Abstract
When adhesives and/or composites are bonded to the tooth, water in the environment can interfere with proper interface formation. Formation of water blisters and phase separation at the adhesive/dentin interface have appeared as new types of bond defects. To better understand this problem, we determined the near-equilibrium partition of the hydrophobic/hydrophilic components when exposed to over-wet environments. Model methacrylate-based adhesives were mixed with different amounts of water to yield well-separated aqueous and resin phases. It was found that less than 0.1% BisGMA but nearly one-third of the HEMA diffused into the aqueous phase, leaving the remaining resin phase relatively hydrophobic. A partial phase diagram was created for the ternary BisGMA/HEMA/water system. All the experimental phase partitioning data were plotted, and the points lay on a binodal curve that separated the single-phase region from the two-phase region. We obtained the 3 tie lines by connecting the 2 points of each conjugate pair of the phase partitioning data from the 3 sets of tripartite mixtures. Information about solubility, water miscibility, distribution ratio, and phase partitioning behavior could be obtained quantitatively. This type of phase diagram will provide a more thorough understanding of current adhesive performance and elucidate directions for further improvement.
Keywords: dentin bonding agents, water, ternary phase diagram, methacrylate, hydrophilicity, hydrophobicity
Introduction
There have been reports of the sensitivity of our current dentin adhesives to excess moisture, e.g., water blisters in adhesives placed on over-wet surfaces and phase separation with concomitant limited infiltration of the critical dimethacrylate component into the demineralized dentin matrix (Spencer et al., 2000; Spencer and Wang, 2002; Tay et al., 2002). Large fluid shifts that occur during the bonding process may allow dentinal fluid to mix with the hydrophilic comonomers, creating nanoleakage pathways within the adhesives (Hashimoto et al., 2004). Such defects have been detected for both total-etch and self-etch adhesive systems in studies using the silver nitrate tracer method and SEM or TEM characterization (Eliades et al., 2001; Tay et al., 2002, 2004). However, such morphologic studies are only qualitative and provide no detailed information on the composition of the aqueous droplets, diffusion/distribution of resin compounds, and their phase partitioning behavior. There has been limited investigation of phase behavior of methacrylate-based dentin adhesives in the presence of wet environments (Spencer and Wang, 2002; Van Landuyt et al., 2005; Finger et al., 2007).
A phase diagram is typically a chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium. Phase diagrams have not been used in the dental research field, except for dental alloys (Fischer, 2000; Luciano et al., 2005). The aim of the present investigation was to create, for the first time, a ternary phase diagram of a model dentin adhesive composed of hydrophobic BisGMA, hydrophilic HEMA, and water. A ternary diagram plot, three-dimensional but illustrated in two dimensions for ease of drawing and interpretation, may depict and potentially predict chemical composition of the various phases and the boundaries of these phases.
Materials & Methods
The model adhesive consisted of hydroxyethylmethacrylate (HEMA, Acros Organics, Fair Lawn, NJ, USA) and 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]-propane (BisGMA, Polysciences Inc., Washington, PA, USA) with a mass ratio of 45/55 (HEMA/BisGMA) (Ye et al., 2007, 2009a). Water (HPLC grade) was added to the neat resins at various amounts: 0%, 10%, 16%, 33%, and 50%. Shaking and sonication were required to yield well-dispersed solutions, especially for the latter 3 turbid mixtures. These turbid mixtures were further centrifuged (20 min at 10,000 g) to obtain separated aqueous and resin phase solutions. All the operations were carried out in triplicate at ambient temperature (24 ± 1°C).
The compositional analysis was carried out in a reverse-phase high-performance liquid chromatography (RP-HPLC) system (Shimadzu LC-2010 HTC, Shimadzu, Columbia, MD, USA) equipped with a photodiode array detector and EZStart chromatography software. Separation was performed on a reverse-phase column [Phenomenex Luna 5 µm C18 4.6 x 250 (Phenomenex, Torrance, CA, USA)] by elution with CH3CN: 20 mM ammonium acetate buffer. For each monomer, the wavelength was optimized based on the UV absorption spectra and the concentrations in different phases (see Fig. 1). For all experimental groups, the differences between compound concentrations were evaluated by one-way analysis of variance (ANOVA), together with Tukey’s test at α = 0.05 to identify significant differences.
Figure 1.
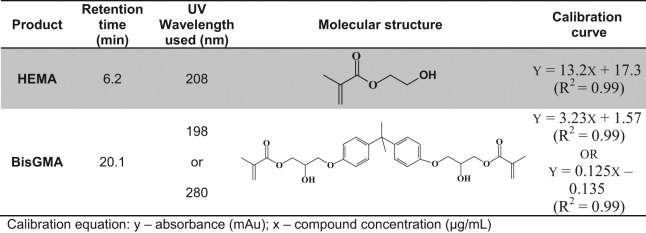
The chemical structures of HEMA and BisGMA, and their specific retention time, UV detection wavelength, and calibrations used for HPLC analysis.
The ternary phase diagram was created with one of the 2D graph tools in OriginLab OriginPro software (Version 8, OriginLab Corporation, Northampton, MA, USA). This was used to represent the fractions of 3 components (BisGMA, HEMA, and water), with each side of this equilateral triangle representing a component.
Results
The compositions determined for both aqueous and resin phase solutions are listed in the Table. The amount of hydrophobic BisGMA in the aqueous phase is very small, less than 0.06 mass%. This BisGMA content represented less than 0.1% of the amount of BisGMA from the original neat resin. The HEMA content in the aqueous phase ranged from 18.3% to 14.7%. The amount of both BisGMA and HEMA in the aqueous phase decreased with the increase of the initial water content (p < 0.01). When the water amount is greater than 10%, the separated resin phase contains an increased amount of BisGMA (p < 0.05), and a decreased amount of HEMA and water (p < 0.05). The partition ratios of HEMA content measured in the aqueous and resin phases ranged from 0.44 to 0.46 (listed in the Table.)
Table.
Aqueous Phase Composition and Resin-rich Phase Composition
| Aqueous Phase Composition (n = 3) at (mass%) |
Resin Phase Composition (n = 3) at (mass%) |
||||||
|---|---|---|---|---|---|---|---|
| Initial Water Content | BisGMA | HEMA | Water | BisGMA | HEMA | Water | Partition Ratio of HEMAap/rp |
| 0% $ | N/A | 55.0 | 45.0 | 0 | N/A | ||
| 10.1% | N/A | 49.4 (0.5) | 41.5 (0.3) | 10.1 (0.2) | N/A | ||
| 16.0% | 0.054 (0.002) | 18.3 (0.8) | 81.6 (0.6) | 50.8# (0.5) | 39.7# (0.4) | 9.5# (0.3) | 0.46 |
| 33.0% | 0.030* (0.002) | 16.8* (0.7) | 83.1* (0.8) | 53.9# (0.3) | 37.4# (0.3) | 8.6# (0.2) | 0.45 |
| 50.0% | 0.014* (0.001) | 14.7* (1.2) | 85.3* (1.1) | 58.9# (0.4) | 33.1# (0.3) | 7.9# (0.2) | 0.44 |
Values are means ± (SD).
Neat resin.
Significantly different from the concentration of the aqueous phase prepared with initial 16% water in the same column at α = 0.05.
Significantly different from the concentration of the aqueous phase prepared with initial 10% water in the same column at α = 0.05.
Abbreviations: ap, aqueous phase; rp, resin phase; N/A, not available.
Based on the experimental data obtained from this study as well as from our previous work (Spencer and Wang, 2002; Ye et al., 2008, 2009b), a partial phase diagram for ternary mixtures comprised of water, BisGMA, and HEMA was plotted (Fig. 2). The bottom line represents the BisGMA mass fractions, and the remaining 2 sides indicate the mass fractions of HEMA and water. Note that the summation of mass fraction for any point in a ternary phase diagram is equal to 1.00. Both aqueous and resin phase compositions, e.g., the experimental phase partitioning data, lie on the binodal curve, which separates the single-phase region from the two-phase region. The composition of each liquid phase is indicated by the end-points of the tie line that goes through the initial composition point. Only 3 tie lines (dashed) were obtained when the 2 points of each conjugate pair of the phase partitioning data were connected from the 3 sets of ternary mixtures. It was found that these tie lines presented similar slopes, but they were not parallel to each other. (Please note that Fig. 3 was also illustrated in different formats of Fig. 2 for discussion.)
Figure 2.
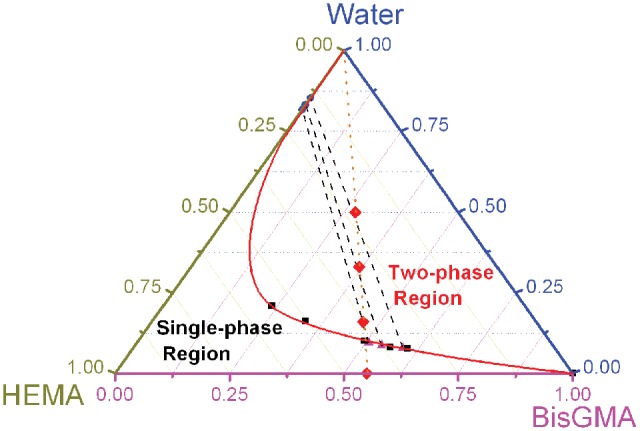
Partial phase diagram for ternary mixtures comprised of water, BisGMA, and HEMA. Filled circles and triangles represent experimental phase partitioning datapoints, and dashed lines represent experimental tie lines. Extra filled squares represent data previously collected by the ‘cloud point’ method (Spencer and Wang, 2002; Ye et al., 2008, 2009b). Note that the solid phase boundary line, drawn as a binodal curve, is only for demonstration, because only a portion of this line corresponds to experimental observation. The dotted line represents all the initial compositions with mass ratio of BisGMA/HEMA fixed at 55/45 and goes through the maximum single-phase water content (10.1%) and also the 3 filled diamonds (representing the initial compositions with water fraction at 16%, 33%, and 50% investigated in this work).
Figure 3.
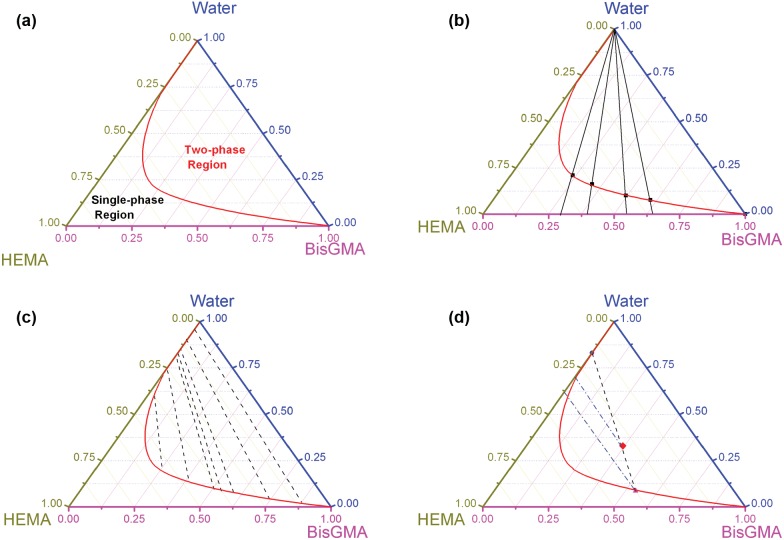
Information provided from a ternary phase diagram of the BisGMA/HEMA/water system. (a) Prediction of liquid/liquid phase separation for any given overall system composition; the binodal curve separates the single-phase region from the two-phase region. (b) Prediction of maximum single-phase water content at any ratio of BisGMA/HEMA resin; straight mass fraction changing lines connected with the specific point on the bottom axis corresponding to the specific ratio of BisGMA/HEMA. (c) Prediction of each phase composition when liquid/liquid phase separation occurs. (d) Partition ratio of each component between water-rich and BisGMA-rich phases and degree of phase separation. A 60o line drawn through the origin represents equal distribution of HEMA.
Discussion
With wet-bonding techniques, the channels between the demineralized dentin collagen fibrils are filled with water, solvent, conditioner, and/or oral fluids. Under in vivo conditions, there is little control over the amount of water left on the tooth. As a result, it is possible to leave the dentin surface so wet that the adhesive undergoes physical separation into hydrophobic and hydrophilic-rich phases (Spencer and Wang, 2002). Equilibrium phase partitioning behavior of a three-component liquid-liquid system is typically described by means of a ternary phase diagram. There is some critical, useful information that can be extracted from the diagram, so as to optimize the resin formulation and reduce the detrimental effects associated with adhesive phase separation. (Please note that further information is provided in the Appendix.)
Prediction of Liquid/Liquid Phase Separation for Any Given Overall System Composition
If the 3 components are mixed to give an overall system composition that falls in the two-phase region, the system will separate into 2 phases: presumably a phase rich in water and another rich in BisGMA (see Fig. 3a). The phase boundary line is a binodal curve, which is asymmetrical. The binodal curve drawn here was simulated to show the overall trend of the phase boundary, since only a portion of this line corresponds to experimental observation. It should be noted that the binodal curve presented in this diagram is purely empirical, unlike the approach of existing theoretical thermodynamic models. Weibull models are usually used for the mathematical modeling of a binodal curve, due to their flexibility in handling a skewed curve (Dodson, 2006).
Solubility of Each Component in the Other Component
In the absence of water, HEMA is a good solvent for BisGMA, so a relatively homogeneous solution can be formed. Water is also a good solvent for HEMA, but a non-solvent for BisGMA. Solubility of BisGMA in water and solubility of water in BisGMA could be theoretically obtained based on the 2 points in the phase diagram that lie along the right axis (BisGMA-water) as “the starting points for the arc” (see Fig. 3a). It was reported that when immersed in water at 37ºC for 7 days, BisGMA dissolved in water at a concentration of 4.1 ppm (Kim and Chung, 2005). This low solubility may explain why hydrophobic BisGMA was minimally distributed in the aqueous phase when phase separation occurred in this study.
Prediction of Maximum Single-phase Water Content at Any Ratio of BisGMA/HEMA Resin
The procedure is to draw a straight mass fraction changing line from the peak of the triangle ‘water’ to the specific point on the bottom axis ‘HEMA to BisGMA’, which could correspond to the specific ratio of BisGMA/HEMA (Fig. 3b). The composition of every datapoint on this mass fraction changing line has the same BisGMA/HEMA ratio. The maximum amount of water as water is incrementally added to neat resin (BisGMA/HEMA) can be determined mathematically by the intersection of the binodal curve and this straight mass fraction changing line.
Prediction of Each Composition Phase When Liquid/Liquid Phase Separation Occurs
The composition of each liquid phase is indicated by the end-points of the tie line that goes through the initial composition point. In other words, if the system was initially in the two-phase region, the tie line uniquely connects the points along the phase boundary line. This could predict the monomer content in the water-rich phase, the water content in the BisGMA-rich phase, the hydrophobic/hydrophilic monomer ratio in the BisGMA-rich phase, etc. Note that only a few tie lines are typically plotted to illustrate the equilibrium phase partitioning trend of a system. If the overall system composition is not on any of the tie lines, but between 2 tie lines which are close, then an artificial tie line could be drawn to go through the overall system composition and follow a similar trend of the close tie lines.
Partition Ratio of Each Component between Water-rich and BisGMA-rich Phases
A 60o line drawn through the origin represents equal distribution of components (see Fig. 3d). From this, the distribution ratio in this system is extracted from the mass fractions in the aqueous and resin phases for the conjugate solutions. HEMA serves as a co-solvent, which is independently miscible with water as well as with BisGMA. However, HEMA distribution in the aqueous and resin phases had not been determined previously. The slope of the tie lines indicates that HEMA preferentially partitions to the BisGMA-rich phase relative to the water-rich phase (as shown in Fig. 3d). Both HEMA and BisGMA contain methacrylate and hydroxyl groups in their molecular structure (see Fig. 1). This similar molecular structure suggests that HEMA distribution in the BisGMA-rich phase would be more than that in the aqueous phase. The partition ratios of HEMA differ among the 3 experimental groups (Table). HEMA content in the aqueous phase of each group is substantial, with over 30% of the original 45 mass% of HEMA diffused into the water from the neat resin. In cases where hydrophobic camphorquinone (CQ) is used in the photoinitiator system (Ye et al., 2009a), and the aqueous phase does not polymerize very well, most of the unreacted HEMA would leach into the aqueous environment over time.
It should be noted that every point on a tie line corresponds with a unique conjugate pair, e.g., the same phase partitioning behavior. The quantity of the water-rich phase volume compared with the BisGMA-rich phase may be linked to the relative distance between the origin and the intersection of the tie lines. This could present how serious phase separation would occur.
Consideration of Adhesive Development and Directions to be Taken for Further Improvement
It is noted that, in the aqueous phase, the amount of BisGMA determined in this study exceeded its solubility in pure water (Kim and Chung, 2005). This is partly attributable to higher solubility of BisGMA in the partial organic solvent (15~18% HEMA) than in pure water. For the same reason, the amount of BisGMA decreased with the increase of the initial water content, where the HEMA amount in the aqueous phase decreased. Nonetheless, the amount of BisGMA in the aqueous phase is limited such that it is unable to sufficiently crosslink the polymer network in the aqueous phase.
The model adhesive could mix with up to 10% water to form a homogeneous solution. When the water amount is further increased and becomes oversaturated, the separated resin phase contains more BisGMA but less HEMA and water. In other words, the resin phase became more hydrophobic than the mixture containing 10% water; this change in composition with a potential increase in viscosity may affect resin infiltration into the wet, demineralized dentin matrix.
Analysis of our data suggests that water-compatible components in adhesive formulation must be considered, especially the partition of these components in the aqueous environment. Future adhesive systems need to be designed carefully to achieve a more homogeneous monomer distribution and conversion in the hybrid layer to overcome the defects associated with phase separation. In our lab, several approaches—such as optimization of hydrophobic/hydrophilic composition (Spencer and Wang, 2002; Ye et al., 2009b; Spencer et al., 2010), new crosslinkable monomers with branched structure (Park et al., 2008, 2009a,b), solubility enhancer (Guo et al., 2007, 2008), and water-soluble photoinitiators (Wang et al., 2006; Ye et al., 2009a)—have been utilized to improve adhesive performance.
To build a ternary phase diagram, one could obtain the phase boundary line by connecting the cloud points at different ratios of BisGMA/HEMA. Visual examination of the samples is the most widely used method. However, the tie lines that show the phase partitioning information are missing if the cloud points are obtained only for the phase boundary line. To get tie lines for phase partitioning, oversaturated water may be added and sufficient time allowed for the 2 conjugate phases to separate into 2 layers. Note that only a few tie lines are typically plotted to illustrate the equilibrium phase partitioning trend of a system. Several tie lines could be obtained experimentally by preparing conjugate solutions having a wide range of compositions. The compositional analysis of the conjugate solutions could be carried out with HPLC, as in this study. However, the procedure for HPLC analysis is relatively time-consuming. There are ongoing investigations in our lab to pursue an efficient characterization using vibrational spectroscopy.
It may be difficult to eliminate phase separation of dentin adhesives in the oral environment. However, the understanding garnered by using the type of ternary phase diagram presented in this article could elucidate strategies for reducing the detrimental effects associated with adhesive phase separation. In addition, an understanding of phase partitioning behavior is critical in estimating and predicting the spread and extent of mixing each component within the complex system. Mapping such phase behavior could benefit materials design in many biomedical engineering applications. For example, it could be of particular benefit in those applications where a material must provide optimum function and performance in a wet environment. Finally, it should be noted that this investigation did not involve the demineralized dentin matrix. The size of interfibrillar spaces (~ 20 nm) may physically exclude phase-separated structures, i.e., resin globules > 10-20 µm, as suggested by our previous work (Spencer and Wang, 2002; Spencer et al., 2010).
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This investigation was supported by Research Grants R01DE14392 and R01DE14392-08S1 (PI: Spencer) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
A preliminary report of this work was presented at the 89th General Session of the International Association for Dental Research, San Diego, CA, USA (March 18, 2011; abstract #1700, http://iadr.confex.com/iadr/2011sandiego/webprogramschedule/Paper145555.html).
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Dodson B. (2006). The Weibull analysis handbook. Milwaukee, WI: ASQ Quality Press [Google Scholar]
- Eliades G, Vougiouklakis G, Palaghias G. (2001). Heterogeneous distribution of single-bottle adhesive monomers in the resin-dentin interdiffusion zone. Dent Mater 17:277-283 [DOI] [PubMed] [Google Scholar]
- Finger WJ, Shao B, Hoffmann M, Kanehira M, Endo T, Komatsu M. (2007). Does application of phase-separated self-etching adhesives affect bond strength? J Adhes Dent 9:169-173 [PubMed] [Google Scholar]
- Fischer J. (2000). Mechanical, thermal, and chemical analyses of the binary system Au-Ti in the development of a dental alloy. J Biomed Mater Res 52:678-686 [DOI] [PubMed] [Google Scholar]
- Guo X, Spencer P, Wang Y, Ye Q, Yao X, Williams K. (2007). Effects of a solubility enhancer on penetration of hydrophobic component in model adhesives into wet demineralized dentin. Dent Mater 23:1473-1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang Y, Spencer P, Ye Q, Yao X. (2008). Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater 24:824-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Ito S, Tay FR, Svizero NR, Sano H, Kaga M, et al. (2004). Fluid movement across the resin-dentin interface during and after bonding. J Dent Res 83:843-848 [DOI] [PubMed] [Google Scholar]
- Kim JG, Chung CM. (2005). Elution from light-cured dental composites: comparison of trimethacrylate and dimethacrylate as base monomers. J Biomed Mater Res B Appl Biomater 72:328-333 [DOI] [PubMed] [Google Scholar]
- Luciano RH, Shiraishi T, Udoh K, Tanaka Y, Hisatsune K. (2005). Microstructures and coherent phase diagram for the pseudobinary system (AuCu)(1-x)Pd-x with x <= 0.10. J Alloys Compounds 392:142-148 [Google Scholar]
- Park JG, Ye Q, Topp EM, Kostoryz EL, Wang Y, Kieweg SL, et al. (2008). Preparation and properties of novel dentin adhesives with esterase resistance. J Appli Polym Sci 107:3588-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Ye Q, Topp EM, Misra A, Spencer P. (2009a). Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dent Mater 25:1569-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Ye Q, Topp EM, Spencer P. (2009b). Enzyme-catalyzed hydrolysis of dentin adhesives containing a new urethane-based trimethacrylate monomer. J Biomed Mater Res B Appl Biomater 91:562-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P, Wang Y. (2002). Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447-456 [DOI] [PubMed] [Google Scholar]
- Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. (2000). Interfacial chemistry of the dentin/adhesive bond. J Dent Res 79:1458-1463 [DOI] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. (2010). Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng 38:1989-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yoshiyama M. (2002). Two modes of nanoleakage expression in single-step adhesives. J Dent Res 81:472-476 [DOI] [PubMed] [Google Scholar]
- Tay FR, Lai CN, Chersoni S, Pashley DH, Mak YF, Suppa P, et al. (2004). Osmotic blistering in enamel bonded with one-step self-etch adhesives. J Dent Res 83:290-295 [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. (2005). Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res 84:183-188 [DOI] [PubMed] [Google Scholar]
- Wang Y, Spencer P, Yao X, Ye Q. (2006). Effect of coinitiator and water on the photoreactivity and photopolymerization of HEMA/camphorquinone-based reactant mixtures. J Biomed Mater Res A 78:721-728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Spencer P, Wang Y, Misra A. (2007). Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res A 80:342-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. (2008). In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res 87:829-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Park J, Topp E, Spencer P. (2009a). Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater 25:452-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang Y, Spencer P. (2009b). Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B Appl Biomater 88:339-348 [DOI] [PMC free article] [PubMed] [Google Scholar]