Abstract
Although the carboxylic acid moiety is prevalent in many biologically produced molecules, including natural products and proteins, methods to chemoselectively target this functional group have remained elusive. Generally, strategies that utilize carboxylate nucleophilicity also promote reactions with other nucleophiles such as amines and hydroxyls. A reagent was sought to facilitate the selective isolation of carboxylic acid-containing compounds from complex mixtures. Here, the development of siloxyl-ether functionalized solid supports is described.
The search for molecules possessing the features required to modulate biological processes is a longstanding scientific goal. Natural products have been a continual source of inspiration, yielding many therapeutic agents and targets for (bio)synthetic studies. Natural products and their derivatives account for nearly half of the drugs currently on the market1 and have been used extensively as chemical probes.2 Despite recent advances,3 the isolation of novel natural products remains challenging as the use of traditional isolation methods often results in rediscovery of known compounds and/or the loss of bioactivity.4
Current purification methods, such as high performance liquid chromatography (HPLC) or size exclusion chromatography, separate molecules by their physicochemical properties including polarity, charge, and size. New technologies are needed that isolate molecules based upon alternative characteristics than those presently used for natural product discovery, which are biased towards abundant molecules. We have reported development of chemoselective isolation strategies to facilitate enrichment of a subclass of biological molecules including metabolites5 and alcohol-containing natural products.6 For natural products discovery, we employ a controllably reversible reaction to immobilize the targeted functional group class onto solid support. Following an extensive wash protocol, the enriched subpool is tracelessly released to enable immediate characterization of the natural products of interest.
Generation of a chemoselective isolation reagent toolkit is significant because it will provide a means for discovery of natural products that are unlikely to be identified by traditional strategies due to low isolation yields, poor compound resolution and/or the presence of interfering compounds. Furthermore, a functional group targeted method can offer insight into the structural content of compounds prior to characterization efforts.
Our first reversible enrichment tag was for the hydroxyl functional group, which was targeted by formation of a silyl ether bond using a chlorodiethylsiloxane polystyrene resin.6 The isolated compound pool was readily released from the resin by treatment with a fluoride source. Here, we describe a chemoselective strategy for the carboxylic acid. This functional group is present in approximately 15% of natural products and 25% of drugs,7 making it an important group to target with our reversible enrichment method. A previous study reports use of an anion exchange resin to separate a subpool of natural products, including carboxylic acids.3b However, other acidic groups, such as phenols,7 were readily isolated using the reported strategy. Additionally, the non-covalent interaction that this method is dependent upon is not strong enough to tolerate extensive washing protocols resulting in substantial carryover between fractions. Clearly, development of a covalent and chemoselective enrichment strategy is warranted.
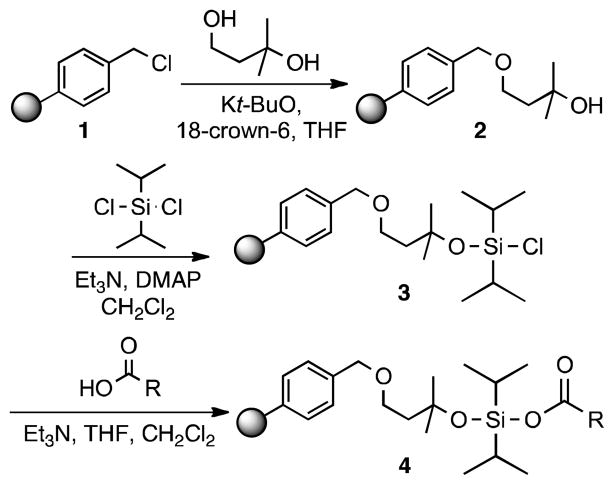
Few transformations that selectively target carboxylic acids have been reported.8 During the development of our hydroxyl group enrichment tag, we identified a silicon-functionalized resin architecture (3) that displayed significant preference for reaction with carboxylic acid-containing molecules. This resin utilizes a diisopropylsiloxane capture moiety, which forms the corresponding siloxyl ester bond with carboxylic acids (4; Scheme 1).9 Following capture, unaltered compounds are readily released from resin using a fluoride source. This result was surprising given the general instability of silyl esters;10 however, carboxylic acids were enriched using resin 3 with yields of ~90% (Table S1). This resin also promoted isolation of alcohol-containing compounds (yields 6–18%) suggesting that optimization would be required to achieve chemoselectivity. Amine- and thiol-containing compounds were not captured to an appreciable extent by this resin (0–3%; Table S1).
Scheme 1.
Synthesis of the diisopropyl siloxyl chloride resin.
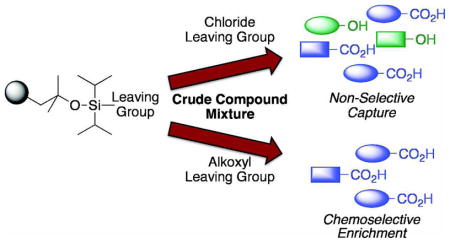
With the ultimate goal of generating a chemoselective capture resin, we first sought to identify the chemical characteristics that resulted in formation of a stable ester species while also affording selectivity towards carboxylic acids. A series of resin derivatives was synthesized and assessed for their ability to capture a standard set of compounds. We anticipated that the steric environment about the silicon atom would be critical to ester stability. Resin 3 displays relatively hindered isopropyl groups. Thus, we wished to examine resin variants with altered steric properties. The more hindered derivative, a di-t-butyl resin, could not be synthesized. We expect that this is a result of the bulky nature of the intended product. Both the dimethyl- and diethyl-substituted resins, S1 and S2, respectively, favored enrichment of alcohol-containing compounds with yields of ~70% and exhibited dramatically lower yields of carboxylic acids (~20%; Table S1). These data suggest that the nature of the alkyl substituents on the silicon atom is critical to preferential functional group capture.
These results are consistent with what is known about silyl and siloxyl ester stability in that bulkier esters are significantly more stable.10 However, silylating reagents are generally not chemoselective for carboxylic acids often forming more stable bonds with the hydroxyl group.11 Recently, a bulky reagent, di-tert-butylisobutylsilyl triflate, was reported to selectively protect carboxylic acids over alcohols in some substrates.12 This protecting group also readily forms stable conjugates with amines. Intriguingly, we found that the presence of the diisopropyl substituents on the silicon was not enough to afford a selective reagent. The oxygen linker between the resin and the silicon atom is also important for selectivity as a diisopropylsilyl-functionalized resin facilitated the isolation of alcohols, carboxylic acids, thiols and amines (S3, Table S1 and Ref 6). Thus, our studies indicate that privileged capture of carboxylic acids is best accomplished with the diisopropyl siloxyl scaffold.
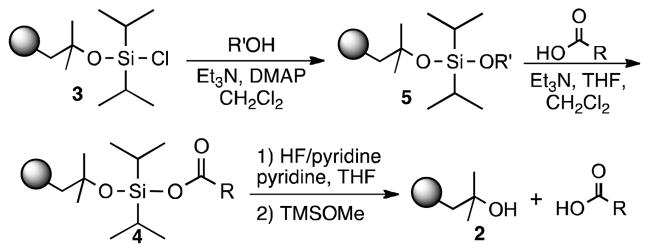
Efforts to achieve the desired chemoselectivity by preferentially cleaving either the captured alcohols or acids from resin 3 were unsuccessful (data not shown). We reasoned that tuning of the leaving group would enable selective capture of the more nucleophilic carboxylate. Accordingly, a series of dialkyldisiloxane resins was generated by alcoholysis of the silyl chloride (5, Scheme 2). First, we examined the loading capacity and enrichment yields of three resins, methoxy- (6), isopropoxy- (7), and t-butoxy-substituted (8), with a model set of carboxylic acids (Tables 1, S2, and S3). The best combination of loading capacity and breadth of carboxylic acid capture was obtained with the isopropoxy derivative (7). The t-butoxy derivative (8) was effective for capture of less hindered substrates but was too bulky to enable efficient enrichment of sterically encumbered compounds, while the methoxy derivative (6) showed moderate to low yields likely because of its relatively poor leaving group potential. Importantly, all three derivatives displayed complete carboxylic acid chemoselectivity (Table 1). Further studies will be required to determine if chemoselectivity is due exclusively to the nucleophilicity of the carboxylate, or to other characteristics such as an increased ability of the carboxylate to form a pentavalent silicon complex.
Scheme 2.
Generation of dialkyldisiloxyl resin derivatives useful for chemoselective capture of carboxylic acids. Upon cleavage, the resin can be regenerated (Table S4).
Table 1.
Enrichment yields of model carboxylic acid-, alcohol-, amine- and thiol-containing molecules with derivatives of 5.
| Model Compound | Enrichment Yields | ||||
|---|---|---|---|---|---|
|
6 |
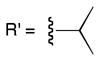 7 |
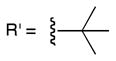 8 |
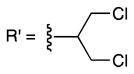 9 |
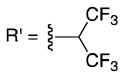 10 |
|
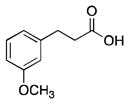 11 |
25% | 95% | 75% | 95% | 95% |
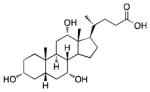 12 |
73% | 94% | 53% | 81% | 47% |
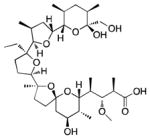 13 |
43% | 13% | 82% | 72% | 80% |
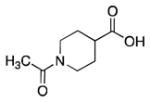 14 |
52% | 75% | 67% | 95% | 90% |
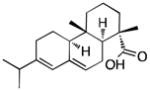 15 |
35% | 42% | 22% | 84% | 63% |
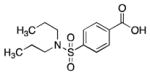 16 |
55% | 50% | 49% | 85% | 69% |
|
| |||||
|
17 |
0% | 0% | 0% | 0% | 0% |
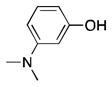 18 |
0% | 0% | 0% | 0% | 0% |
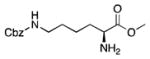 19 |
0% | 0% | 0% | 0% | 0% |
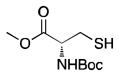 20 |
0% | 0% | 0% | 0% | 0% |
To identify a resin with increased loading capacity and consistently high enrichment yields, we explored alkoxyl moieties with electron withdrawing groups (EWG) or electron donating groups (EDG). A variety of alcohols, 22 in all, were tested to see which properties (i.e. sterics, EWG, or EDG) yielded resins with the highest loading capacity with three carboxylic acids (Table S2). The highest loading values were obtained by activation with secondary alcohols. Efficient isolation of tertiary carboxylic acids was only seen with resins derived from electron poor alcohols verifying the influence of the leaving group on capture potential (e.g. 9, 10, S23, S24).
From this resin set, 9 and 10 were selected for further characterization based upon both loading capacity and cost of the reagents. These resins were subjected to coupling with a large model set of carboxylic acids ranging in steric hinderance and molecular complexity (20 compounds; Table 1 and S3). Amine-, thiol- and alcohol-containing compounds were included to assess chemoselectivity (Table 1). High and consistent enrichment yields of a wide array of carboxylic acids were observed with resin 9, which was activated with a 1,3-dichloro-2-propoxyl group. This resin was also found to be completely chemoselective and was chosen as our carboxylic acid enrichment tag.
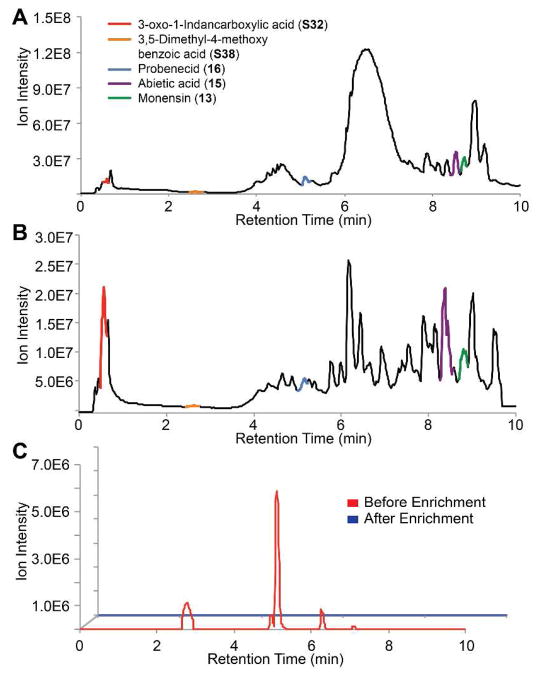
We next sought to show the utility of this resin for the isolation of carboxylic acids from complex mixtures. Monensin (13) is a polyether ionphore antibiotic secreted by Streptomyces cinnamonensis.13 The growth media of this bacterium was collected to yield a crude natural product extract. Prior to enrichment, the extract was doped with four model carboxylic acids and two alcohols, two amines, and a thiol (17–20, S9) to ensure chemoselectivity in a biological setting. We analyzed the crude extract by LC-MS, yielding trace A (Figure 1).
Figure 1.
Enrichment from the extract of Streptomyces cinnamonensis. A. TIC of the crude natural product extract with monensin and four doped model carboxylic acids, which are highlighted. B. TIC of the carboxylic acid-containing molecules enriched from the extract. C. Two alcohols (17, 18), two amines (19, S9), and a thiol (20) were spiked into the extract. Red trace is the extracted ion chromatogram of the compounds prior to enrichment. Blue trace indicates that none of the compounds are present following capture and release of the extract.
The extract was subjected to enrichment with resin 9. The resulting material was analyzed by LC-MS using the same gradient (trace B, Figure 1). Monensin (13) was recovered in a 64% yield along with 3-oxo-1-indancarboxylic acid (S32), 3,5-dimethyl-4-methoxybenzoic acid (S38), probenecid (16), and abietic acid (15) recovered in a 96%, 87%, 75%, and 57% yield, respectively. None of the chemoselective model compounds were detected, confirming that 9 remains specific for carbxoylic acid-containing compounds in a biological background (Figures 1C and S3). It is evident from the chromatograms that many of highly abundant compounds were eliminated and that fewer compounds are present after carboxylic acid capture enabling better resolution of the remaining components. Additionally, some features represent a greater proportion of the carboxylic acid fraction than of the crude extract demonstrating enrichment. We quantified the extent of carboxylic acid enrichment in comparison to compounds containing other functional groups that were spiked into the sample. Carboxylic acids were enriched by an average of ~300-fold over other functional groups (Table S5).
To show that enrichment occurs equivalently independent of the proportion of the sample that the targeted compounds comprise, we spiked three carboxylic acids into the Streptomyces cinnamonensis extract at varying concentrations. An average recovery yield of ~80% was observed across ratios of natural product background to targeted acid ranging from 2:1 to 800:1 (8 μmol-15 nmol; Table S7). This provides strong evidence that our enrichment strategy will enable detection of low abundance species.
Finally, we confirmed the utility of resin 9 for isolation of carboxylic acids following solution phase synthesis. We converted the methyl ester derivative of serine to the corresponding carboxylic acid and subjected the crude reaction to 9, resulting in isolation of the product in good yield and high purity (Figure S5). Thus, our resin can be used to purify synthetically-derived carboxylic acids, mostly notably, polar compounds that are difficult to isolate using standard chromatography methods.
We have synthesized a chemoselective enrichment tag for carboxylic acid-containing molecules and applied it to a variety of systems. We determined that a diisopropyl siloxyl architechure is required for carboxylic acid preference and that an alkoxyl leaving group is essential to achieve complete chemoselectivity. After determining the loading capacities of 22 diisopropyldisiloxane resins, we established that resin 9 had all of the desired properties to serve as our enrichment reagent. This is the first example of a chemoselective method for solid-phase enrichment of carboxylic acids. In addition, to the best of our knowledge, formation of silyl or siloxyl esters via the siloxyl ether has not been previously reported.10,14 We expect that this carboxylic acid enrichment tag, along with our previously developed tag for hydroxyl-containing compounds will find utility in many applications, including the discovery of natural products.
Supplementary Material
Acknowledgments
This work was supported by NIH R00 GM82983 and a Pew Biomedical Scholar Award (EEC). We thank L. Brown and M. K. Brown for their thorough reading of the manuscript.
Footnotes
Supporting Information Available Methods for resin synthesis, compound capture and cleavage; methods for determination of loading capacities and enrichment yields; Streptomyces cinnamonensis enrichment data; characterization of resins and mass spectrometry data for enrichment experiments.
References
- 1.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 2.(a) Carlson EE. ACS Chem Biol. 2010;5:639. doi: 10.1021/cb100105c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bottcher T, Pitscheider M, Sieber SA. Angew Chem Int Ed. 2010;49:2680. doi: 10.1002/anie.200905352. [DOI] [PubMed] [Google Scholar]
- 3.(a) Månsson M, Phipps RK, Gram L, Munro MH, Larsen TO, Nielsen KF. J Nat Prod. 2010;73:1126. doi: 10.1021/np100151y. [DOI] [PubMed] [Google Scholar]; (b) Araya JJ, Montenegro G, Mitscher LA, Timmermann BN. J Nat Prod. 2010;73:1568. doi: 10.1021/np100465h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watve MG, Tickoo R, Jog MM, Bhole BD. Arch Microbiol. 2001;176:386. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- 5.(a) Carlson EE, Cravatt BF. Nat Methods. 2007;4:429. doi: 10.1038/nmeth1038. [DOI] [PubMed] [Google Scholar]; (b) Carlson EE, Cravatt BF. J Am Chem Soc. 2007;129:15780. doi: 10.1021/ja0779506. [DOI] [PubMed] [Google Scholar]
- 6.Odendaal AY, Trader DJ, Carlson EE. Chem Sci. 2011;2:760. doi: 10.1039/C0SC00620C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henkel T, Brunne RM, Müller H, Reichel F. Angew Chem Int Ed. 1999;38:643. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Hermanson GT. Bioconjugate Techniques. 2. Elsevier Inc; Rockford, Illinois: 2008. [Google Scholar]
- 9.Meloni MM, White PD, Armour D, Brown RCD. Tetrahedron. 2007;63:299. [Google Scholar]
- 10.(a) Weinberg JM, Gitto SP, Wooley KL. Macromolecules. 1998;31:15. [Google Scholar]; (b) Wang M, Weinberg JM, Wooley KL. Macromolecules. 1998;31:7606. [Google Scholar]; (c) Kocienski PJ. Protecting Groups. 3. Thieme; Stuttgart: 2005. [Google Scholar]
- 11.Ojima Y, Yamaguchi K, Mizuno N. Adv Synth Catal. 2009;351:1405. [Google Scholar]
- 12.Liang H, Hu L, Corey EJ. Org Lett. 2011;13:4120. doi: 10.1021/ol201640y. [DOI] [PubMed] [Google Scholar]
- 13.Huczynski A, Stefanska J, Przybylski P, Brzezinski B, Bartl F. Bioorg Med Chem Lett. 2008;18:2585. doi: 10.1016/j.bmcl.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 14.(a) Mbah GC, Speier JL. J Organomet Chem. 1984;271:77. [Google Scholar]; (b) Chauhan M, Chauhan BPS, Boudjouk P. Org Lett. 2000;2:1027. doi: 10.1021/ol005507t. [DOI] [PubMed] [Google Scholar]; (c) Huang X, Craita C, Awad L, Vogel P. Chem Commun. 2005:1297. doi: 10.1039/b417894g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.