading, it is imperative to consider the effects of electrical signals on the early stages of osseointegration as well as on long-term outcome. The concern of reducing implant corrosion might be addressed and is being addressed by different methods such as: new formulations of metallic alloys that improve the mechanical and corrosion properties of the implant (Yamazoe et al., 2007; Mareci et al., 2009; Oliveira and Guastaldi, 2009); surface modifications that stabilize the reactivity of the surface (Papakyriacou et al., 2000); or electrical protection (i.e., stimulation) of implants. However, a fundamental understanding of the consequences of abnormal electrical signals on the growth and development of cells and tissues is required for the design of appropriate solutions and adequate treatment for affected individuals.
Footnotes
R.A.G. is partially supported by a fellowship from IFARHU-SENACYT. The authors thank the National Institutes of Health [AR052102] for their support of our work.
The authors declare no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. (2004). Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res 419:30-37 [DOI] [PubMed] [Google Scholar]
- Aitken AP, Blackett CW, Cincoth JJ. (1939). Overgrowth of the femoral shaft following fracture in childhood. J Bone Joint Surg Am 21:334-338 [Google Scholar]
- Baan R, Straif K, Grosse Y, Secretan W, El Ghissassi F, Cogliano V, et al. (2006). Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol 7:295-296 [DOI] [PubMed] [Google Scholar]
- Bassett CA, Becker RO. (1962). Generation of electric potentials by bone in response to mechanical stress. Science 137:1063-1064 [DOI] [PubMed] [Google Scholar]
- Bassett CA, Pawluk RJ, Becker RO. (1964). Effects of electric currents on bone in vivo. Nature 204:652-654 [DOI] [PubMed] [Google Scholar]
- Becker RO, Spadaro JA, Marino AA. (1977). Clinical experiences with low intensity direct-current stimulation of bone-growth. Clin Orthop Relat Res 124:75-83 [PubMed] [Google Scholar]
- Bhattarai SR, Khalil KA, Dewidar M, Hwang PH, Yi HK, Kim HY. (2008). Novel production method and in-vitro cell compatibility of porous Ti-6Al-4V alloy disk for hard tissue engineering. J Biomed Mater Res A 86:289-299 [DOI] [PubMed] [Google Scholar]
- Black J. (1986). Electrical stimulation: its role in growth, repair, and remodeling of the musculoskeletal system. New York: Praeger Publishers [Google Scholar]
- Black J, Baranowski TJ, Brighton CT. (1984). Electrochemical aspects of DC stimulation of osteogenesis. Bioelectrochemistry and Bioenergetics 12:323-327 [Google Scholar]
- Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, et al. (1998). Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Commun 250:458-461 [DOI] [PubMed] [Google Scholar]
- Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR. (1999). Effect of Faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun 264:657-661 [DOI] [PubMed] [Google Scholar]
- Borgens RB, Jaffe LF, Cohen MJ. (1980). Large and persistent electrical currents enter the transected lamprey spinal-cord. Proc Natl Acad Sci USA 77:1209-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezniak N, Wasserstein A. (2002). Orthodontically induced inflammatory root resorption. Part II: The clinical aspects. Angle Orthod 72:180-184 [DOI] [PubMed] [Google Scholar]
- Brighton CT, Hunt RM. (1986). Ultrastructure of electrically induced osteogenesis in the rabbit medullary canal. J Orthop Res 4:27-36 [DOI] [PubMed] [Google Scholar]
- Brighton CT, Tadduni GT, Pollack SR. (1985). Treatment of sciatic denervation disuse osteoporosis in the rat tibia with capacitively coupled electrical-stimulation—dose-response and duty cycle. J Bone Joint Surg Am 67:1022-1028 [PubMed] [Google Scholar]
- Brighton CT, Fisher JR, Levine SE, Corsetti JR, Reilly T, Landsman AS, et al. (1996). The biochemical pathway mediating the proliferative response of bone cells to a mechanical stimulus. J Bone Joint Surg Am 78:1337-1347 [DOI] [PubMed] [Google Scholar]
- Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. (2001). Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am 83:1514-1523 [DOI] [PubMed] [Google Scholar]
- Brunette D, Tengvall P, Textor M, Thomsen P. (2001). Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. 1st ed. Berlin: Springer [Google Scholar]
- Bundy KJ, Marek M, Hochman RF. (1983). In vivo and in vitro studies of the stress-corrosion cracking behavior of surgical implant alloys. J Biomed Mater Res 17:467-487 [DOI] [PubMed] [Google Scholar]
- Burr DB, Robling AG, Turner CH. (2002). Effects of biomechanical stress on bones in animals. Bone 30:781-786 [DOI] [PubMed] [Google Scholar]
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. (2004). Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 83:529-533 [DOI] [PubMed] [Google Scholar]
- Charles AE, Ness MG. (2006). Crevice corrosion of implants recovered after tibial plateau leveling osteotomy in dogs. Vet Surg 35:438-444 [DOI] [PubMed] [Google Scholar]
- Cobo J, Sicilia A, Arguelles J, Suarez D, Vijande M. (1993). Initial stress-induced in periodontal tissue with diverse degrees of bone loss by an orthodontic force—tridimensional analysis by means of the finite-element method. Am J Orthod Dentofacial Orthop 104:448-454 [DOI] [PubMed] [Google Scholar]
- Cochran GV, Pawluk RJ, Bassett CA. (1967). Stress generated electric potentials in mandible and teeth. Arch Oral Biol 12:917-920 [DOI] [PubMed] [Google Scholar]
- Curtze S, Dembo M, Miron M, Jones DB. (2004). Dynamic changes in traction forces with DC electric field in osteoblast-like cells. J Cell Sci 117(Pt 13):2721-2729 [DOI] [PubMed] [Google Scholar]
- Denaro V, Papapietro N, Sgambato A, Barnaba SA, Ruzzini L, De Paola B, et al. (2008a). Periprosthetic electrochemical corrosion of titanium and titanium-based alloys as a cause of spinal fusion failure. Spine 33:8-13 [DOI] [PubMed] [Google Scholar]
- Denaro V, Cittadini A, Barnaba SA, Ruzzini L, Denaro L, Rettino A, et al. (2008b). Static electromagnetic fields generated by corrosion currents inhibit human osteoblast differentiation. Spine 33:955-959 [DOI] [PubMed] [Google Scholar]
- Dodge GR, Bowen JR, Oh CW, Tokmakova K, Simon BJ, Aroojis A, et al. (2007). Electrical stimulation of the growth plate: a potential approach to an epiphysiodesis. Bioelectromagnetics 28:463-470 [DOI] [PubMed] [Google Scholar]
- Doran A, Law FC, Allen MJ, Rushton N. (1998). Neoplastic transformation of cells by soluble but not particulate forms of metals used in orthopaedic implants. Biomaterials 19:751-759 [DOI] [PubMed] [Google Scholar]
- Dorr LD, Bloebaum R, Emmanual J, Meldrum R. (1990). Histologic, biochemical, and ion analysis of tissue and fluids retrieved during total hip-arthroplasty. Clin Orthop Relat Res 261:82-95 [PubMed] [Google Scholar]
- Duncan RL, Turner CH. (1995). Mechanotransduction and the functional-response of bone to mechanical strain. Calcif Tissue Int 57:344-358 [DOI] [PubMed] [Google Scholar]
- Ercan B, Webster TJ. (2008). Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int J Nanomedicine 3:477-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan B, Webster TJ. (2010). The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials 31:3684-3693 [DOI] [PubMed] [Google Scholar]
- Evans UR. (1960). The corrosion and oxidation of metals: scientific principles and practical applications. 1st ed. London: Edward Arnold Publishers [Google Scholar]
- Faes TJ, van der Meij HA, de Munck JC, Heethaar RM. (1999). The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas 20:R1-R10 [DOI] [PubMed] [Google Scholar]
- Ferrier J, Ross SM, Kanehisa J, Aubin JE. (1986). Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical-field. J Cell Physiol 129:283-288 [DOI] [PubMed] [Google Scholar]
- Finet B, Weber G, Cloots R. (2000). Titanium release from dental implants: an in vivo study on sheep. Materials Letters 43:159-165 [Google Scholar]
- Friedenberg ZB, Brighton CT. (1966). Bioelectric potentials in bone. J Bone Joint Surg Am 48:915-923 [PubMed] [Google Scholar]
- Frisken KW, Dandie GW, Lugowski S, Jordan G. (2002). A study of titanium release into body organs following the insertion of single threaded screw implants into the mandibles of sheep. Aust Dent J 47:214-217 [DOI] [PubMed] [Google Scholar]
- Fukada E, Yasuda I. (1957). On the piezoelectric effect of bone. J Phys Soc Jpn 12:1158-1162 [Google Scholar]
- Gaber S, Fischerauer EE, Frohlich E, Janezic G, Amerstorfer F, Weinberg AM. (2009). Chondrocyte apoptosis enhanced at the growth plate: a physeal response to a diaphyseal fracture. Cell Tissue Res 335:539-549 [DOI] [PubMed] [Google Scholar]
- Gan JC, Glazer PA. (2006). Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J 15:1301-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JL, Mehta M, Pinder B. (2009). Fretting crevice corrosion of stainless steel stem-CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Rest B: Applied Biomater 88:162-173 [DOI] [PubMed] [Google Scholar]
- Goldberg JR, Gilbert JL. (1997). Electrochemical response of CoCrMo to high-speed fracture of its metal oxide using an electrochemical scratch test method. J Biomed Mater Res 37:421-431 [DOI] [PubMed] [Google Scholar]
- Grosgogeat B, Reclaru L, Lissac M, Dalard F. (1999). Measurement and evaluation of galvanic corrosion between titanium/Ti6Al4V implants and dental alloys by electrochemical techniques and auger spectrometry. Biomaterials 20:933-941 [DOI] [PubMed] [Google Scholar]
- Guerkov HH, Lohmann CH, Liu Y, Dean DD, Simon BJ, Heckman JD, et al. (2001). Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin Orthop Relat Res 384:265-279 [DOI] [PubMed] [Google Scholar]
- Guzelsu N, Demiray H. (1979). Electro-mechanical properties and related models of bone tissues—review. Int J Engineering Science 17:813-851 [Google Scholar]
- Hanawa T. (2004). Metal ion release from metal implants. Mater Sci Engineering C: Biomimetic and Supramolecular Syst 24:745-752 [Google Scholar]
- Hazar Yoruc AB, Kelesoglu E. (2009). Fatigue behaviour of the chemically treated titanium grade 4 implant material. J Optoelectr Biomed Mater 1:200-208 [Google Scholar]
- Hou B, Fukai N, Olsen BR. (2007). Mechanical force-induced midpalatal suture remodeling in mice. Bone 40:1483-1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham E, Fisher J. (2000). Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng [H] 214:21-37 [DOI] [PubMed] [Google Scholar]
- Isaacson RJ, Lindauer SJ, Davidovitch M. (1993). On tooth movement. Angle Orthod 63:305-309 [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, et al. (2008). A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 105:7970-7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Gilbert JL, Urban RM. (1998). Corrosion of metal orthopaedic implants. J Bone Joint Surg Am 80:268-282 [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. (2001). Osteolysis: basic science. Clin Orthop Relat Res 393:71-77 [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. (2009). Metal-on-metal bearing surfaces. J Am Acad Orthop Surg 17:69-76 [DOI] [PubMed] [Google Scholar]
- Kaesche H. (2003). Corrosion of metals: physicochemical principles and current problems. 1st ed. Berlin: Springer [Google Scholar]
- Kieswetter K, Schwartz Z, Dean DD, Boyan BD. (1996). The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med 7:329-345 [DOI] [PubMed] [Google Scholar]
- Kim IS, Song JK, Zhang YL, Lee TH, Cho TH, Song YM, et al. (2006). Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochim Biophys Acta 1763:907-916 [DOI] [PubMed] [Google Scholar]
- Lai WM, Mow VC, Sun DD, Ateshian GA. (2000). On the electric potentials inside a charged soft hydrated biological tissue: streaming potential versus diffusion potential. J Biomech Eng 122:336-346 [DOI] [PubMed] [Google Scholar]
- Landolt D. (2007). Corrosion and surface chemistry of metals. 1st ed. Lausanne, Switzerland: EPFL Press [Google Scholar]
- Lassus J, Salo J, Jiranek WA, Santavirta S, Nevalainen J, Matucci-Cerinic M, et al. (1998). Macrophage activation results in bone resorption. Clin Orthop Relat Res 352:7-15 [PubMed] [Google Scholar]
- Levin M. (2007). Large-scale biophysics: ion flows and regeneration. Trends Cell Biol 17:261-270 [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. (2002). Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111:77-89 [DOI] [PubMed] [Google Scholar]
- Lewis AC, Kilburn MR, Papageorgiou I, Allen GC, Case CP. (2005). Effect of synovial fluid, phosphate-buffered saline solution, and water on the dissolution and corrosion properties of CoCrMo alloys as used in orthopedic implants. J Biomed Mater Res A 73:456-467 [DOI] [PubMed] [Google Scholar]
- Lirani-Galvao AP, Chavassieux P, Portero-Muzy N, Bergamaschi CT, Silva OL, Carvalho AB, et al. (2009). Low-intensity electrical stimulation counteracts the effects of ovariectomy on bone tissue of rats: effects on bone microarchitecture, viability of osteocytes, and nitric oxide expression. Calcif Tissue Int 84:502-509 [DOI] [PubMed] [Google Scholar]
- Lohmann CH, Schwartz Z, Koster G, Jahn U, Buchhorn GH, MacDougall MJ, et al. (2000a). Phagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials 21:551-561 [DOI] [PubMed] [Google Scholar]
- Lohmann CH, Schwartz Z, Liu Y, Guerkov H, Dean DD, Simon B, et al. (2000b). Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Orthop Res 18:637-646 [DOI] [PubMed] [Google Scholar]
- Lokietek W, Pawluk RJ, Bassett CA. (1974). Muscle injury potentials—source of voltage in undeformed rabbit tibia. J Bone Joint Surg Br 56:361-369 [PubMed] [Google Scholar]
- Long M, Rack HJ. (1998). Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 19:1621-1639 [DOI] [PubMed] [Google Scholar]
- Lorich DG, Brighton CT, Gupta R, Corsetti JR, Levine SE, Gelb ID, et al. (1998). Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin Orthop Relat Res 350:246-256 [PubMed] [Google Scholar]
- Lugowski SJ, Smith DC, McHugh AD, Van Loon JC. (1991). Release of metal-ions from dental implant materials in vivo—determination of Al, Co, Cr, Mo, Ni, V, and Ti in organ tissue. J Biomed Mater Res 25:1443-1458 [DOI] [PubMed] [Google Scholar]
- Mareci D, Chelariu R, Gordin DM, Ungureanu G, Gloriant T. (2009). Comparative corrosion study of Ti-Ta alloys for dental applications. Acta Biomater 5:3625-3639 [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. (2005). Controlling cell behavior electrically: Current views and future potential. Physiol Rev 85:943-978 [DOI] [PubMed] [Google Scholar]
- McGuff HS, Heim-Hall J, Holsinger FC, Jones AA, O’Dell DS, Hafemeister AC. (2008). Maxillary osteosarcoma associated with a dental implant: report of a case and review of the literature regarding implant-related sarcomas. J Am Dent Assoc 139:1052-1059 [DOI] [PubMed] [Google Scholar]
- McGuinness NJ, Wilson AN, Jones ML, Middleton J. (1991). A stress-analysis of the periodontal-ligament under various orthodontic loadings. Eur J Orthod 13:231-242 [DOI] [PubMed] [Google Scholar]
- Meikle MC. (2006). The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28:221-240 [DOI] [PubMed] [Google Scholar]
- Mudali UK, Sridhar TM, Raj B. (2003). Corrosion of bio implants. Sadhana-Acad Proc Engineering Sci 28:601-637 [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. (2005). Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435:1239-1243 [DOI] [PubMed] [Google Scholar]
- Norton LA. (1975). Implications of bioelectric growth-control in orthodontics and dentistry. Angle Orthod 45:34-42 [DOI] [PubMed] [Google Scholar]
- Norton LA, Hanley KJ, Turkewicz J. (1984). Bioelectric perturbations of bone—research directions and clinical-applications. Angle Orthod 54:73-87 [DOI] [PubMed] [Google Scholar]
- Oliveira NT, Guastaldi AC. (2009). Electrochemical stability and corrosion resistance of Ti-Mo alloys for biomedical applications. Acta Biomater 5:399-405 [DOI] [PubMed] [Google Scholar]
- Papakyriacou M, Mayer H, Pypen C, Plenk H, Stanzl-Tschegg S. (2000). Effects of surface treatments on high cycle corrosion fatigue of metallic implant materials. Int J Fatigue 22:873-886 [Google Scholar]
- Piccolino M. (1997). Luigi Galvani and animal electricity: two centuries after the foundation of electrophysiology. Trends Neurosci 20:443-448 [DOI] [PubMed] [Google Scholar]
- Pioletti DP, Takei H, Kwon SY, Wood D, Sung KL. (1999). The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J Biomed Mater Res 46:399-407 [DOI] [PubMed] [Google Scholar]
- Poggio CE. (2007). Plasmacytoma of the mandible associated with a dental implant failure: a clinical report. Clin Oral Implants Res 18:540-543 [DOI] [PubMed] [Google Scholar]
- Rahal MD, Delorme D, Brånemark PI, Osmond DG. (2000). Myelointegration of titanium implants: B lymphopoiesis and hemopoietic cell proliferation in mouse bone marrow exposed to titanium implants. Int J Oral Maxillofac Implants 15:175-184 [PubMed] [Google Scholar]
- Rajnicek AM, Foubister LE, McCaig CD. (2007). Prioritising guidance cues: directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch. Dev Biol 312:448-460 [DOI] [PubMed] [Google Scholar]
- Reclaru L, Meyer JM. (1994). Study of corrosion between a titanium implant and dental alloys. J Dent 22:159-168 [DOI] [PubMed] [Google Scholar]
- Rubinacci A, Black J, Brighton CT, Friedenberg ZB. (1988). Changes in bioelectric potentials on bone associated with direct-current stimulation of osteogenesis. J Orthop Res 6:335-345 [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Boyan BD. (1994). Underlying mechanisms at the bone-biomaterial interface. J Cell Biochem 56:340-347 [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Simon BJ, Duran MA, Barabino G, Chaudhri R, Boyan BD. (2008). Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res 26:1250-1255 [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Fisher M, Lohmann CH, Simon BJ, Boyan BD. (2009). Osteoprotegerin (OPG) production by cells in the osteoblast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann Biomed Eng 37:437-444 [DOI] [PubMed] [Google Scholar]
- Serhan H, Slivka M, Albert T, Kwak SD. (2004). Is galvanic corrosion between titanium alloy and stainless steel spinal implants a clinical concern? Spine J 4:379-387 [DOI] [PubMed] [Google Scholar]
- Shafer DM, Rogerson K, Norton L, Bennett J. (1995). The effect of electrical perturbation on osseointegration of titanium dental implants—a preliminary-study. J Oral Maxillofac Surg 53:1063-1068 [DOI] [PubMed] [Google Scholar]
- Shigino T, Ochi M, Hirose Y, Hirayama H, Sakaguchi K. (2001). Enhancing osseointegration by capacitively coupled electric field: a pilot study on early occlusal loading in the dog mandible. Int J Oral Maxillofac Implants 16:841-850 [PubMed] [Google Scholar]
- Smith DC, Lugowski S, McHugh A, Deporter D, Watson PA, Chipman M. (1997). Systemic metal ion levels in dental implant patients. Int J Oral Maxillofac Implants 12:828-834 [PubMed] [Google Scholar]
- Song JK, Cho TH, Pan H, Song YM, Kim IS, Lee TH, et al. (2009). An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics 30:374-38419288541 [Google Scholar]
- Sul YT, Johansson C, Byon E, Albrektsson T. (2005). The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials 26:6720-6730 [DOI] [PubMed] [Google Scholar]
- Sun ZL, Wataha JC, Hanks CT. (1997). Effects of metal ions on osteoblast-like cell metabolism and differentiation. J Biomed Mater Res 34:29-37 [DOI] [PubMed] [Google Scholar]
- Teoh SH. (2000). Fatigue of biomaterials: a review. Intl J Fatigue 22:825-837 [Google Scholar]
- Wang N, Butler JP, Ingber DE. (1993). Mechanotransduction across the cell-surface and through the cytoskeleton. Science 260:1124-1127 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhong S, Jun O, Jiang L, Zhang Z, Xie Y, et al. (1998). Osteogenesis of electrically stimulated bone cells mediated in part by calcium ions. Clin Orthop Relat Res 348:259-268 [PubMed] [Google Scholar]
- Wang XX, Yan W, Hayakawa S, Tsuru K, Osaka A. (2003). Apatite deposition on thermally and anodically oxidized titanium surfaces in a simulated body fluid. Biomaterials 24:4631-4637 [DOI] [PubMed] [Google Scholar]
- Wang Z, Clark CC, Brighton CT. (2006). Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am 88:1053-1065 [DOI] [PubMed] [Google Scholar]
- Wray JB, Goodman HO. (1961). Post-fracture vascular phenomena and long-bone overgrowth in the immature skeleton of the rat. J Bone Joint Surg Am 43(A):1047-1055 [PubMed] [Google Scholar]
- Yamazoe J, Nakagawa M, Matono Y, Takeuchi A, Ishikawa K. (2007). The development of Ti alloys for dental implant with high corrosion resistance and mechanical strength. Dent Mater J 26:260-267 [DOI] [PubMed] [Google Scholar]
- Zengo AN, Pawluk RJ, Bassett CA. (1973). Stress-induced bioelectric potentials in dentoalveolar complex. Am J Orthod 64:17-27 [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442:457-460 [DOI] [PubMed] [Google Scholar]