Abstract
Long term complications following hematopoietic cell transplantation (HCT) have undergone comprehensive study. Although virtually every organ system can be adversely affected after HCT, the underlying pathophysiology of these late effects are incompletely understood. This manuscript describes current understanding of the pathophysiology of late effects involving the gastrointestinal, renal, cardiac, pulmonary systems along with a discussion of post-HCT metabolic syndrome studies. The patient’s underlying disease, pretransplant exposures, transplant conditioning regimens, graft versus host disease (GVHD) and other therapies contribute to these problems. Because organ systems are interdependent, long term complications with similar pathophysiology often involve multiple organ systems. Current data suggest that post-HCT organ complications occur as a result of cellular damage that leads to a cascade of complex events. The interplay between inflammatory processes and dysregulated cellular repair likely contributes to end-organ fibrosis and dysfunction. Though many long term problems cannot be prevented, appropriate monitoring can lead to detection and organ-preserving medical management at earlier stages. Currently, management strategies are aimed at minimizing symptoms and optimizing function.
There remain significant gaps in knowledge regarding pathophysiology of therapy-related organ toxicities disease following HCT. These gaps can be filled by closely examining disease biology and defining which patients are at highest risk of adverse outcomes. In addition, strategies should be developed for targeted disease prevention and health promotion efforts for individuals at high risk because of their genetic makeup or specific exposure profile.
Introduction
The incidence of and risk factors for long term complications following hematopoietic cell transplantation (HCT) have undergone comprehensive study. Virtually every organ system can be adversely affected in some way after HCT and although much is known about these potential toxicities, the underlying pathophysiology of most are incompletely understood.
In April 2011 an NCI/NHLBI sponsored consensus conference of international experts in clinical and biological research into late effects after HCT convened to review the state of the science of pediatric studies and identify key areas for future research. This manuscript will describe the conclusions shared at that conference relating to the pathophysiology of late effects involving the gastrointestinal, renal, cardiac, metabolic, and pulmonary systems. The patient’s underlying disease, pretransplant exposures, transplant conditioning regimens, graft versus host disease (GVHD) and other therapies all contribute to these problems. Since no organ system functions independently, it is clear that long term complications are usually inter-related and rarely limited to one system.
Iron Overload
Secondary iron overload is a nearly universal complication of HCT; the most troublesome complications are not hepatic, but cardiac, pancreatic, pituitary and thyroid-related. It develops from repeated red blood cell transfusions and increased gastrointestinal iron absorption in the setting of ineffective erythropoiesis and inflammatory conditions, including GVHD.1 The iron burden among patients presenting for transplantation for chronic anemias or protracted hematologic malignancy can be substantial.2,3 Iron overload after transplantation for hematologic malignancy is very common, ranging from 1832–13120g/g dry weight (measured biochemically) before day 100 after HCT.4 Except in patients transplanted for thalassemia5, the effects of iron overload on morbidity in transplant survivors have not been fully investigated.
Recent studies using serum ferritin as a marker suggest that iron levels fall slowly over time after transplant, reaching normal levels years later.6,7 Humans cannot excrete excess iron; iron mobilization and removal is needed to accelerate this process when prolonged iron excess could lead to excessive morbidity.8 Phlebotomy can mobilize iron from overloaded tissues if patients have recovered normal erythropoiesis.9 In heavily iron-overloaded patients, iron reduction therapy may improve transplantation outcomes10 and cardiac function5, but few data are available for transplant survivors from underlying diseases other than thalassemia. Table 1 provides a literature summary of the adverse health outcomes from excess body iron.
Table 1.
Potential clinical consequences of iron overload in transplant survivors, based on data from studies of iron burdens in non-transplant populations.
| Organ involved | Potential consequences | References |
|---|---|---|
| Heart | Cardiac failure, arrhythmias, death | 11–13 |
| Pituitary | Hypogonadism, delayed puberty, growth hormone deficiency |
14–16 |
| Thyroid | Hypothyroidism | 14,15 |
| Pancreas | Insulin-dependent diabetes mellitus | 14,17 –20 |
| Brain | Neurocognitive defects | 21,22 |
|
Secondary malignancy |
Solid tumor development | 23–26 |
Application of iron-specific MRI to the study of transplant survivors is lacking. Risk factor analyses of survivors, with iron burden as a co-factor, have not been carried out with regard to cardiac events, growth and development, gonadal development, fertility, endocrine dysfunction, fibrotic liver disease, and secondary malignancy. The threshold of cardiac iron concentration for cardiac events is unknown. The most important recent development has been standardization of the T2* MRI method of quantifying tissue iron. This methodology will provide a foundation for future studies of transplant survivors. Using T2* MRI, epidemiologic studies using several study designs (prospective, cross-sectional, and disease-specific) are urgently needed to better understand the role of iron overload on long-term transplant outcomes. Intervention studies should then follow.
Gastrointestinal, Hepatobiliary, and Pancreatic Dysfunction
Gut symptoms in the years following transplant are usually a continuation of problems during the first year (protracted acute GVHD, chronic GVHD, medication side effects, and infection related to immune suppression). The frequency and severity of these problems wanes with time—but new problems involving the gut and liver may arise decades later. Table 2 describes the symptoms and causes of gastrointestinal problems associated with transplantation.
Table 2.
Causes of gastrointestinal, hepatobiliary, and pancreatic problems in long-term transplant survivors.
| Problem areas | Common causes | Less common causes |
|---|---|---|
Esophageal symptoms: 27–32
|
|
|
|
Upper gut symptoms33–37 Anorexia, nausea, vomiting |
|
|
|
Mid-gut and colonic symptoms: Diarrhea and abdominal pain38,39 |
|
|
| Liver problems40–50 |
|
|
|
Biliary and pancreatic problems51–54 |
|
|
The majority of GI late effects are GVHD-related. Unfortunately, the significant current knowledge gaps include the mystery of why some patients fail to develop graft tolerance and why others suffer from refractory chronic GVHD. Current research on GVHD biomarkers may help identify flares so that pre-emptive therapy can be given. In the future, focus areas should include acceleration of immune reconstitution, development of tolerance, and discovery of markers of incipient GVHD. New therapies for protracted acute and chronic GVHD are urgently needed.
Chronic Kidney Disease (CKD)
CKD is frequently diagnosed after transplant. There are multiple clinical forms of CKD but the most commonly described ones include thrombotic microangiopathy, nephrotic syndrome, calcineurin inhibitor toxicity, acute kidney injury and GVHD-related CKD. Various risk factors associated with the development of CKD have been described; however, outlined below are recent studies that suggest that GVHD may also be a proximal cause of renal injury.
In a systematic review of 9317 adults and children who underwent HCT from 28 study cohorts, approximately 16.6% (3.6% to 89%) of patients developed CKD defined as a decrease in estimated glomerular filtration rate (eGFR) of at least 24.5 mL/min/1.73 m2 within the first year after transplant55. The cumulative incidence of CKD developing approximately 5 years after transplant ranges from 4.4%–44.3% depending on type of transplant and stage of CKD.56,57 Mortality rates among patients with CKD in this setting are significantly higher than in transplant recipients who retain normal renal function, even when controlled for co-morbidities58 Patients who develop CKD after HCT have a range of possible outcomes, including end-stage renal disease (ESRD) requiring chronic dialysis and renal transplant.
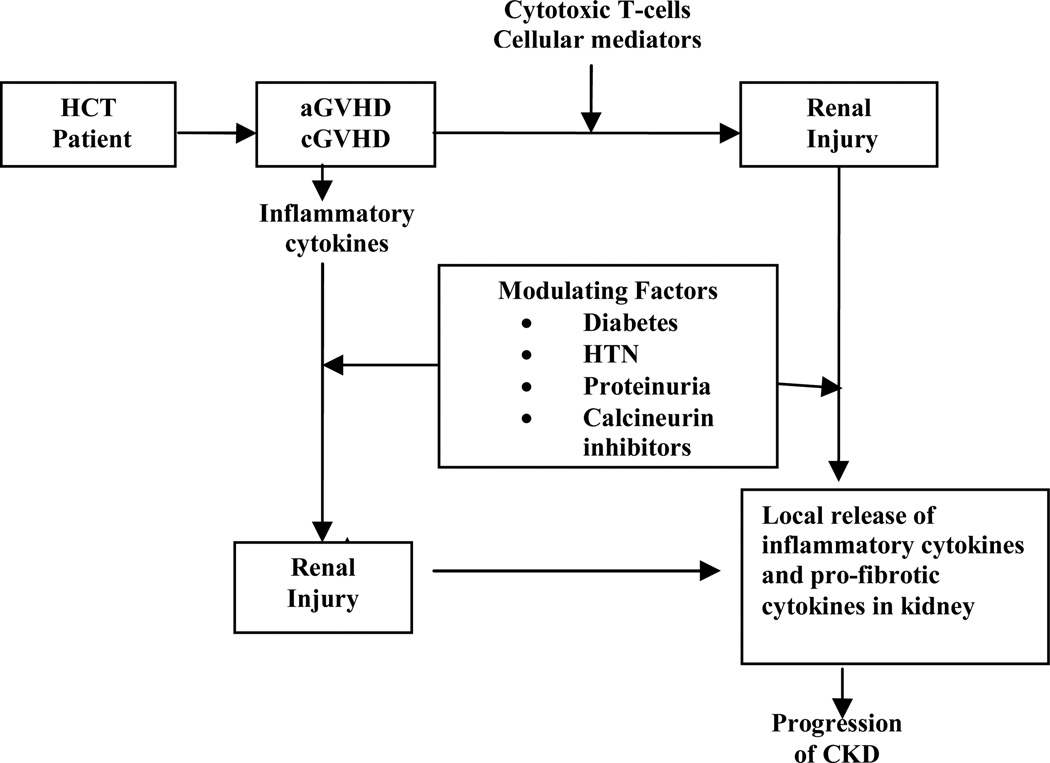
The mechanisms of HCT-related chronic renal dysfunction are still unknown. Although many clinical factors have been associated with the development of CKD, findings from the Seattle group and others have refuted previous traditional risk factors such as TBI. 59–61 These new data suggest that both acute and chronic graft vs. host disease (GVHD) are the primary pathogenic mechanisms. Early studies focused on patients receiving TBI as part of their conditioning regimen who later developed hemolytic uremic syndrome (HUS) 62–67 or on patients who developed nephrotic syndrome after HCT 68. However, these are specific subtypes of renal disease and likely do not account for the majority of cases of CKD. Current thought regarding the pathophysiology of CKD implicates GVHD and/or the therapies used to manage GVHD. (Figure 1).
Figure 1.
Proposed conceptual representation of pathogenesis of CKD in HCT recipients (aGVHD: acute GVHD, cGVHD: chronic GVHD, HTN: hypertension)
From: Hingorani, S., Chapter 97: Kidney and Bladder Complications of Hematopoietic Cell Transplantation. Thomas ED, Appelbaum FR, Forman,SG, Negrin, RS, Blume, KG, editors. Hematopoietic Cell Transplantation. Fourth ed. Wiley Blackwell Science; 2009. p. 1473.
This new paradigm positing HCT-related CKD as a renal manifestation of GVHD could occur through two different mechanisms: the kidney could be a direct target of T-cell mediated renal damage or the chronic systemic inflammatory state of GVHD could lead secondarily to cytokine-mediated nephropathy. A third potential explanation is that chronic exposure to calcineurin inhibitors, such as cyclosporine and tacrolimus used for GVHD prevention and/or treatment, causes CKD. These are not mutually exclusive hypotheses as T-cell mediated injury in GVHD is intertwined with cytokine effects,69 and the effects of cyclosporine can be potentiated in the presence of a chronic inflammatory state. In an autopsy study of 26 autologous and allogeneic transplant patients, renal tubulitis identical to that seen in renal allograft rejection was present in 67% of patients. 70 In a report of minimal change nephrotic syndrome that developed after HCT, large numbers of CD8+ donor T-cells were found infiltrating the interstitium and periglomerular areas of the kidney.71 A mouse model of GVHD kidney disease has shown that progressive venulitis, endothelialitis and tubulitis can begin within 2 weeks of transplant.72
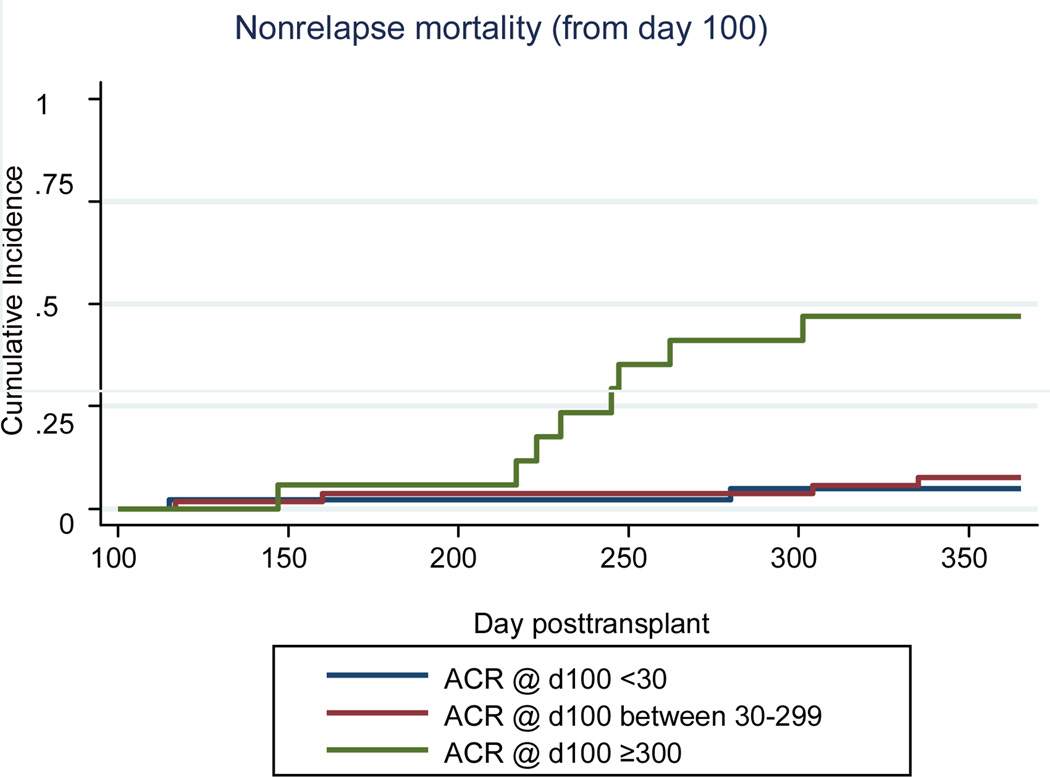
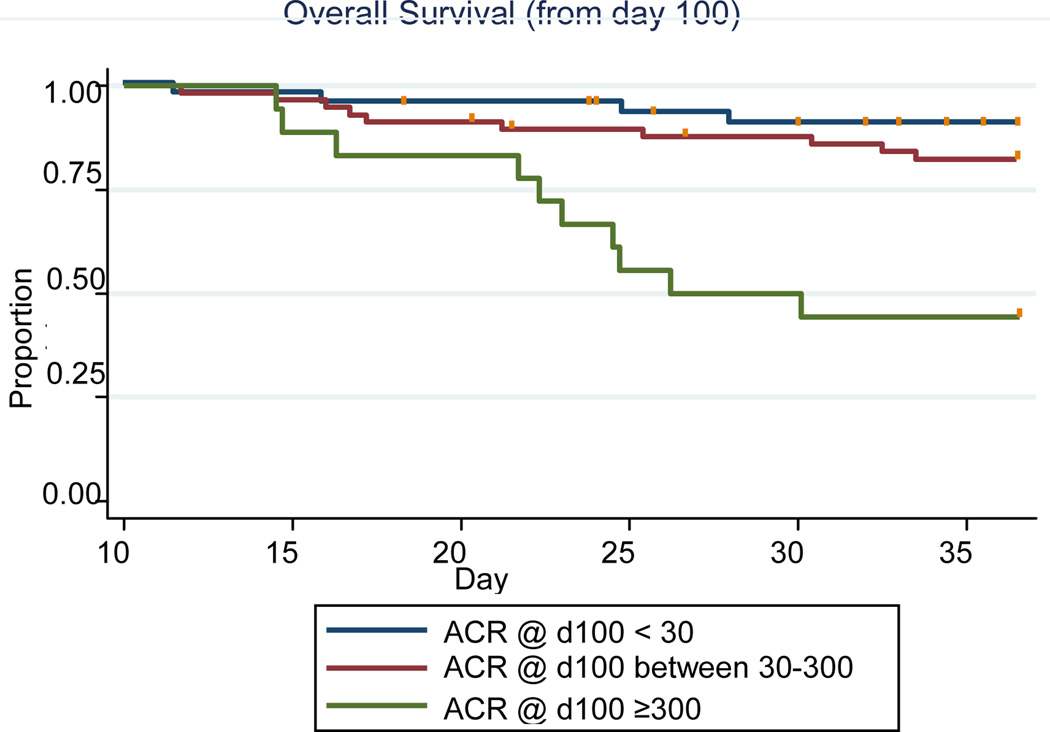
Although albuminuria and other conventional risk factors for renal disease progression have been identified in other patient populations, little is known about risk factors for CKD progression or why CKD and proteinuria increase nonrelapse mortality in the HCT patient population. In a cohort of 142 patients (median age 47 years) undergoing their first HCT, albuminuria and proteinuria at day 100 were associated with an increased risk of CKD and non-relapse (HR=12.8; 95% CI 2.7–60.6) and overall mortality (HR=7.7; 95%CI 2.4–24.7) respectively 1 year after transplant (Figure 2 and 3)73. In a cohort of 376 patients with CKD at 1 year, (defined as a GFR <60 ml/min/1.73 m2), 8% of the 109 patients for whom follow-up data was available (up to 8 years), progressed to ESRD.
Figure 2.
Cumulative incidence curves of albuminuria and non-relapse mortality from day 100 to 1 year post-transplant. N=43 for ACR <30 N=54 for ACR 30–299; N=17 for ACR≥300.
From: Hingorani, S., et al., Albuminuria in Hematopoietic Cell Transplant (HCT) Patients: Prevalence and Risk Factors. Journal of the American Society of Nephrology, 2006. 17: p. 405A.
Figure 3.
Kaplan-Meier curves of albuminuria and overall survival from day 100 to 1 year post-HCT. N=44 for ACR<30; N=58 for ACR 30–299; N=18 for ACR≥300.
From: Hingorani, S., et al., Albuminuria in Hematopoietic Cell Transplant (HCT) Patients: Prevalence and Risk Factors. Journal of the American Society of Nephrology, 2006. 17: p. 405A.
Albuminuria and proteinuria may reflect GVHD-induced endothelial injury, inflammatory tubular and interstitial damage and progressive CKD; however, it is not known if albuminuria or proteinuria by themselves cause the increased morbidity and mortality of HCT, or merely reflect other processes. Recent research has focused on the direct role of albuminuria and proteinuria on progression of CKD [see review]74. It is thought that albuminuria triggers the release of pro-inflammatory cytokines and chemokines that recruit macrophages and other inflammatory cells into the interstitium causing fibrosis and progression of CKD. It may be that in HCT patients, inflammatory damage to the tubules from GVHD leads to albuminuria and the latter is a manifestation of renal GVHD. Establishing such a mechanism would have important therapeutic implications. Thus, it is critical to determine if albuminuria and proteinuria are epiphenomenon or true independent risk factors for progression and mortality in the HCT population before changes in management can be proposed in a prospective clinical trial. Longer term follow-up is needed in these patients to determine whether patients progress from albuminuria to overt proteinuria and then to ESRD or if these conditions resolve when their GVHD and its associated inflammation are successfully treated.
Extrapolating from the studies in the diabetic population, we speculate that ACEI and ARB would be useful in patients with albuminuria and hypertension after HCT. In fact, Cohen and colleagues led a single institution trial in which patients were randomized to receive either captopril (n=28) or placebo (n=27) starting at Day + 35 after HCT.75 Patients who received placebo had a 15% incidence at one year of developing hemolytic uremic syndrome, compared to a 4% incidence in treated patients. Five year survival was 20% in the placebo group and 45% in the captopril group.
Current evidence suggests a need to think differently about CKD in patients after HCT. Most post-HCT CKD is not secondary to TBI or cyclosporine use but rather can be a consequence of nephropathic processes such as GVHD and the chronic inflammatory state that accompanies it (Figure 1). It is clear that we first need to better define the scope of the problem of CKD in this patient population using accurate and sensitive measures of kidney function. Identifying those patients at risk for the development of CKD will be important for early intervention and even prevention clinical trials in this patient population. In order to determine how to minimize and treat CKD, future studies will need to focus on the mechanisms by which GVHD leads to renal injury, determining if albuminuria is an indicator of disease or a target for therapy, optimizing prevention strategies and deciding how best to measure post-transplant renal function.
Cardiovascular Disease
Cardiovascular complications are a leading cause of therapy-related morbidity and mortality in long-term survivors of childhood malignancy.76–79 In patients who do not undergo hematopoietic cell transplantation (HCT), it is well-recognized that there is a strong dose-dependent association between anthracycline exposure and risk of CHF; this risk is modified by younger age at exposure, female gender, and chest irradiation.80–83 Less is known regarding the incidence and predictors of CHF following HCT in childhood. Potentially cardiotoxic exposures unique to HCT include conditioning with high-dose chemotherapy (especially cyclophosphamide) and total body irradiation (TBI).83 In addition, HCT survivors are at increased risk of developing cardiovascular risk factors such as hypertension and diabetes due, in part, to exposure to TBI, prolonged immunosuppressive therapy following allogeneic HCT, or other health conditions such as hypothyroidism or growth hormone deficiency.83,84 The modifying influence of these cardiovascular risk factors on risk of CHF following cardiotoxic therapy has also not been fully investigated.
The independent role of pre-HCT exposure to therapeutic agents, transplant-related conditioning and co-morbidities in the development of late CHF after HCT has been recently examined.85 From a cohort of nearly 3,000 1+ year survivors who underwent HCT, patients with late CHF were identified. Pre-HCT exposure to anthracyclines and the presence of post-HCT co-morbidities were primarily responsible for the risk of late CHF after HCT. Conditioning-related exposures did not appear to contribute significantly to this risk. The cardiotoxic effect of anthracyclines was highest for autologous HCT recipients, with a cumulative dose of ≥250 mg/m2 being associated with a 30-fold increased risk of late CHF. Overall survival was less than 50% at 2 years after CHF diagnosis. A subsequent study86 evaluating long-term health-related outcomes in three cohorts - conventionally treated childhood cancer survivors, survivors of childhood HCT, and sibling controls – revealed that while survivors of HCT were at a 13-fold risk of severe or life-threatening cardiovascular complications when compared to healthy controls, the risk was equivalent to that seen in conventionally treated patients. One possible explanation for this finding is that, as seen in previous studies,87,88 the risk for late-occurring cardiovascular complications following HCT may be largely due to pre-HCT therapeutic exposures, with little additional risk from conditioning-related exposures or GvHD.
It is becoming increasingly recognized that risks for many diseases result from an interaction between inherited gene variants and environmental factors, including chemical, physical, and behavioral factors. However, there continue to be large gaps in knowledge with regards to the pathogenesis of therapy-related adverse events. There is emerging evidence to suggest that individual genetic susceptibility could be a determinant of therapy-related CHF.89,90 Significant cardiotoxicity has been reported at cumulative doses of less than 250 mg/m2,9 while doses that exceed 1000 mg/m2 have been tolerated without long-term sequelae by a few individuals.91 Among long-term HCT survivors, 40% of cases with clinical CHF had received a cumulative dose of less than 250 mg/m2.11 This heterogeneity could be explained, in part, by the presence of genetic polymorphisms that alter the metabolism of anthracyclines, the myocardial response to the drug, as well as other factors thought to play a role in susceptibility to de novo disease.89,90,92
Using a nested case-control study design the role of functional SNPs in genes involved in free radical metabolism (NAD[P]H oxidase: subunits NCF4, RAC2, CYBA) as well as those impacting synthesis of cardiotoxic anthracycline alcohol metabolites (carbonyl reductase: CBR1 and CBR3) in modifying of risk of CHF following HCT was recently examined.93 Patients with CHF and controls (without CHF) were matched by: age at HCT, type of HCT, ethnicity, anthracycline dose, and length of follow-up. Multivariate conditional logistic regression revealed that a polymorphism in the NAD(P)H oxidase subunit RAC2 (rs13058338, 7508T→A) conferred a 3.2-fold risk of A-CHF (Odds Ratio [OR]: 3.2; p=0.05), and there was a 10.8-fold risk (OR: 10.8, p=0.04) for those with the GG genotype of rs9024 (1096G→A) in CBR1. These preliminary findings support the “unifying hypothesis”94 that A-CHF could develop as a result of oxidative stress or metabolic derangements induced by cardiotoxic alcohol metabolites, and that the high-risk variants of RAC2 and CBR1 play a critical role in modifying this risk. If these findings are replicated and confirmed by others in independent study samples, they could set the stage for identifying a subgroup of patients up front who could perhaps receive alternative treatment for management of their cancer; while for those who have already received the anthracyclines, identification of high-risk alleles would warrant closer surveillance for cardiotoxicity and use of medications that modulate cardiac function.
Insulin Resistance and Abnormal Body Composition
Survivors after allogeneic HCT have a risk of premature cardiovascular (CV) related death that is increased 2.3-fold compared to the general population.96,97 The exact etiology of CV risk and subsequent death is largely unknown, although development of “metabolic syndrome” (the constellation of central obesity, insulin resistance, glucose intolerance, dyslipidemia, and hypertension, that is associated with a substantially increased risk for type 2 diabetes mellitus and atherosclerotic CV disease; see Table 3) and more specifically, insulin resistance as a consequence of HCT has been suggested.98–100 In studies of conventionally treated leukemia survivors compared to those who underwent HCT, transplant survivors are significantly more likely to manifest metabolic syndrome or multiple adverse cardiac risk factors including central adiposity, hypertension (HTN), insulin resistance and dyslipidemia.102,103 The concern over time is that survivors who develop metabolic syndrome after HCT will be at higher risk for developing significant CV related events and/or premature death from CV related causes. The Bone Marrow Transplant Survivor Study examined diabetes, hypertension, and cardiovascular events in 1089 patients who were two or more year survivors after HCT.103 At a mean age of 39 years and with a mean follow-up after HCT of nearly 9 years, survivors of allogeneic HCT were 3.6 times more likely to report diabetes than siblings and 2 times more likely to report hypertension. TBI exposure was also associated with an increased risk of diabetes (OR=3.42; 95% CI, 1.55– 7.52). Rates of CV outcomes also have been examined among nearly 1,500 >2 year transplant survivors treated in Seattle from 1985–2006 relative to an age, year, and sex matched population-based comparison group.104 Using state hospital and death registry data defining key CV outcomes, survivors experienced increased rates of cardiovascular death and had an increased cumulative incidence of ischemic heart disease, cardiomyopathy/heart failure, stroke, vascular diseases, and rhythm disorders. Survivors also had an increased cumulative incidence of related conditions that predispose towards more serious cardiovascular disease: hypertension, renal disease, dyslipidemia, and diabetes.
Table 3.
ATP III criteria for metabolic syndrome – indicated by 3 or more positive findings:
| Criterion | Adults | Adolescents* |
|---|---|---|
| High Triglyceride Level, mg/dL | ≥150 | ≥110 |
| Low HDL-C level, mg/dL | ||
| Males | <40 | ≤40 |
| Females | <50 | ≤40 |
| Abdominal obesity, waist circumference, cm | ||
| Males | >102 | ≥90th Percentile |
| Females | >88 | ≥90th Percentile |
| High fasting glucose level, mg/dL | ≥100** | ≥100** |
| High blood pressure, mm Hg | ≥ 130/85 | ≥90th Percentile |
Many gaps exist in our current knowledge of why HCT survivors have a higher risk of adverse CV outcomes. While descriptive and epidemiologic based studies have at least made us aware of the problem, they have thus far provided little information into the underlying pathophysiology of CV disease in HCT survivors or why these events are happening in HCT survivors at greater frequency than in the general population. We also know little if anything about whether there are preventative strategies or other interventions that may modify this risk.
The association of obesity with diabetes and CV disease risk in the general population is well established, but obesity as determined by body mass index (BMI) is uncommon in long term survivors after HCT.103 However, despite having a normal BMI, HCT survivors develop significantly altered body composition that results in both an increase in total percent fat mass (PFM) as well as a reduction in lean body mass (LBM). This finding is termed “sarcopenic obesity” and results in a loss of myocyte insulin receptors and an increase in adipocyte insulin receptors, the latter of which are less efficient in binding insulin and clearing glucose ultimately contributing to insulin resistance.104–106 Preliminary data from 119 children and young adults (current mean age 26.1(±0.8) yr, 61.3% male) who had received HCT at a mean age of 12.2 yr, ±0.6) and 81 healthy sibling controls (current mean age 22.8(±0.9)yr, 49.4% male) found that compared to siblings, HCT survivors had significantly lower weight but no differences in BMI or waist circumference.107 HCT survivors had significantly higher PFM and lower LBM. Insulin resistance was measured by means of euglycemic hyperinslulinemic clamp studies and results were adjusted for PFM. HCT survivors were significantly more insulin resistant than controls and they also had other CV risk factors (significantly higher levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides). Interestingly, these differences were only found in patients who had received TBI as part of their transplant conditioning regimen. These preliminary data are thus revealing that even at a relatively young age HCT survivors have increased CV risk factors which are independent of obesity, but may be related to alterations in body composition (↓LBM and ↑PFM), insulin resistance and exposure to TBI.
While it is becoming more evident that HCT survivors are at risk for developing insulin resistance, the mechanistic pathways and risk factors leading to this are still undefined and thus determining more specifically what these are at a cellular and genetic level will be critical. Additionally, further definition of the role that alteration in body composition plays in insulin resistance and CV risk in HCT survivors is needed. Whether CV risk and abnormal body composition is related primarily to TBI exposure, corticosteroid exposure, a chronic inflammatory state (mediated or related to graft vs. host disease), or some other mechanism needs to be carefully examined. Finally, the post-HCT time course of development of adverse CV risk factors and changes in body composition begins will be important to determine and will guide us in devising preventative strategies and interventions.
Chronic Pulmonary Dysfunction
Decline in lung function is a significant complication in the months and years following successful allogeneic-HCT. Two forms of chronic pulmonary dysfunction are commonly observed: obstructive lung disease (OLD) and restrictive lung disease (RLD).113–117 The incidence of both forms of lung toxicity can range from 10% to 40% depending upon donor source, the time interval after HCT, definition applied and presence of chronic GVHD.113 In each scenario, collagen deposition and the development of fibrosis either in the interstitial (RLD) or peri-bronchiolar (OLD) space are believed to contribute to the patterns of lung dysfunction displayed on pulmonary function tests (PFTs).118
The most common form of OLD following allogeneic-HSCT is bronchiolitis obliterans (BO).115,119,120 First reported in the 1980’s, BO is a serious and potentially life-threatening late effect that is characterized by an inflammatory process resulting in bronchiolar obliteration, fibrosis and progressive obstructive lung disease.113 Historically, the term BO has been used to describe “chronic GVHD of the lung” and begins 6–20 months after HSCT. Patients with BO may be initially asymptomatic, but typically present with a cough, wheezing or dyspnea on exertion.118 PFTs show OLD with general preservation of forced vital capacity (FVC), reductions in forced expiratory volume in one second (FEV1) and associated decreases in the FEV1/FVC ratio with or with out significant declines in the DLCO.121 The diagnosis of OLD without histologic confirmation is commonly referred to as bronchiolitis obliterans syndrome or BOS. More recently, “air flow obstruction” has been defined as a more than 5% per year decline in percent predicted FEV1 with the lowest post-transplant FEV1/FVC ratio less than 0.8.122 Risk factors for BO include lower pre-transplant FEV1/FVC values, concomitant pulmonary infections, chronic aspiration, acute and chronic GVHD, older recipient age, the use of mismatched donors and high dose (vs. reduced intensity) conditioning.113,119 The clinical course of BO is variable, but patients frequently develop progressive and debilitating respiratory failure despite the initiation of enhanced immunosuppression.113
RLD is defined by reductions in FVC, total lung capacity (TLC) and diffusion capacity of the lung for carbon monoxide (DLCO). In contrast to OLD, the FEV1/FVC ratio is maintained near 100%. RLD is common after HSCT and has been reported in as many as 25% to 45% of patients by day 100.113 Importantly, declines in TLC or FVC occurring at 100 days and one year after HCT are associated with an increase in non-relapse mortality. Early reports suggested that the incidence of RLD increases with advancing recipient age, but more recent studies have revealed significant RLD in children receiving HCT.123 The most recognizable form of RLD is bronchiolitis obliterans organizing pneumonia (BOOP). Clinical features include dry cough, shortness of breath and fever. Radiographic findings show diffuse, peripheral, fluffy infiltrates consistent with airspace consolidation. Although reported in less than 10% of HSCT recipients, the development of BOOP is strongly associated with prior acute and chronic GVHD.124
The complex pathophysiology of chronic lung injury after HCT is poorly understood and represents the most significant gap in current knowledge for this spectrum of late effects. This limitation stems from the paucity of 1) correlative data obtained from afflicted HCT recipients, 2) controlled clinical trials and 3) suitable animal models for either RLD or OLD. RLD and OLD after HCT likely involve an initial insult to pulmonary vascular endothelium and leukocyte recruitment into the lung parenchyma followed by a dysregulated reparative response characterized by the interplay between recruited donor-derived leukocytes, bronchiolar and interstitial epithelial cells and lung fibroblasts and the ultimate deposition of collagen.113 The possible role of innate immunity in the development of OLD was recently highlighted by two clinical studies. Investigators have found that genetic variations in the bactericidal/permeability-increasing (BPI) protein and nucleotide-binding oligomerization domain containing 2 / caspase recruitment domain family member 15 (NOD2/CARD15) influence the risk of airflow obstruction and BO after allogeneic-HCT.125,126
A tri-phasic model of RLD and OLD after HCT has been proposed wherein alloantigen recognition is the inciting stimulus for pulmonary inflammation.113 In phase I, an acute pneumonitis develops as a consequence of an allo-immune response, resulting in the sequential influx of lymphocytes, macrophages and neutrophils into an inflamed lung parenchyma. In phase II, the persistence of an inflammatory signal in the setting of exuberant repair mechanisms promotes the transition from acute to chronic injury. If the inciting injurious stimuli predominantly involves bronchiolar epithelial cells, phase II is associated with the concentric infiltration of lymphocytes and collagen deposition in the peri-bronchiolar areas resulting in the development of chronic bronchiolitis. If, by contrast, the principal target of early damage is the alveolar epithelium, leukocyte recruitment and matrix deposition during phase II are confined primarily to the interstitial space. Activated lymphocytes then migrate into the airway mucosa and contribute to epithelial injury. As chronic inflammation proceeds to phase III, lung fibroblasts increase in number and contribute to the enhanced deposition of collagen and granulation tissue in and around bronchial structures, ultimately resulting in complete obliteration of small airways and fixed obstructive defects. Similarly, fibroblast proliferation and intra-septal collagen deposition during phase III ultimately results in interstitial thickening, septal fibrosis, significant volume loss and severe restrictive lung disease.
Clinical and experimental data suggest that the progression to a chronic, pro-fibrotic form of pulmonary toxicity involves the secretion of immunomodulatory proteins, and in this context, TNFα may be a central factor in the tri-phasic model outlined above. Strong evidence for a role of TNFα in the transition from acute to chronic lung injury comes from a study using transgenic mice with targeted over-expression of TNFα in the lungs. Early lung histopathology includes a robust leukocytic infiltrate whereas prolonged exposure to TNFα results in chronic inflammation and fibrosis127.
Patients with more severe disease at the time of diagnosis tend to have a poor prognosis; early recognition and treatment may be important to successful outcomes. Hence, increased surveillance for lung dysfunction by serial PFTs (including an assessment of lung volumes, spirometry and diffusion capacity) for the first two years following HCT should be considered whenever feasible. Given the significant morbidity and mortality associated with advanced OLD and RLD, a careful, comprehensive evaluation is recommended once persistent signs or symptoms of pulmonary dysfunction are detected.119,120 Testing should include a high-resolution, computer-assisted tomography (CT) scan of the chest and broncho-alveolar lavage to exclude opportunistic infections. Lung biopsy can also be quite helpful in making a definitive diagnosis.
The “standard” therapy for OLD combines enhanced immunosuppression in conjunction with supportive care including antimicrobial prophylaxis, bronchodilator therapy and supplemental oxygen when indicated. While the approach to RLD is less well defined, increasing evidence suggests that this form of pulmonary dysfunction may also be immunologically mediated.124 Unfortunately, the response to multiple agents including corticosteroids, cyclosporine, tacrolimus and azathioprine is limited and tends to occur only early in the course of treatment.113 The potential role for TNFα in the pathogenesis of both OLD and RLD suggests that neutralizing agents such as etanercept (Enbrel ®, Amgen Inc) may have promise.128 The combination of azithromycin, montelukast (FAM) and inhaled fluticasone is currently being investigated to prevent progression of newly-diagnosed BOS.129
Non-infectious lung injury remains a significant problem following allogeneic-HSCT. It is extremely important to determine if the lung is a target of GVHD. Similarities between the histopathologic features of BO seen in association with OLD after allogeneic-HSCT and during lung allograft rejection, together with reports of improvement in lung function with immunosuppression, strongly suggest that pathways of allo-immune activation are operative. Further research into mechanisms of chronic lung injury after HSCT is paramount for improving our understanding of this debilitating spectrum of late effects and the development of novel therapeutic strategies for treatment and prevention. Studying a tri-phasic model of chronic, non-infectious lung injury after HCT that involves T cell activation, leukocyte recruitment, the deposition of collagen and the development of fibrosis may lead to improvements in therapy. Finally, discovering what determines the anatomic specificity (peribronchiolar vs. interstitial) of chronic lung injury and understanding the role of acute inflammation in the initial damage to the alveolar or bronchiolar epithelium will enhance our understanding of post transplant pulmonary dysfunction.
CONCLUSION
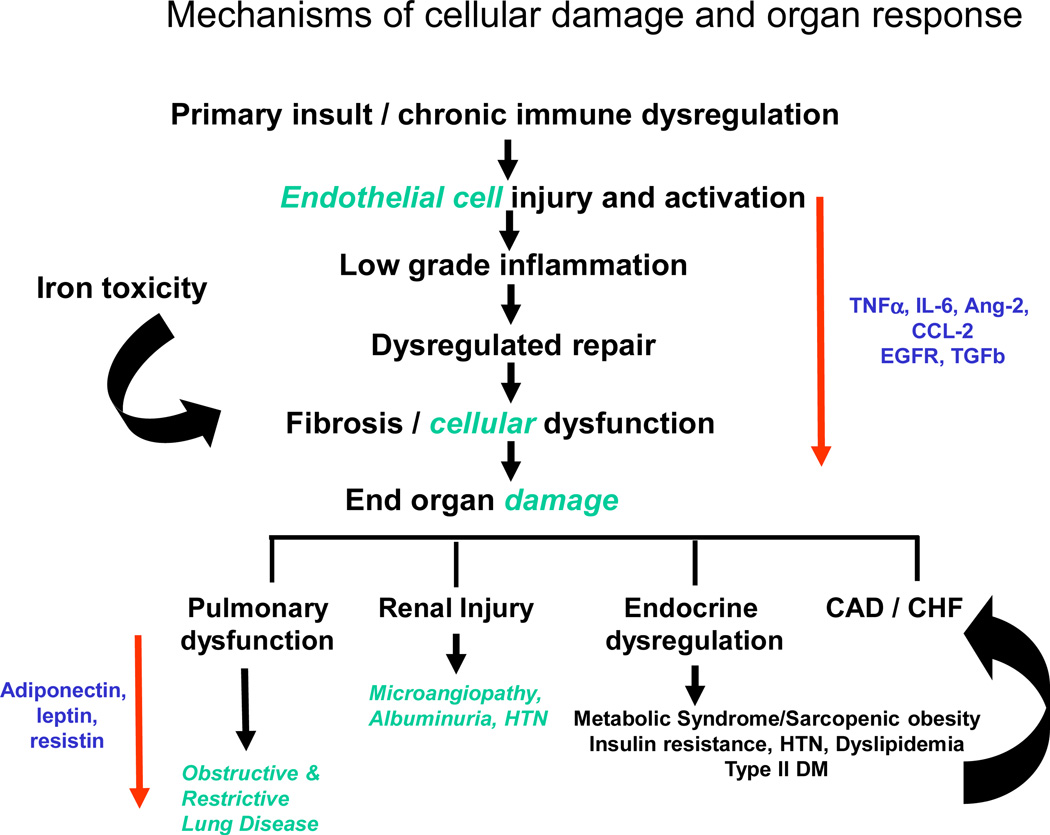
Current data suggest that post-HCT organ complications occur as a result of cellular damage that leads to a cascade of complex events. The degree of cellular damage that occurs is related to the overall health status, presence of other co-morbidities and baseline organ function of the pre-HCT recipient, with additional impact related to the intensity of the conditioning regimen, infections, drug exposures, and delayed immune tolerance. The interplay inflammatory processes and dysregulated cellular repair likely contributes to end-organ fibrosis and dysfunction (Figure 4).
Figure 4.
Mechanisms of cellular damage and organ response
HCT survivors have a high burden of morbidity; especially as it relates to development of organ specific late effects after HCT. However, there remain significant gaps in knowledge regarding pathophysiology of therapy-related organ toxicities disease following HCT. These gaps can be filled by closely examining disease biology and defining which patients are at highest risk of these adverse outcomes. In addition, strategies should be developed for targeted disease prevention and health promotion efforts for individuals at high risk because of their genetic makeup or specific exposure profile.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 2.Armand P, Kim HT, Rhodes J, et al. Iron Overload in Patients with Acute Leukemia or MDS Undergoing Myeloablative Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majhail NS, Lazarus HM, Burns LJ. A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2010;16:832–837. doi: 10.1016/j.bbmt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Strasser SI, Kowdley KV, Sale GE, McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant. 1998;22:167–173. doi: 10.1038/sj.bmt.1701301. [DOI] [PubMed] [Google Scholar]
- 5.Mariotti E, Angelucci E, Agostini A, Baronciani D, Sgarbi E, Lucarelli G. Evaluation of cardiac status in iron-loaded thalassaemia patients following bone marrow transplantation: improvement in cardiac function during reduction in body iron burden. British Journal of Haematology. 1998;103:916–921. doi: 10.1046/j.1365-2141.1998.01099.x. [DOI] [PubMed] [Google Scholar]
- 6.Lucarelli G, Angelucci E, Giardini C, et al. Fate of iron stores in thalassaemia after bone-marrow transplantation. Lancet. 1993;342:1388–1391. doi: 10.1016/0140-6736(93)92753-g. [DOI] [PubMed] [Google Scholar]
- 7.Chotsampancharoen T, Gan K, Kasow KA, Barfield RC, Hale GA, Leung W. Iron overload in survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatr Transplant. 2009;13:348–352. doi: 10.1111/j.1399-3046.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 8.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelucci E, Muretto P, Lucarelli G, et al. Treatment of iron overload in the “ex-thalassemic” Report from the phlebotomy program. Ann N Y Acad Sci. 1998;850:288–293. doi: 10.1111/j.1749-6632.1998.tb10485.x. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci E, Muretto P, Lucarelli G, et al. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994–998. [PubMed] [Google Scholar]
- 11.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European Heart Journal. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 13.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 14.Delvecchio M, Cavallo L. Growth and endocrine function in thalassemia major in childhood and adolescence. J Endocrinol Invest. 2010;33:61–68. doi: 10.1007/BF03346551. [DOI] [PubMed] [Google Scholar]
- 15.Toumba M, Sergis A, Kanaris C, Skordis N. Endocrine complications in patients with Thalassaemia Major. Pediatr Endocrinol Rev. 2007;5:642–648. [PubMed] [Google Scholar]
- 16.De Sanctis V. Growth and puberty and its management in thalassaemia. Horm Res. 2002;58 Suppl l:72–79. doi: 10.1159/000064766. [DOI] [PubMed] [Google Scholar]
- 17.Kattamis C, Ladis V, Tsoussis D, Kaloumenou I, Theodoridis C. Evolution of glucose intolerance and diabetes in transfused patients with thalassemia. Pediatr Endocrinol Rev. 2004;(2 Suppl 2):267–271. [PubMed] [Google Scholar]
- 18.Angelopoulos NG, Zervas A, Livadas S, et al. Reduced insulin secretion in normoglycaemic patients with beta-thalassaemia major. Diabet Med. 2006;23:1327–1331. doi: 10.1111/j.1464-5491.2006.01988.x. [DOI] [PubMed] [Google Scholar]
- 19.Au WY, Lam WW, Chu W, et al. A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica. 2008;93:116–119. doi: 10.3324/haematol.11768. [DOI] [PubMed] [Google Scholar]
- 20.Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong FD. Thalassemia and learning: Neurocognitive functioning in children. Ann N Y Acad Sci. 2005;1054:283–289. doi: 10.1196/annals.1345.036. [DOI] [PubMed] [Google Scholar]
- 22.Metafratzi Z, Argyropoulou MI, Kiortsis DN, Tsampoulas C, Chaliassos N, Efremidis SC. T(2) relaxation rate of basal ganglia and cortex in patients with beta-thalassaemia major. Br J Radiol. 2001;74:407–410. doi: 10.1259/bjr.74.881.740407. [DOI] [PubMed] [Google Scholar]
- 23.Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer. 1994;56:364–369. doi: 10.1002/ijc.2910560312. [DOI] [PubMed] [Google Scholar]
- 24.Fracanzani AL, Conte D, Fraquelli M, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology. 2001;33:647–651. doi: 10.1053/jhep.2001.22506. [DOI] [PubMed] [Google Scholar]
- 25.Osborne NJ, Gurrin LC, Allen KJ, et al. HFE C282Y homozygotes are at increased risk of breast and colorectal cancer. Hepatology. 2010;51:1311–1318. doi: 10.1002/hep.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 27.Mackey JR, Desai S, Larratt L, Cwik V, Nabholtz JM. Myasthenia gravis in association with allogeneic bone marrow transplantation: clinical observations, therapeutic implications and review of literature. Bone Marrow Transplant. 1997;19:939–42. doi: 10.1038/sj.bmt.1700759. [DOI] [PubMed] [Google Scholar]
- 28.Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplantation. 2005;36:115–121. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- 29.McDonald GB, Sullivan KM, Schuffler MD, Shulman HM, Thomas ED. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914–921. [PubMed] [Google Scholar]
- 30.McDonald GB, Sullivan KM, Plumley TF. Radiographic features of esophageal involvement in chronic graft-versus-host disease. Am J Roentgenol. 1984;142:501–506. doi: 10.2214/ajr.142.3.501. [DOI] [PubMed] [Google Scholar]
- 31.Schima W, Pokieser P, Forstinger C, et al. Videofluoroscopy of the pharynx and esophagus in chronic graft-versus-host disease. Abdom Imaging. 1994;19:191–194. doi: 10.1007/BF00203504. [DOI] [PubMed] [Google Scholar]
- 32.Minocha A, Mandanas RA, Kida M, Jazzar A. Bullous esophagitis due to chronic graft-versus-host disease. Am J Gastroenterol. 1997;92:529–530. [PubMed] [Google Scholar]
- 33.Patey-Mariaud de Serre N, Reijasse D, Verkarre V, et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplantation. 2002;29:223–230. doi: 10.1038/sj.bmt.1703329. [DOI] [PubMed] [Google Scholar]
- 34.Akpek G, Chinratanalab W, Lee LA, et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biology of Blood & Marrow Transplantation. 2003;9:46–51. doi: 10.1053/bbmt.2003.49999. [DOI] [PubMed] [Google Scholar]
- 35.Filipovich A, Weisdorf D, Pavletic S, et al. National Institutes of health consensus developement project on criteria for clinical trials in chronic graft-versus-host disease I Diagnosis of staging working group report. Biology of Blood & Marrow Transplantation. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 36.McDonald GB, Bouvier M, Hockenbery DM, et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology. 1998;115:28–35. doi: 10.1016/s0016-5085(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 37.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prenisone-sparing therapy for gastrointestinal graft-versus-host diesease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 38.Iyer RV, Hahn T, Roy HN, et al. Long-term use of Oral Beclomethasone Dipropionate for the treatment of gastrointestinal graft-versus-host disease. Biology of Blood & Marrow Transplantation. 2005;11:587–592. doi: 10.1016/j.bbmt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Borgaonkar MR, Duggan PR, Adams G. Differing clinical manifestations of celiac disease transmitted by bone marrow transplantation. Digestive Diseases & Sciences. 2006;51:210–212. doi: 10.1007/s10620-006-3109-z. [DOI] [PubMed] [Google Scholar]
- 40.McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–1460. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 42.Tomas JF, Pinilla I, Garcia-Buey ML, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: Clinical features and course in 61 patients. Bone Marrow Transplantation. 2000;26:649–655. doi: 10.1038/sj.bmt.1702532. [DOI] [PubMed] [Google Scholar]
- 43.Strasser SI, Shulman HM, Flowers ME, et al. Chronic graft-vs-host disease of the liver: presentation as an acute hepatitis. Hepatology. 2000;32:1265–1271. doi: 10.1053/jhep.2000.20067. [DOI] [PubMed] [Google Scholar]
- 44.Malik AH, Collins JRH, Saboorian MH, Lee WM. Chronic graft versus host disease (GVHD) following hematopoietic cell transplantation (HCT) presenting as an acute hepatitis. American Journal of Gastroenterology. 2001;96:588–590. doi: 10.1111/j.1572-0241.2001.03563.x. [DOI] [PubMed] [Google Scholar]
- 45.Akpek G, Boitnott JK, Lee LA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002;100:3903–3907. doi: 10.1182/blood-2002-03-0857. [DOI] [PubMed] [Google Scholar]
- 46.Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB. A coded histologic study of hepatic graft-versus-host disease after human marrow transplantation. Hepatology. 1988;8:463–470. doi: 10.1002/hep.1840080305. [DOI] [PubMed] [Google Scholar]
- 47.Strasser SI, Myerson D, Spurgeon CL, et al. Hepatitis C virus infection after bone marrow transplantation: A cohort study with 10 year follow-up. Hepatology. 1999;29:1893–1899. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- 48.Ljungman P, Johansson N, Aschan J, et al. Long-term effects of hepatitis C virus infection in allogeneic bone marrow transplant recipients. Blood. 1995;86:1614–1618. [PubMed] [Google Scholar]
- 49.Strasser SI, Sullivan KM, Myerson D, et al. Cirrhosis of the liver in long-term marrow transplant survivors. Blood. 1999;93:3259–3266. [PubMed] [Google Scholar]
- 50.Peffault de Latour R, Levy V, Asselah T, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 51.Ko CW, Murakami C, Sekijima JH, Kim MH, McDonald GB, Lee SP. Chemical composition of gallbladder sludge in patients after marrow transplantation. Am J Gastroenterol. 1996;91:1207–1210. [PubMed] [Google Scholar]
- 52.Akpek G, Valladares JL, Lee L, Margolis J, Vogelsang GB. Pancreatic insufficiency in patients with chronic graft-versus-host disease. Bone Marrow Transplantation. 2001;27:163–166. doi: 10.1038/sj.bmt.1702769. [DOI] [PubMed] [Google Scholar]
- 53.Maringhini A, Gertz MA, DiMagno EP. Exocrine pancreatic insufficiency after allogeneic bone marrow transplantation. International Journal of Pancreatology. 1995;17:243–247. doi: 10.1007/BF02785821. [DOI] [PubMed] [Google Scholar]
- 54.Radu B, Allez M, Gornet JM, et al. Chronic diarrhoea after allogenic bone marrow transplantation. Gut. 2005;54:161. doi: 10.1136/gut.2004.041335. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellis MJ, et al. Chronic kidney disease after hematopoietic cell transplantation: a systematic review. Am J Transplant. 2008;8(11):2378–2390. doi: 10.1111/j.1600-6143.2008.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi M, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. 2008;113(7):1580–1587. doi: 10.1002/cncr.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ando M, et al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant. 25(1):278–282. doi: 10.1093/ndt/gfp485. [DOI] [PubMed] [Google Scholar]
- 58.Cohen EP, et al. End-stage renal disease (ESRD)after bone marrow transplantation: poor survival compar ed to other causes of ESRD. Nephron. 1998;79(4):408–412. doi: 10.1159/000045085. [DOI] [PubMed] [Google Scholar]
- 59.Hingorani S, et al. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39(4):223–229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 60.Miralbell R, et al. Renal Toxicity After Allogeneic Bone Marrow Transplantation: The Combined Effects of Total-Body Irradiation and Graft-Versus-Host Disease. Journal of Clinical Oncology. 1996;14(2):579–585. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 61.Weiss AS, et al. Chronic Kidney Disease Following Non-Myeloablative Hematopoietic Cell Transplantation. American Journal of Transplantation. 2006;6:89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 62.Steele B, Lirenman D. Acute radiation nephritis and the hemolytic uremic syndrome. Clinical Nephrology. 1979;11(5):272–274. [PubMed] [Google Scholar]
- 63.Tarbell N, et al. Late Onset of Renal Dysfunction in Survivors of Bone Marrow Transplantation. Int. J.Radiation Oncology BiolPhys. 1988;15:99–104. doi: 10.1016/0360-3016(88)90352-5. [DOI] [PubMed] [Google Scholar]
- 64.Guinan E, et al. Intravascular Hemolysis and Renal Insufficiency After Bone Marrow Transplantation. Blood. 1988;72:451–455. [PubMed] [Google Scholar]
- 65.Cohen E, Lawton C, Moulder J. Bone Marrow Transplant Nephropathy: Radiation Nephritis Revisited. Nephron. 1995;70:217–222. doi: 10.1159/000188587. [DOI] [PubMed] [Google Scholar]
- 66.Cohen E. Radiation nephropathy after bone marrow transplantation. Kidney International. 2000;58:903–918. doi: 10.1046/j.1523-1755.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 67.Antignac C, et al. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney International. 1989;35:1336–1344. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 68.Rao P. Nephrotic syndrome in patients with peripheral blood stem cell transplant. Am J Kidney Dis. 2005;45(4):780–785. doi: 10.1053/j.ajkd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Ferrara J. Pathogenesis of acute graft versus-host disease:cytokines and cellular effectors. J Hematother Stem Cell Res. 2000;9(3):299–306. doi: 10.1089/15258160050079407. [DOI] [PubMed] [Google Scholar]
- 70.El Seisi S, et al. Renal Pathology at Autopsy in Patients Who Died after Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2003;9:683–688. doi: 10.1016/s1083-8791(03)00243-x. [DOI] [PubMed] [Google Scholar]
- 71.Romagnani P, et al. Nephrotic Syndrome and Renal Failure After Allogeniec Stem Cell Transplantation: Novel Molecular Diagnostic Tools for a Challenging Differential Diagnosis. Am J Kidney Dis. 2005;46(3):550–556. doi: 10.1053/j.ajkd.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 72.Niculescu F, et al. Both apoptosis complement membrane attack complex deposition are major features of murine acute graft vs host disease. Experimental and Molecular Pathology. 2005;73 doi: 10.1016/j.yexmp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Hingorani SR, Seidel K, Lindner A, Aneja T, Schoch G, McDonald G. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biol Blood Marrow Transplant. 2008 Dec;14(12):1365–1372. doi: 10.1016/j.bbmt.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 75.Cohen EP, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70(5):1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22:305–318. doi: 10.1016/j.hoc.2008.01.010. vii. [DOI] [PubMed] [Google Scholar]
- 77.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 78.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 79.van der Pal HJ, van Dalen EC, Kremer LC, et al. Risk of morbidity mortality from cardiovascular disease following radiotherapy for childhood cancer a systematic review. Cancer Treat Rev. 2005;31:173–185. doi: 10.1016/j.ctrv.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 81.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 82.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline-and radiation-associated cardiomyopathiesimplications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 83.van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 84.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 85.Armenian SH, Sun CL, Francisco L, et al. Late Congestive Heart Failure After Hematopoietic Cell Transplantation. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2008.17.7428. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with hematopoietic cell transplantation (HCT) versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011 doi: 10.1182/blood-2011-01-331835. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wojnowski L, Kulle B, Schirmer M, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 90.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 91.Henderson IC, Allegra JC, Woodcock T, et al. Randomized clinical trial comparing mitoxantrone with doxorubicin in previously treated patients with metastatic breast cancer. J Clin Oncol. 1989;7:560–571. doi: 10.1200/JCO.1989.7.5.560. [DOI] [PubMed] [Google Scholar]
- 92.Deng S, Wojnowski L. Genotyping the risk of anthracycline-induced cardiotoxicity. Cardiovasc Toxicol. 2007;7:129–134. doi: 10.1007/s12012-007-0024-2. [DOI] [PubMed] [Google Scholar]
- 93.Armenian SH, Ding Y, Sun CL, et al. Genetic susceptibility to anthracycline-related congestive heart failure (A-CHF) in survivors of hematopoietic cell transplantation (HCT), BMT Tandem Meetings. Honolullu, HI: Elsevier; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minotti G, Recalcati S, Menna P, et al. Doxorubicin cardiotoxicity the control of iron metabolism quinone-dependent and independent mechanisms. Methods Enzymol. 2004;378:340–361. doi: 10.1016/S0076-6879(04)78025-8. [DOI] [PubMed] [Google Scholar]
- 95.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening preventive practices for long-term survivors after hematopoietic cell transplantation joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reusch JE. Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. Am J Cardiol. 2002;90(5A):19–26. doi: 10.1016/s0002-9149(02)02555-9. [DOI] [PubMed] [Google Scholar]
- 99.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X mortality: a population-based study Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998;148(10):958–966. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 100.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 101.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16(12):1674–1681. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone- marrow transplantation in childhood. Lancet. 2000;356(9234):993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 103.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narici MV, Maffulli NSarcopenia. characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 105.Eaton SB, Cordain L, Sparling PB. Evolution, body composition, insulin receptor competition, and insulin resistance. Prev Med. 2009;49(4):283–285. doi: 10.1016/j.ypmed.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13(8):717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 107.Baker KS, Chow EJ, Koves I, et al. Adverse Impact of Hematopoietic Cell Transplantation (HCT) on Body Composition and Insulin Resistance is Associated with Increased Cardiovascular Risk. Biol Blood Marrow Transplant. 2011;17 Supplement 2:174. [Google Scholar]
- 108.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12(2):138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT) Bone Marrow Transplant. 2006;37(3):249–261. doi: 10.1038/sj.bmt.1705243. [DOI] [PubMed] [Google Scholar]
- 110.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 111.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 112.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 113.CookeKR YG. Foreman A, Negrin Blume, editors. Lung Injury Following Hematopoietic Stem Cell Transplantation. Thomas’ Hematopoietic Cell Transplantation. (4th Edition) 2009:1456–1472. [Google Scholar]
- 114.Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. 57:297–322. doi: 10.1016/j.pcl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duncan CN, Buonanno MR, Barry EV, Myers K, Peritz D, Lehmann L. Bronchiolitis obliterans following pediatric allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:971–975. doi: 10.1038/bmt.2008.19. [DOI] [PubMed] [Google Scholar]
- 117.Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43:42–52. doi: 10.1053/j.seminhematol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 119.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 16:106–114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hildebrandt GC, Fazekas T, Lawitschka A, Bertz H, Greinix H, Halter J, Pavletic SZ, Holler E, Wolff D. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. doi: 10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner ME, Vogelsang G, Flowers M. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 122.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, Clark JG. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 123.Cerveri I, Zoia MC, Fulgoni P, Corsico A, Casali L, Tinelli C, Zecca M, Giorgiani G Locatelli F. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax. 1999;54:131–135. doi: 10.1136/thx.54.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–3828. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]
- 125.Chien JW, Zhao LP, Hansen JA, Fan WH, Parimon T, Clark JG. Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood. 2006;107:2200–2207. doi: 10.1182/blood-2005-06-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hildebrandt GC, Granell M, Urbano-Ispizua A, Wolff D, Hertenstein B, Greinix HT, Brenmoehl J, Schulz C, Dickinson AM, Hahn J, Rogler G, Andreesen R, Holler E. Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:67–74. doi: 10.1016/j.bbmt.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 127.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a Tumor necrosis factor-a transgene in murine lung causes lymphocytic and fibrosing alveolitis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yanik GA, M S, Levine JE, Kitko CL, White ES, VanderLug MT, Harris AC, Braun T, Cooke KR. Submitted for publication. Soluble Tumor Necrosis Factor Receptor: Enbrel (Etanercept) For Sub-Acute Pulmonary Dysfunction Following Allogeneic Stem Cell Transplantation. doi: 10.1016/j.bbmt.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Norman BC, Jacobsohn DA, Williams KM, Au BK, Au MA, Lee SJ, Moravec CK, Chien JW. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. doi: 10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]