Abstract
Recent studies have reported collagen cross-linking after exposure to riboflavin followed by ultraviolet-A (UVA) exposure. This study is the first to investigate the effect of a riboflavin-containing primer on adhesive interface stability and dentinal matrix metalloproteinase activity. Human dentin was etched with 35% phosphoric acid, treated with 0.1% riboflavin, exposed to UVA for 2 min, and bonded with a two-step etch-and-rinse adhesive. Adhesive was applied to control specimens without riboflavin/UVA. Specimens were subjected to microtensile bond strength tests and pulled to failure after storage for 24 hrs, 6 mos, or 1 yr. Interfacial nanoleakage was evaluated by light and transmission electron microscopy. To investigate dentinal matrix metalloproteinase activity, we performed correlative zymographic assays on protein extracts obtained from phosphoric-acid-etched dentin powder with or without riboflavin/UVA treatment and XP Bond. Ultraviolet-activated riboflavin treatment increased the immediate bond strength to dentin at all aging intervals (p < 0.05 vs. control) and decreased interfacial nanoleakage in aged specimens (1 yr; p < 0.05). Zymograms revealed that riboflavin/UVA pre-treatment inhibited dentinal matrix metalloproteinase activity (especially MMP-9). In conclusion, dentinal collagen cross-linking induced by riboflavin/UVA increased immediate bond strength, stabilized the adhesive interface, and inhibited dentin matrix metalloproteinases, thereby increasing the durability of resin-dentin bonds.
Keywords: riboflavin, dentin bonding agent, collagen cross-linking, matrix metalloproteinases, nanoleakage, dentin matrix
Introduction
In etch-and-rinse or self-etch adhesive bonding systems, the stability and integrity of collagen fibrils within the hybrid layer are crucial for the maintenance of bond effectiveness over time (Breschi et al., 2008; Liu et al., 2011). Several approaches have been used to improve bond durability, including modification of dentin substrate with different cross-linking agents to strengthen the collagen network (Bedran-Russo et al., 2007, 2008, 2011; Liu et al., 2011).
The available approaches to increase collagen cross-linking can be divided into chemical methods, wherein different cross-linking solutions such as glutaraldehyde, formaldehyde, transglutaminase, carbodiimide, genepin, and proanthocyanidin are used (Munksgaard and Asmussen, 1984; Han et al., 2003; Bedran-Russo et al., 2007, 2008; Castellan et al., 2010); or a physical method (also called photo-oxidative) that uses light exposure, especially ultraviolet radiation (Foote, 1968; Barnard et al., 1987).
Glutaraldehyde is a dialdehyde cross-linking agent used commonly as a fixative due to its affinity for active nitrogen groups of amino acids (Cheung et al., 1985; Pashley et al., 2001), and it is probably the most common chemical used to stiffen dentin collagen fibrils. Although priming with glutaraldehyde solution increases in vitro resin-dentin bond strengths, it may not increase the ultimate tensile strength of dentin as well as do proanthocyanidin and genipin (Bedran-Russo et al., 2007, 2008; Castellan et al., 2010). Moreover, being common components of fruits, vegetables, and seeds, genipin and proanthocyanidin have an advantage of not producing the toxic effects (Han et al., 2003) that are reported with glutaraldehyde (Cheung et al., 1985).
For the photo-oxidative collagen cross-linking method, the presence of singlet oxygen is required, and riboflavin (vitamin B2) is one of the most potent producers of these oxygen radicals (Snibson, 2010). Riboflavin is not toxic and is also used as a food dye. Because of its lack of toxicity, collagen cross-linking induced by ultraviolet A (UVA) radiation of photosensitive riboflavin has been reported as a successful treatment for human ophthalmic diseases such as keratoconus (Wollensak et al., 2003; Snibson, 2010), wherein the recovery of collagen stiffness is necessary. While previous studies have emphasized the importance of strengthening the collagen network to improve adhesion, the use of UVA-activated riboflavin on dentinal collagen has not been previously tested.
The aim of this study was to evaluate the ability of an UVA-activated, riboflavin-containing experimental primer to cross-link dentinal collagen to improve bond strength and stabilize the adhesive interface. Since the activity of dentinal MMPs has been implicated in the degradation of resin-dentin bonds (Breschi et al., 2010; Liu et al., 2011), the effect of riboflavin/UVA on the activity of MMPs was also investigated. The tested hypotheses were that the application of this primer to acid-etched dentin before bonding: (1) would not affect immediate bond strength or interfacial nanoleakage expression; (2) would prevent adhesive interface degradation over time; and (3) would inhibit dentinal MMPs activity.
Materials & Methods
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless stated otherwise.
Specimen Preparation
Sixty freshly extracted, non-carious, human third molars were used. Patients provided informed consent for the use of their teeth in this research, and the study protocol was approved by the Human Assurance Committee of the University of Bologna (Italy).
Tooth crowns were flattened with the use of a low-speed diamond saw (Micromet; Remet, Bologna, Italy) under water irrigation, and a standardized smear layer was created with wet 600-grit silicon-carbide (SiC) paper. Dentin was etched for 15 sec with 35% phosphoric-acid gel (Dentsply DeTrey GmbH, Konstanz, Germany), rinsed with water, and kept moist in accordance with the wet-bonding technique.
Specimens were allocated randomly to treatment and control groups (n = 30). The etched dentin surfaces of Group 1 were treated for 60 sec with a water solution of 0.1% riboflavin-5- phosphate (2 mmol/L), gently air-dried, and exposed to UVA rays for 2 min under a UV lamp (Philips, Hamburg, Germany; λ = 370 nm at 3 mW/cm2 or 5.4 joule/cm2) placed 1 cm from the dentin surface (Wollensak et al., 2003). After riboflavin pre-treatment, the dentin surface was kept moist in accordance with the wet-bonding technique. XP Bond adhesive (Dentsply) was applied following the manufacturer’s instructions. XP Bond was applied to specimens in Group 2 (control group) in accordance with the manufacturer’s instructions, without riboflavin or UV treatment.
Each bonded specimen was light-cured for 20 sec (Curing Light 2500; 3M ESPE, St. Paul, MN, USA). Four 1-mm-thick layers of microhybrid resin composite (Filtek Z250; 3M ESPE) were placed and polymerized individually for 20 sec. The teeth were then stored overnight in water at 25°C.
Microtensile Bond Strength Test
We sectioned the bonded specimens serially in both x and y directions across the adhesive interface to obtain approximately 1-mm-thick beams in accordance with the microtensile non-trimming technique. The dimension of each stick was measured with digital calipers (± 0.01 mm), and the bonded area was calculated for subsequent conversion of microtensile strength values into units of stress (MPa). Beams were pulled to failure after 24 hrs (T0), 6 mos (T6), or 1 yr (T12) of storage in artificial saliva at 37°C. The artificial saliva contained (mmol/L): CaCl2 (0.7), MgCl2·6H2O (0.2), KH2PO4 (4.0), KCl (30), NaN3 (0.3), and HEPES buffer (20.0). Bonded beams were stressed to failure under tension in a simplified universal testing machine (Bisco, Inc., Schaumburg, IL, USA) at a crosshead speed of 1 mm/min. Each specimen was observed under a stereomicroscope (Stemi 2000-C; Carl Zeiss Jena GmbH, Göttingen, Germany) at 50× magnification to determine the mode of failure, classified as adhesive (A), cohesive in composite (CC), cohesive in dentin (CD), or mixed (M).
Because a Kolmogorov–Smirnov test determined that values were normally distributed, data were analyzed by two-way (surface treatment, storage) analysis of variance (ANOVA) and post hoc Tukey tests. P values of 0.05 were considered to indicate statistical significance.
Interfacial Nanoleakage Evaluation
Twelve additional teeth (n = 6/group) were processed for interfacial nanoleakage evaluation. Middle/deep dentin was selected and bonded as described previously. A 1-mm-thick flowable composite (Filtek Flow; 3M ESPE) was applied to bonded disks and light-cured. Bonded specimens were then cut vertically into 1-mm-thick slabs to expose the bonded surfaces and stored for 24 hrs (T0), 6 mos (T6), or 1 yr (T12) in artificial saliva at 37°C.
Specimens were prepared for correlative light (LM) and transmission electron microscopy (TEM) analyses, following the procedures described by Suppa et al. (2006). Briefly, specimens were immersed in 50 wt% ammoniacal silver nitrate (AgNO3) for 24 hrs, then immersed in photo-developing solution, dehydrated in ascending alcohols, and embedded in epoxy resin. LM preparation was performed in accordance with Saboia et al. (2008); specimens were fixed on glass slides with cyanoacrylate glue and flattened to approximately 40 μm in thickness with 600-, 800-, 1200-, and 2400-grit SiC paper under running water (LS2, Remet). Specimens were then stained with 0.5% acid fuchsin for 15 min and observed under LM (Nikon E 800; Tokyo, Japan).
Images of the adhesive interfaces were obtained (original magnification: 1000×), and the degree of interfacial nanoleakage was scored on a scale of 0 to 4 by two observers as described by Saboia et al. (2008). Interfacial nanoleakage was scored based on the percentage of the adhesive surface showing silver nitrate deposition: 0, no nanoleakage; 1, < 25% nanoleakage; 2, 25 to ≤ 50% nanoleakage; 3, 50 to ≤ 75% nanoleakage; and 4, > 75% nanoleakage. Differences among specimens in each scoring category were analyzed by chi-square tests (significance was set at p < 0.05). Inter-observer agreement was measured by Cohen’s kappa test.
Representative embedded specimens from each group were also cut into thin sections and processed for interfacial nanoleakage evaluation with TEM (CM-10; Philips, Eindhoven, The Netherlands).
Zymographic Analysis
Ten additional teeth were powdered as described by Mazzoni et al. (2007). Four 1-g aliquots of dentin powder were partially demineralized with 1% phosphoric acid at 4°C for 10 min, then assigned to one of the following treatment groups: Group A, untreated; Group B, incubated for 30 min with XP Bond; Group C, incubated for 60 sec with 0.1% riboflavin, followed by UVA-photopolymerization for 2 min; and Group D, incubated with 0.1% riboflavin for 60 min, followed by UVA photopolymerization for 2 min and application of XP Bond for 30 min in the dark.
Proteins were then extracted for zymographic assays as described by Breschi et al. (2010). Briefly, specimens were rinsed with acetone, re-suspended in extraction buffer for 24 hrs, and centrifuged. Supernatants were collected, and protein content was precipitated with 25% trichloroacetic acid and resolubilized in loading buffer [Trizma and sodium dodecyl sulfate (SDS) in water; pH 8.8].
Total protein concentrations of the dentin extracts were determined by Bradford assay. In addition, purified human recombinant MMP-2 and MMP-9 (Calbiochem, Darmstadt, Germany) were included as positive controls. Proteins were subjected to electrophoresis under non-reducing conditions on 7.5% SDS-polyacrylamide gels copolymerized with 2 g/L gelatin. The gelatinase proforms were activated with 2 mM p-aminophenylmercuric acetate (APMA) at 37°C for 1 hr. The SDS-polyacrylamide gels were then incubated at 37°C for 24 hrs in zymography buffer, stained with 0.2% Coomassie Brilliant Blue R-250, and processed in destaining buffer (Breschi et al., 2010).
Control zymograms were incubated in the presence of 5 mM ethylenediaminetetraacetic acid (EDTA) or 2 mM 1,10-phenanthroline to inhibit gelatinases.
Results
Microtensile Bond Strength
The Table presents the means and standard deviations of microtensile bond strength (in MPa) at T0, T6, and T12 and failure modes of Groups 1 and 2. The use of the experimental primer containing UVA-activated riboflavin before XP Bond application (Group 1) increased the immediate bond strength compared with control specimens (Group 2; Table). Over the next 6 mos, the riboflavin cross-linked (Group 1) specimens showed small but significant (p < 0.05) 19.8% reductions in bond strength, while the untreated control fell 41%. After storage for 1 yr, the riboflavin cross-linked specimens showed no further significant loss of bond strength (30.4%), while the control specimens fell to a cumulative loss of 52.5% of their original values.
Table.
Means and Standard Deviations of Microtensile Bond Strength of Group 1 (0.1% riboflavin pre-treatment) and Group 2 (control) Immediately after Bonding (T0), after 6 mos (T6) and 1 yr (T12) of Aging in Artificial Saliva at 37°C
| Nanoleakage Expression (under LM observation) | ||||||
|---|---|---|---|---|---|---|
| Group | Microtensile Bond Strength and Failure Mode % | Score 0-4 | N of Images (total of images) | % Relative Nanoleakage Score | Statistical Difference | |
| Riboflavin 0.1% | T0 | 44.4 ± 10.4aMPa A:56; CC:0; CD:2; M:42 [92/8] |
0 1 2 3 4 |
26 (81) 33 (81) 11 (81) 10 (81) 1 (81) |
32.1 40.7 13.6 12.4 1.2 |
A |
| T6 |
35.6 ± 11.2bMPa A:61; CC:2; CD:3; M:34 [85/6] |
0 1 2 3 4 |
21 (64) 18 (64) 11 (64) 5 (64) 9 (64) |
32.8 28.1 17.1 7.8 14.2 |
B | |
| T12 | 30.9 ± 12.2bMPa A:68; CC:0; CD:3; M:29 [106/5] |
0 1 2 3 4 |
6 (70) 11 (70) 18 (70) 20 (70) 15 (70) |
8.6 15.7 25.7 28.6 21.4 |
C | |
| Group Control |
T0 | 37.3 ± 10.3bMPa A:69; CC:8; CD:3; M:20 [92/4] |
0 1 2 3 4 |
25 (76) 29 (76) 8 (76) 10 (76) 4 (76) |
32.9 38.2 10.5 13.2 5.2 |
A |
| T6 | 22.0 ± 7.0cMPa A:70; CC:7; CD:4; M:19 [75/4] |
0 1 2 3 4 |
3 (71) 35 (71) 18 (71) 9 (71) 6 (71) |
4.2 49.3 25.3 12.7 8.5 |
B | |
| T12 | 17.7 ± 9cMPa A:59; CC:5; CD:0; M:36 [102/5] |
0 1 2 3 4 |
0 (96) 6 (96) 7 (96) 33 (96) 50 (96) |
0.0 6.3 7.3 34.4 52.0 |
D | |
Values of microtensile bond strength are means ± standard deviations [number of intact sticks tested/premature failures]. Groups with the same superscripts are not statistically significant (p > 0.05). Different capital letters indicate statistical differences in interfacial nanoleakage expression (chi-square test; p < 0.05).
Percentages of the failure modes after microtensile test analyzed by stereomicroscopy were classified as: A, adhesive; CC, cohesive in composite; CD, cohesive in dentin; and M, mixed failure. The total numbers of beams and pre-test failures are in brackets.
For each group, numbers of sections and assigned nanoleakage scores are also reported. Interfacial nanoleakage expression under LM was scored based on the percentage of the adhesive surface showing silver nitrate deposition in accordance with Saboia et al. (2008): 0 = no nanoleakage; 1 = < 25% with nanoleakage; 2 = 25% to ≤ 50% with nanoleakage; 3 = 50% to ≤ 75% with nanoleakage; and 4 = > 75% with nanoleakage. Increasing gray relates to increased nanoleakage expression.
Interfacial Nanoleakage Expression
The Table summarizes the extent of LM interfacial nanoleakage. The quantity of interfacial silver uptake did not differ between groups at T0 or T6 (p > 0.05; Fig. 1). At T12, fewer interfacial silver deposits were found in the riboflavin-treated specimens (Group 1) than in control specimens (Group 2; p < 0.05; Fig. 1). Inter-observer agreement was nearly perfect (κ = 0.81). TEM observation (Fig. 2) revealed similar findings of LM analysis.
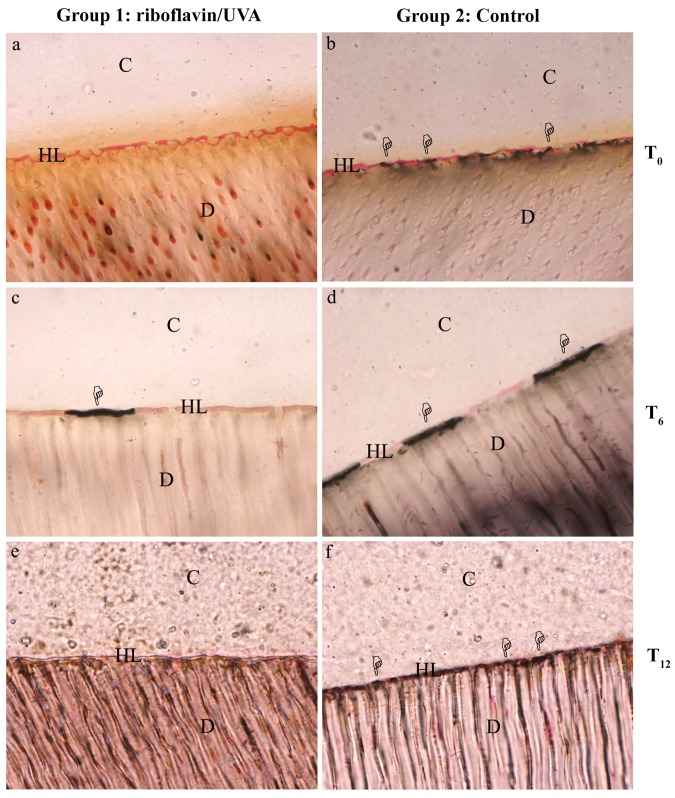
Figure 1.
Light microscopy images (1000×) showing interfacial nanoleakage expression (pointers). Similar deposits of silver nitrate were found at time 0 (T0; Figs. 1a, 1b) and after 6 mos of storage in artificial saliva (T6; Figs. 1c, 1d) in riboflavin/UVA-treated specimens compared with controls. Decreased nanoleakage expression was found in riboflavin/UVA-treated specimens after 12 mos of aging (T12; Figs. 1e, 1f) compared with controls. C, composite resin; HL, hybrid layer; D, dentin. (a) Representative image of a nanoleakage-free area (score = 0) within the adhesive interface created by XP Bond after treatment with riboflavin/UVA immediately after bonding (T0). (b) Representative image of deposits of silver nitrate (score = 3) within the control adhesive interface immediately after bonding (T0; control). (c) Representative image of score = 1 of the riboflavin/UVA-treated interface aged for 6 mos in artificial saliva (T6). (d) Representative image of score = 3 of a control specimen at T6. After 12 mos of storage in artificial saliva at 37°C, the riboflavin/UVA-treated interface showed large areas of the hybrid layer nanoleakage-free (e) representative image of score 0; T12 with minor deposits compared with control specimens (f) representative image of score = 4.
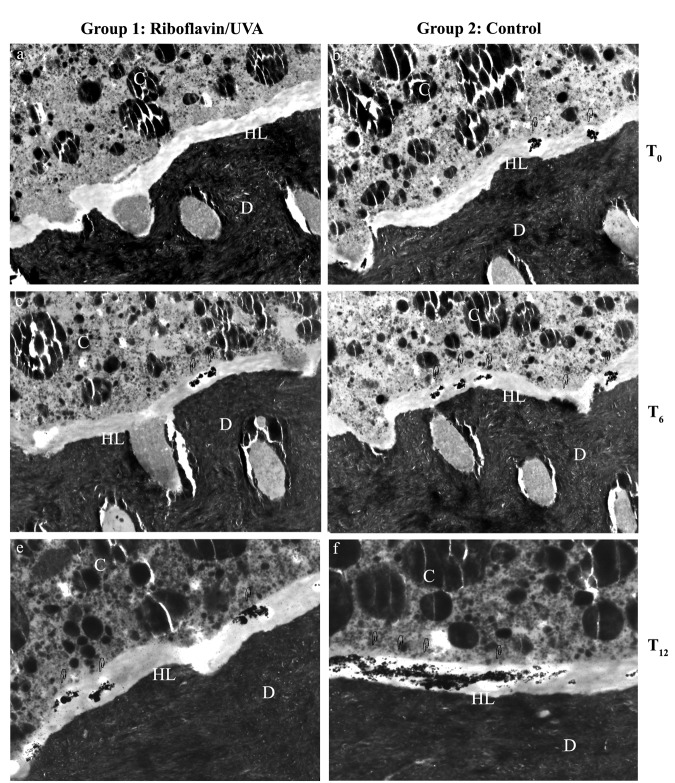
Figure 2.
Transmission electron microscopy images showing interfacial nanoleakage expression. D, dentin; HL, hybrid layer; C, composite resin. Original magnification: 4600×. Adhesive interfaces created by XP Bond after riboflavin/UVA treatment (Figs. 2a, 2c, 2e) or on control (Figs. 2b, 2d, 2f), respectively. Scattered deposits of silver nitrate were found immediately after bonding on riboflavin/UVA-treated specimens (T0, Fig. 2a) and on control specimens (T0, Fig. 2b). After 6 mos of storage in artificial saliva at 37°C, both the riboflavin/UVA-treated interface (T6, Fig. 2c) and the control specimens (T6, Fig. 2d) showed similar silver deposits with increased nanoleakage compared with T0. After 12 mos of storage, silver deposits were recorded in the riboflavin/UVA-treated group (T12, Fig. 2e), and large areas of silver diffused throughout the hybrid layer were frequently found in controls (T12, Fig. 2f).
Zymographic Analysis
Results of zymographic analysis are shown in Fig. 3. Positive controls of recombinant human MMP-2 and -9 revealing pro- and active forms are shown in lanes 1 and 2, respectively. Phosphoric-acid-demineralized dentin extracts showed multiple forms of gelatinolytic enzymes, including a 72-kDa MMP-2 proform, a fainter 86-kDa band corresponding to the active form of MMP-9, and other minor gelatinolytic bands (Fig. 3, lane 3). The application of XP Bond resulted in similar enzymatic activation (Fig. 3, lane 4), whereas the incubation of demineralized dentin with 0.1% riboflavin resulted in the partial inhibition of the MMP-2 proform and complete inhibition of the 86-kDa active MMP-9 (with or without XP bond; Fig. 3, lanes 5 and 6, respectively).
Figure 3.
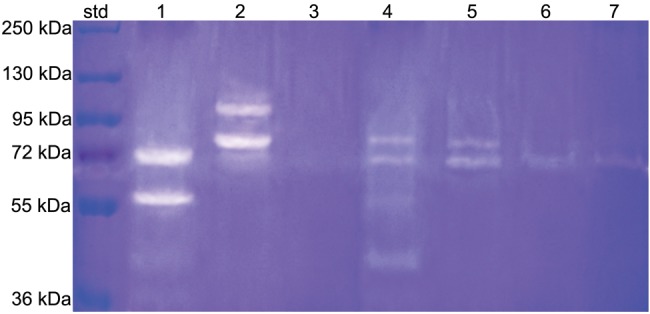
Results of zymographic analysis. Lane 1: recombinant human MMP-2 enzyme showing proform (72 kDa) and active form (64 kDa). Lane 2: recombinant human MMP-9 enzyme showing proform (92 kDa) and active form (86 kDa). Lane 3: Group A, phosphoric-acid-demineralized dentin extracts showed multiple forms of gelatinolytic enzymes, including a 72-kDa MMP-2 proform, a fainter 86-kDa band corresponding to the active form of MMP-9, and other minor gelatinolytic bands with lower molecular weights. Lane 4: Group B, XP Bond-treated dentin (Group 2) showed intense activity related to the MMP-2 proform and fainter activity related to MMP-9. Lane 5: Group C, incubation of phosphoric-acid-demineralized dentin powder treated with 0.1% riboflavin solution plus irradiation with UVA resulted in the partial inactivation of the 72-kDa MMP-2 proform and complete inhibition of the 86-kDa MMP-9. Lane 6: Group D, incubation of phosphoric-acid-demineralized dentin powder with riboflavin followed by incubation with XP Bond also resulted in partial inhibition of the 72-kDa MMP-2 proform and complete inactivation of the 86-kDa MMP-9.
Control zymograms incubated with 5 mM EDTA or 2 mM 1,10-phenanthroline showed no enzymatic activity (data not shown).
Discussion
This was the first study that evaluated the photo-oxidative cross-linking method consisting of the combined application of riboflavin-containing primer and UVA irradiation on the adhesive performance of a two-step etch-and-rinse adhesive. The first null hypothesis was partially accepted, since the application of UVA-activated riboflavin before bonding increased the immediate bond strength of XP Bond, but did not affect immediate interfacial nanoleakage expression. After in vitro aging (i.e., 6 mos or 1 yr), the use of UVA-activated riboflavin primer produced hybrid layers with significantly less nanoleakage compared with control groups, which led to the acceptance of the second study hypothesis. Interestingly, UVA-activated riboflavin primer inhibited the zymographic activity of MMPs, which also led to the acceptance of the third study hypothesis.
Collagen constitutes 90% of the dentin organic matrix, and along with the mineral component, contributes to the biomechanical properties and functional integrity of this tissue. In physiological conditions, collagen is stabilized by the formation of native cross-links, which decreases its solubility as well as provides greater tensile properties. Aggregated forms of collagen fibrils are strengthened by intermolecular cross-links that occur as part of the maturation process of tissues or in response to a disease (Prockop and Fertala, 1998). Lysine and hydroxylysine residues in collagen telopeptides participate in cross-linking (Bella et al., 1994). Post-translational modifications of the collagenous matrix are structurally and mechanically important, and the disruption of cross-linking can result in severe tissue dysfunction (Knott and Bailey, 1998).
Natural cross-linking of collagen over time increases the number of covalent inter- and intramolecular collagen cross-links (Han et al., 2003; Bedran-Russo et al., 2007) by the oxidative deamination of lysine or hydroxylysine in telopeptides, leading to the formation of pyridinium bonds (Barnard et al., 1987).
The basic principle of photo-oxidative cross-linking is the same as that of photopolymerization, that is, UVA radiation causes the release of reactive oxygen species that can induce the formation of covalent cross-links through oxidation (Sionkowska, 2006). In cross-linking therapy with riboflavin/UVA, yellow riboflavin works as a photosensitizer that stimulates the formation of reactive oxygen species, and, at the same time, it also acts as a shield from the penetration of UVA (Snibson, 2010).
The effects of cross-linking agents on stabilizing dentin matrix degradation have been attributed to their capacity to increase the stiffness of dentin collagen. Nevertheless, the present results strongly indicate that riboflavin/UVA pre-treatment can also inactivate MMPs, particularly MMP-9 (Fig. 3). Latent MMPs are present in mineralized dentin and can be activated in low-pH environments (Tjäderhane et al., 1998; Pashley et al., 2004; Mazzoni et al., 2006, 2007). Etching of dentin, followed by the application of an etch-and-rinse adhesive, exposes endogenous MMPs by removing the mineral component, thereby contributing to an activation process related to the acidity of the etchant and adhesive (Mazzoni et al., 2006; Pashley et al., 2011). We speculate that UVA-activated riboflavin may reduce MMPs activity through direct cross-linking of MMPs and by strengthening the collagen fibrils through cross-linking. Telopeptidase activity of osteoclast-derived MMP-9, eliminating collagen molecule telopeptides, is considered essential for collagenase activity against insoluble bone collagen (Okada et al., 1995). The riboflavin-induced MMP-9 inhibition may be responsible for the increased durability of hybrid layers, via reduced MMP-9 telopeptidase activity. Considering the important role of dentinal MMPs in the degradation of hybrid layers over time (Carrilho et al., 2007; Breschi et al., 2008, 2010; Liu et al., 2011), future research should more fully investigate the MMPs inactivation induced by cross-linking agents.
The advantage of inactivating proteolytic enzymes in the dentin matrix by cross-linking is that the mechanism is non-specific—that is, it cross-links all types of MMPs found in dentin as well as cysteine cathepsins (Tersariol et al., 2010; Nascimento et al., 2011) and collagen. These cross-links involve covalent bonds that are stable over time.
Previous attempts to inhibit MMPs with chlorhexidine or galardin (Carrilho et al., 2007; Breschi et al., 2010) required that these inhibitors bind to MMPs irreversibly; however, there is no evidence that the inhibitors will inhibit the enzymes irreversibly. Since MMPs do not turn over in dentin, their inactivation by cross-linking agents should last for a long time and may be even more effective than inhibitors.
In conclusion, the present findings support the use of collagen cross-linkers to increase immediate bond strength stabilizing the adhesive interface over time. Further studies should clarify the role of cross-linking agents on dentinal MMPs.
Footnotes
The study was funded with an FIRB grant (MIUR, Italy), and by grants R21 DE 091213 (P.I. FRT) and R01 DE 015306-07 from the NIH/NIDCR (P.I. DHP).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Barnard K, Light ND, Sims TJ, Bailey AJ. (1987). Chemistry of the collagen cross-links. Origin and partial characterization of a putative mature cross-link of collagen. Biochem J 244:303-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. (2007). Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater 80:268-272 [DOI] [PubMed] [Google Scholar]
- Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. (2008). Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater 86:330-334 [DOI] [PubMed] [Google Scholar]
- Bedran-Russo AK, Castellan CS, Shinohara MS, Hassan L, Antunes A. (2011). Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater 7:1735-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella J, Eaton M, Brodsky B, Berman HM. (1994). Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 266:75-81 [DOI] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. (2008). Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90-101 [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533 [DOI] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. (2010). Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 26:968-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung DT, Perelman N, Ko EC, Nimni ME. (1985). Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connect Tissue Res 13:109-115 [DOI] [PubMed] [Google Scholar]
- Foote CS. (1968). Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which May be important in biological systems. Science 162:963-970 [DOI] [PubMed] [Google Scholar]
- Han B, Jaurequi J, Tang BW, Nimni ME. (2003). Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A 65:118-124 [DOI] [PubMed] [Google Scholar]
- Knott L, Bailey AJ. (1998). Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone 22:181-187 [DOI] [PubMed] [Google Scholar]
- Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. (2006). Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 27:4470-4476 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti G, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res 86:436-440 [DOI] [PubMed] [Google Scholar]
- Munksgaard EC, Asmussen E. (1984). Bond strength between dentin and restorative resins mediated by mixtures of HEMA and glutaraldehyde. J Dent Res 63:1087-1089 [DOI] [PubMed] [Google Scholar]
- Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley D, Tay FR, et al. (2011). Cysteine cathepsins in human carious dentin. J Dent Res 90:506-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, et al. (1995). Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Lab Invest 72:311-322 [PubMed] [Google Scholar]
- Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS. (2001). Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res 56:273-281 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. (2011). State of the art etch-and-rinse adhesives. Dent Mater 27:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Fertala A. (1998). The collagen fibril: the almost crystalline structure. J Struct Biol 122:111-118 [DOI] [PubMed] [Google Scholar]
- Saboia V, Nato F, Mazzoni A, Orsini G, Putignano A, Giannini M, et al. (2008). Adhesion of two-step etch-and-rinse adhesive on collagen-depleted dentin. J Adhes Dent 10:419-422 [PubMed] [Google Scholar]
- Sionkowska A. (2006). Flash photolysis and pulse radiolysis studies on collagen Type I in acetic acid solution. J Photochem Photobiol B 84: 38-45 [DOI] [PubMed] [Google Scholar]
- Snibson GR. (2010). Collagen cross-linking: a new treatment paradigm in corneal disease – a review. Clin Experiment Ophthalmol 38:141-153 [DOI] [PubMed] [Google Scholar]
- Suppa P, Breschi L, Ruggeri A, Mazzotti G, Prati C, Chersoni S, et al. (2005). Nanoleakage within the hybrid layer: a correlative FEISEM/TEM investigation. J Biomed Mater Res B Appl Biomater 73:7-14 [DOI] [PubMed] [Google Scholar]
- Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. (2010). Cysteine cathepsins in human dentin-pulp complex. J Endo 36:475-481 [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. (1998). The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res 77:1622-1629 [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. (2003). Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135:620-627 [DOI] [PubMed] [Google Scholar]