Abstract
P190A and p190B Rho GTPase activating proteins (GAPs) are essential genes that have distinct, but overlapping roles in the developing nervous system. Previous studies from our laboratory demonstrated that p190B is required for mammary gland morphogenesis, and we hypothesized that p190A might have a distinct role in the developing mammary gland. To test this hypothesis, we examined mammary gland development in p190A-deficient mice. P190A expression was detected by in situ hybridization in the developing E14.5 day embryonic mammary bud and within the ducts, terminal end buds (TEBs), and surrounding stroma of the developing virgin mammary gland. In contrast to previous results with p190B, examination of p190A heterozygous mammary glands demonstrated that p190A deficiency disrupted TEB morphology, but did not significantly delay ductal outgrowth indicating haploinsufficiency for TEB development. To examine the effects of homozygous deletion of p190A, embryonic mammary buds were rescued by transplantation into the cleared fat pads of SCID/Beige mice. Complete loss of p190A function inhibited ductal outgrowth in comparison to wildtype transplants (51% vs. 94% fat pad filled). In addition, the transplantation take rate of p190A deficient whole gland transplants from E18.5 embryos was significantly reduced compared to wildtype transplants (31% vs. 90%, respectively). These results suggest that p190A function in both the epithelium and stroma is required for mammary gland development. Immunostaining for p63 demonstrated that the myoepithelial cell layer is disrupted in the p190A deficient glands, which may result from the defective cell adhesion between the cap and body cell layers detected in the TEBs. The number of estrogen- and progesterone receptor-positive cells, as well as the expression levels of these receptors was increased in p190A deficient outgrowths. These data suggest that p190A is required in both the epithelial and stromal compartments for ductal outgrowth and that it may play a role in mammary epithelial cell differentiation.
Keywords: p190RhoGAP, p190A, Grlf1, Mammary, RhoGAP, Embryonic transplant
Introduction
Rho GTPases regulate a diverse set of biological activities including actin organization, focal complex/adhesion assembly, cell motility, cell polarity, gene transcription, differentiation, and cell cycle progression. Aberrant Rho GTPase expression and activation is known to be involved in breast cancer development (Boettner and Van Aelst, 2002; Fritz et al., 2002; Fritz et al., 1999; Sahai and Marshall, 2002; Schnelzer et al., 2000). The mechanisms implicated in the deregulation of Rho GTPases are indirect, requiring changes in Rho regulatory proteins, which regulate the cycle between the GTP-bound active form and the GDP-bound inactive form. Guanine nucleotide exchange factors (GEFs) exchange the bound GDP for GTP and return the GTPase to the active state, while Rho GTPase activating proteins (RhoGAPs) enhance the hydrolysis of the bound GTP.
Of the large RhoGAPs, the most widely studied are the p190 RhoGAPs. Two isoforms of p190 exist, p190A, a putative tumor suppressor and p190B, a putative oncogene (Heckman-Stoddard et al., 2009; McHenry PR, 2010; Wang et al., 1997). These two genes, which share 51% amino acid identity, map to different chromosomes suggesting they diverged early in evolution acquiring distinct functions (Scheffzek et al., 1998).
The functions of p190A have been investigated both in cell culture and in vivo in p190A deficient mice. P190A deficiency in mice results in perinatal lethality due to defects in central nervous system (CNS) development. These mice exhibit extensive neural cell adhesion defects indicating that p190A plays a critical role in cell adhesion (Brouns et al., 2000). Furthermore, extensive accumulation of polymerized actin was observed in the developing neural tube in the mutant embryos, indicating that p190A is an important regulator of the actin cytoskeleton. These studies also resulted in identification of p190A as a substrate of protein kinase C (PKC) due to similar phenotypes observed in the p190A mutant and MARCKS null mice, a known substrate of PKC (Brouns et al., 2000). Despite these defects, mouse embryonic fibroblasts (MEFs) from p190A mutant embryos exhibit normal proliferation rates, morphology, actin organization, migration, spreading, and adhesion to extracellular matrix. This is thought to be due to abundant p190B in these cells, which was previously shown to account for 5-times the level of the RhoGAP activity of p190A in fibroblasts (Vincent and Settleman, 1999). However, recent studies have shown that p190A-null fibroblasts are defective in directional motility, and p190A along with RhoA was shown to act downstream of the PAR-6/aPKC complex in regulating dentritic-spine polarity (Jiang et al., 2008; Zhang and Macara, 2008). P190A further regulates polarity by affecting focal adhesion maturation and interacting with p120RasGAP downstream of FAK at the leading edge of fibroblasts during polarized cell migration (Pullikuth and Catling; Tomar et al., 2009). P190A has also been shown to indirectly regulate gene expression by affecting release of the transcription factor TFII-I, allowing it to translocate to the nucleus to activate gene transcription (Chang et al., 1995; Jiang et al., 2005). A role for p190A in cytokinesis was indicated by its overexpression in MDA-MB-468 cells, which blocked cytokinesis (Mikawa et al., 2008; Su et al., 2003).
In the developing mammary gland, p190B completely prevents ductal outgrowth demonstrating that it is critical for normal development. Consistent with these results, p190B heterozygous virgin mice demonstrate delayed ductal morphogenesis during weeks 3–6 of postnatal development as well as reduced tumor penetrance and delayed tumor onset when crossed to the MMTV-Neu mammary tumor model (Chakravarty et al., 2003; Heckman-Stoddard et al., 2009). Conversely, overexpression of p190B in the mammary epithelium during virgin development disrupts TEB morphogenesis, increases side branching, and delays ductal elongation. This overexpression during pregnancy results in hyperplastic lesions (Vargo-Gogola et al., 2006). In the MMTV-Neu tumor model, p190B overexpression leads to a 2-fold increase in tumor multiplicity, and a 3-fold increase in metastasis (McHenry PR, 2010). P190B is also required for embryonic mammary development, and it plays an important role in both the epithelial and stromal compartments of the developing mammary bud. Deficiency of p190B results in a reduced number of epithelial cells, and the mesenchyme surrounding the buds is disorganized and diminished due decreased proliferation. Similarities between these developmental defects and those that result from loss of both IRS-1 and IRS-2 expression suggested a role for crosstalk of IGFR and p190B signaling in embryonic mammary development (Heckman et al., 2007).
Investigation of the roles of p190A and p190B in CNS development demonstrated that these two genes are required for CNS development and that they have overlapping, but distinct functions (Matheson et al., 2006). Therefore, we hypothesized that p190A, like p190B, is required for mammary gland development, and if so, that it may play a distinct role in regulating TEB morphogenesis and ductal outgrowth. We examined the expression of p190A and tested the effects of loss of p190A function on mammary gland development were examined. The data reported here confirm that p190A is indeed required for mammary development and that it exerts unique effects distinct from p190B in regulating mammary morphogenesis.
Materials and Methods
Mouse Strains
P190A null mice, generated in the laboratory of Dr. Jeffrey Settleman (Harvard Medical School, Cambridge, MA), were maintained on a CD1 × C57Bl/6 × 129Sv background. Mice were fed a conventional diet ad libitum and maintained at 21–22 C with a 12 hr light cycle, 12 hr dark cycle. Animal protocols were approved by the Animal Care and Use Committee of Baylor College of Medicine and were conducted in accordance with the provisions of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Wildtype females were mated with heterozygous males for virgin mammary gland development studies. Wildtype littermates were used as controls. Mice at appropriate age were injected with BrdU (100 mg/kg) intraperitoneally. Two hours later mammary glands (MGs) were dissected and fixed for 2 hrs. in 4% paraformaldehyde (PFA). MGs were dehydrated and embedded in paraffin. Five µm serial sections were then taken in the frontal plane. To obtain embryonic mammary tissue for transplantation experiments, heterozygous females were mated with heterozygous males with detection with detection of a plug marked as E1. At E18.5, embryos were dissected by Cesarean section, stored at 4°C in DMEM/F12, genotyped overnight for p190A (Matheson et al., 2006) and SRY (Chakravarty et al., 2003), and dissected the following morning to isolate the no. 3 and 4 embryonic fat pads containing the rudimentary ductal tree.
In situ hybridization
Riboprobes were labeled with [DIG]-UTP (Boehringer 1277073), using T7 transcription system from Stratagene. To generate antisense riboprobe template, p190B cDNA was amplified from mouse brain by PCR using forward primer 5’-GGATCCTAATACGACTCACTATAGGGAGATATACTCGGTGCCCCACGACA and reverse primer 5’-CTCTGGAGTCACCACTGTTGT. The sense riboprobe template was generated from the same cDNA by PCR using forward primer 5’-TATACTCGGTGCCCCACGACA and reverse primer 5’-GGATCCTAATACGACTCACTATAGGGAGACTCTGGAGTCACCACTGTTGT. Embryos were stained as previously described (Heckman et al., 2007).
For mammary gland sections: 5 µm paraffin sections of 6 week old mammary glands were deparaffinized, rehydrated and washed in PBS. Then treated with PK (25 µg/ml) for 15 min at 37°C and fixed with 4% PFA for 30 min followed by washing with 2xSSC. Pre-warmed hybridization buffer containing the probe at a concentration of 1 µg/ml was added and hybridized overnight at 55°C. Slides were washed with stringency wash (50% 2xSSC, 50% formamide) for 15 min at 42 °C followed by washes with 2xSSC 20 min at RT and digestion with 40 µg/ml of RNase A for 15 min at 37 °C then washed with 0.1xSSC for 15 min at 42 °C and then 10 min at RT. Slides were then washed with Buffer I (100mM Tris pH 7.5, 150mM NaCl) for 5 min at RT and blocked for 2 hrs in Buffer I with 3% sheep serum and 0.3% triton X-100 at RT. The slides were then incubated overnight with 1:200 anti-digoxigenin(DIG)-AP at 4 °C. For color development slides were washed with Buffer I for 10 min at RT and then washed with Buffer II (100mM Tris pH9.5, 100mM NaCl, 50mM MgCl) for 2 min at RT and incubating them in prewarmed BM purple (Roche 1442074) from 30 min to overnight in the dark at RT. Slides were counterstained with nuclear fast red, dehydrated, and mounted using Permount (Sigma, St. Louis, MO).
Whole Mounts
Virgin MGs were prepared as stained whole mounts using hematoxylin as previously described (Chakravarty et al., 2003). Transplant MGs were stained with Neutral Red (Landua et al., 2009). Briefly, glands were washed with acetone 3 times for 1 hr each followed by overnight staining with 10% Neutral Red/1%Acetic Acid (Moraes et al., 2007). The following morning glands were washed with 100% EtOH 3 times for 30 min, and cleared in xylene overnight. Pictures are captured in the same manner as hematoxylin whole mounts using a Leica stereomicroscope. Neutral Red stained glands are then embedded and sectioned as described above to use for immunostaining.
Mammary Tissue Transplantation
Because p190A heterozygous mice were in a mixed background, immuno-compromised 3 week-old SCID/Beige mice (Charles River) were employed as hosts to avoid graft rejection. Both of the inguinal mammary glands were cleared of endogenous epithelium. Wildtype inguinal embryonic epithelial fragments were implanted into an incision made in the cleared fat pad of the recipient gland, with a p190A deficient embryonic epithelial fragment from a littermate placed into the contralateral gland. Thoracic embryonic glands were placed in the interstitial space between the host thoracic and inguinal gland. Mammary gland outgrowths were analyzed 6 weeks after transplantation by whole mount staining.
Immunohistochemistry/ Immunofluorescence
Immunohistochemistry for p63 (Labvision Thermo Fisher), netrin and neogenin (Santa Cruz Biotechnology, Santa Cruz, CA), ERα (Santa Cruz Biotechnology), and PR (Dako, Carpenteria, CA) was performed as previously described (Vargo-Gogola et al., 2006). Immunofluorescence was performed using antibodies recognizing cytokeratin 8 (CK8) (Developmental Studies Hybridoma Bank, University of Iowa) cytokeratin 14 (CK14) (Covance) 1:200/1:200 in 5%BSA, 0.5% blocking buffer plus MOM kit (Vector Laboratories) overnight at RT. Sections were washed in PBS and incubated with anti-mouse Alexa 488 and anti-rabbit Alexa 594 (Invitrogen) secondary antibody diluted 1:250 in 5% BSA, 0.5% blocking buffer for 1 hr at RT. Slides were washed with PBS and staining according to the manufacturer’s instructions with DAPI (Vector Laboratories). As a negative control, slides were incubated with purified rabbit immunoglobulin (The Jackson Laboratory). Masson’s Trichrome (Sigma) was completed as per the manufacturer guidelines.
Results
P190A is expressed in the developing embryonic and virgin mammary gland
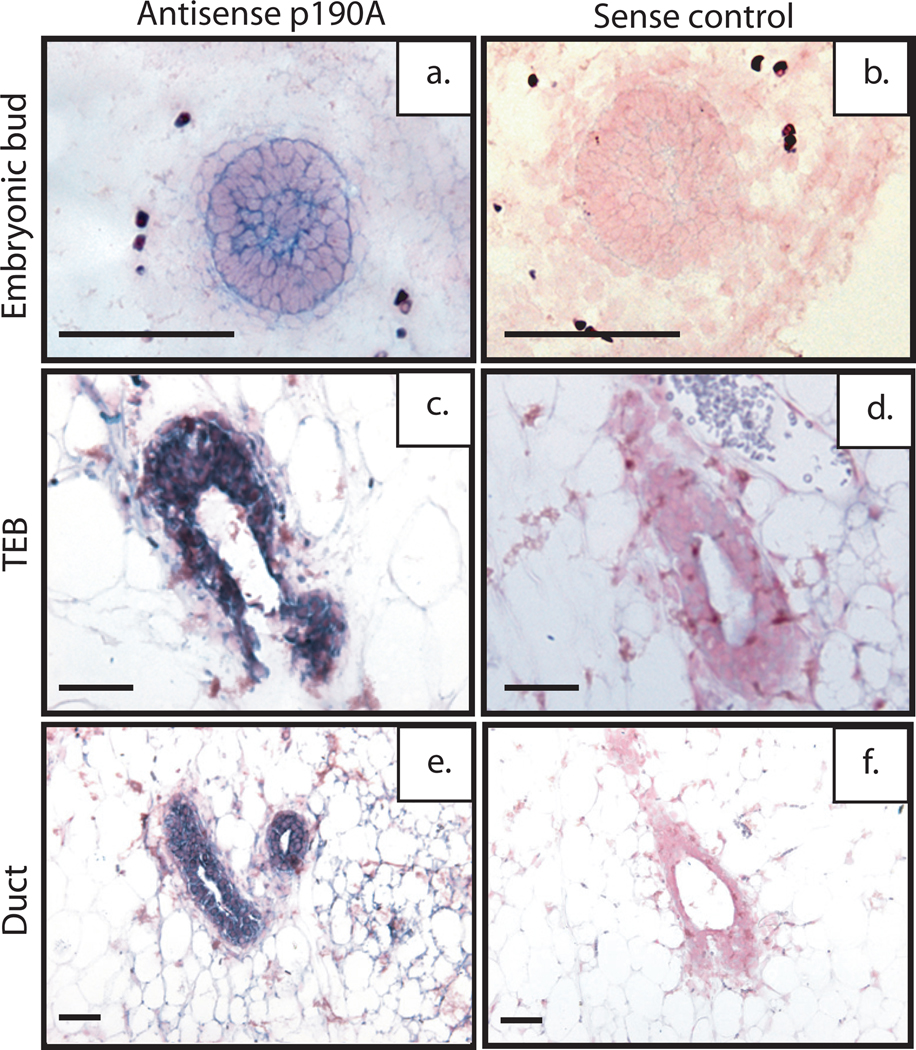
We previously reported that p190B is essential for mammary gland development (Chakravarty et al., 2003). Because p190A and p190B share 51% amino acid identity, we wanted to determine the expression patterns of p190A during mammary gland development. Accordingly, we generated a DIG-labeled riboprobe to the more divergent region of the p190A and p190B transcripts (no stretch of identity greater than 6 nucleotides). In situ hybridization revealed that p190A, like p190B, is expressed within the epithelial compartment of the E14.5 day embryonic mammary bud and at a lower level in the surrounding stroma and skin (Fig. 1a). P190A is also expressed throughout the TEB at comparable levels in both the body and cap cells (Fig. 1c), in contrast to p190B, which is expressed at a higher level in the cap cell layer (Chakravarty et al., 2000), confirmed using dig-labeled p190B probe (data not shown). p190A is also observed in the fibroblasts and stroma surrounding the TEB and uniformly throughout the ducts (Fig 1c,e). No expression was detected when sense control probes were used (Fig. 1b,d,f). These results suggested that p190A, like p190B, may also play a role in embryonic and virgin mammary gland development. There are currently no antibodies for either p190A or p190B that detect protein expression in paraffin-embedded sections, and thus, these results could not be confirmed using immunohistochemical methods.
Figure 1. p190A is expressed throughout embryonic and virgin mammary gland development.
Spatial localization of p190A mRNA in E14.5 mammary buds of wildtype mice using DIG-labeled antisense riboprobe and counter stained with fast red nuclear stain. Shown are representative antisense (a) and sense (b) images with strong transcript expression in the epithelial compartment of the mammary bud and lower expression in the mesenchyme and skin. Spatial localization of p190A mRNA within the developing virgin mammary gland. Shown are representative images of TEB (c) and duct (e) demonstrating strong expression within both, and in the surrounding stroma. Sense control (d and f) is negative for expression. Scale bar 50 µm.
P190A heterozygosity transiently delays virgin outgrowth and disrupts TEB architecture
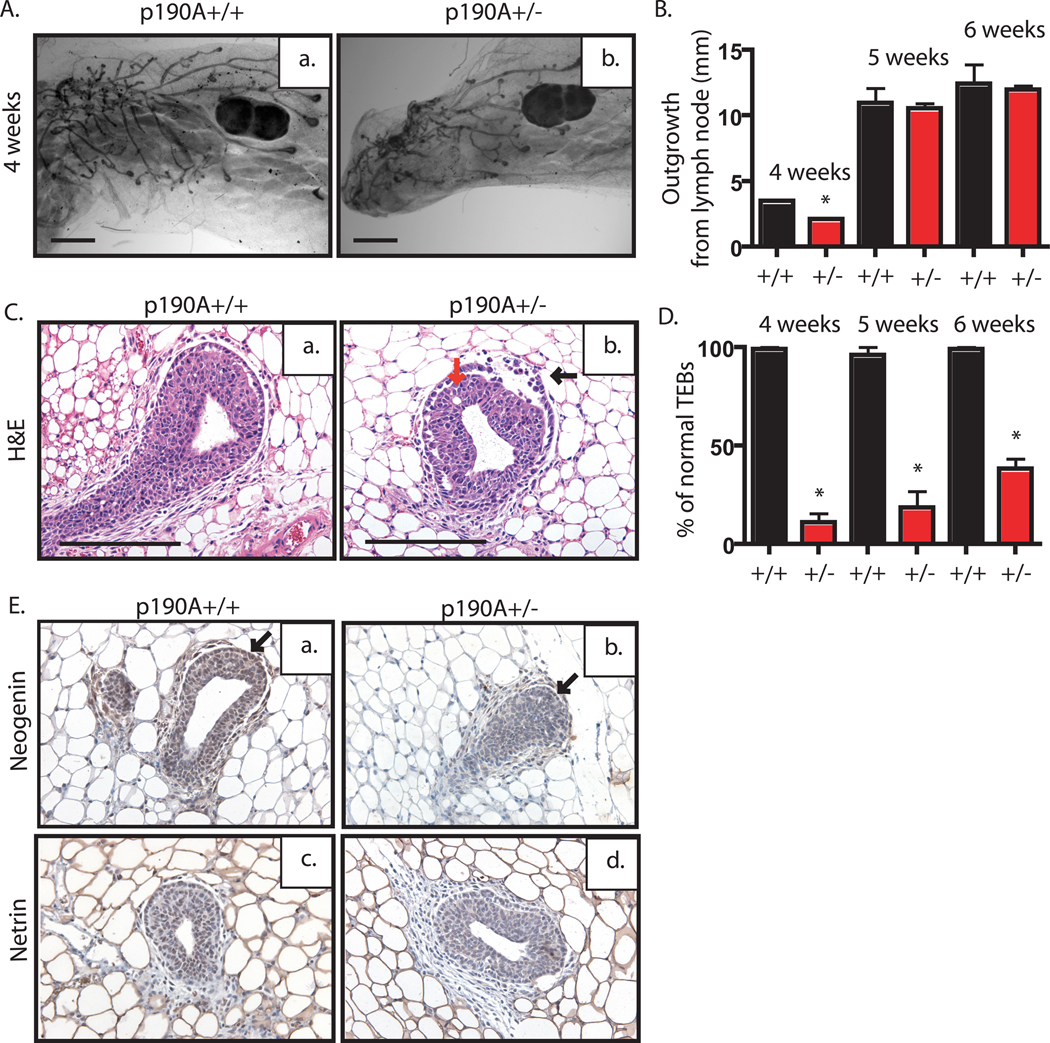
Examination of mature p190A heterozygous females revealed no overt defects during embryonic or postnatal mammary gland development as well as no overt defects during lactation or involution. The heterozygous females are viable, gave birth to normal size litters, and nursed their young. Examination of whole mounts of mammary glands from virgin, midpregnant, and lactating mice did not reveal any apparent differences (data not shown). Because p190B mice had shown a delay in ductal outgrowth in early pubescent mice, we analyzed the rate of ductal outgrowth at similar time points, 4, 5, and 6 weeks of age in wildtype and heterozygous female littermates. P190A heterozygous mice exhibited significantly retarded growth at 4 weeks (p<.0001) (Fig. 2Aa–b; 2B). However, this delay is no longer apparent at 5 and 6 weeks (Fig. 2B and Supplementary Fig. 1a–b). To further investigate this early delay, we examined TEB histology in hematoxylin & eosin (H&E) stained tissue sections. This analysis suggests that p190A heterozygosity leads to a defect in adhesion between the cap and body cell layers (black arrow) (Fig. 2C). To quantify the extent of disruption the TEB structures, the percentage of normal TEB structures was determined at 4, 5, and 6 weeks. Structures were designated as normal if they did not show a disruption of the cap cell layer. In comparison to wildtype, the percentage of normal TEB structures was significantly decreased in the p190A heterozygous glands (Fig. 2D), p<.0001 at all time points (n=5 per time point; 50 TEBs analyzed). To further investigate the defect in adhesion between the cap and body cells, we performed immunohistochemistry to examine expression patterns of the axon guidance molecules netrin and neogenin that have been shown to mediate cap and body cell adhesion (Srinivasan et al., 2003). Neogenin levels in the cap cell layer were reduced in p190A heterozygous TEBs compared to wildtype TEBs (Fig. 2Ea–b), whereas netrin levels were similar (Fig. 2Ec–d) (n=3 mice per genotype; 15 TEBs analyzed). These results indicate that p190A may play a role in early pubertal development by affecting cell adhesion within the TEBs.
Figure 2. p190A heterozygosity, unlike p190B, does not delay virgin outgrowth but does disrupt TEB architecture.
A. Whole mounted mammary glands from sister pairs of 4-week-old wildtype (a) and p190A heterozygous (b) mice used to analyze the extent of ductal outgrowth. B. Using lymph node as a reference (indicated by LN), length of ductal outgrowth was recorded in millimeters (mm) for wildtype (black bar) and p190A heterozygous (red bar) (n=5 per genotype per time point). Plot indicates significantly reduced outgrowth at 4 week time point, only (p<.0001). C. Representative images of TEB from H&E-stained mammary gland tissue sections from wildtype (a) or p190A heterozygous (b) mice. Note the presence of dissociated cap cell layer (black arrow) and body cell layer (red arrow) in the p190A heterozygous TEB. D. The percentage of normal TEBs in wildtype (black bar) and p190A heterozygous (red bar) mice is depicted by a graph. Note the significant decrease in the number of normal TEBs at all time point (p<.0001). E. Representative images of immunohistochemical staining for neogenin and netrin. Neogenin expression appears to be decreased in the cap cell layer (arrows) of p190A heterozygous TEBs (b) compared to wildtype TEBs (a). Netrin staining intensity is similar in wildtype (c) and p190A heterozygous (d) TEBs.
P190A is required in both the epithelium and stroma for mammary morphogenesis
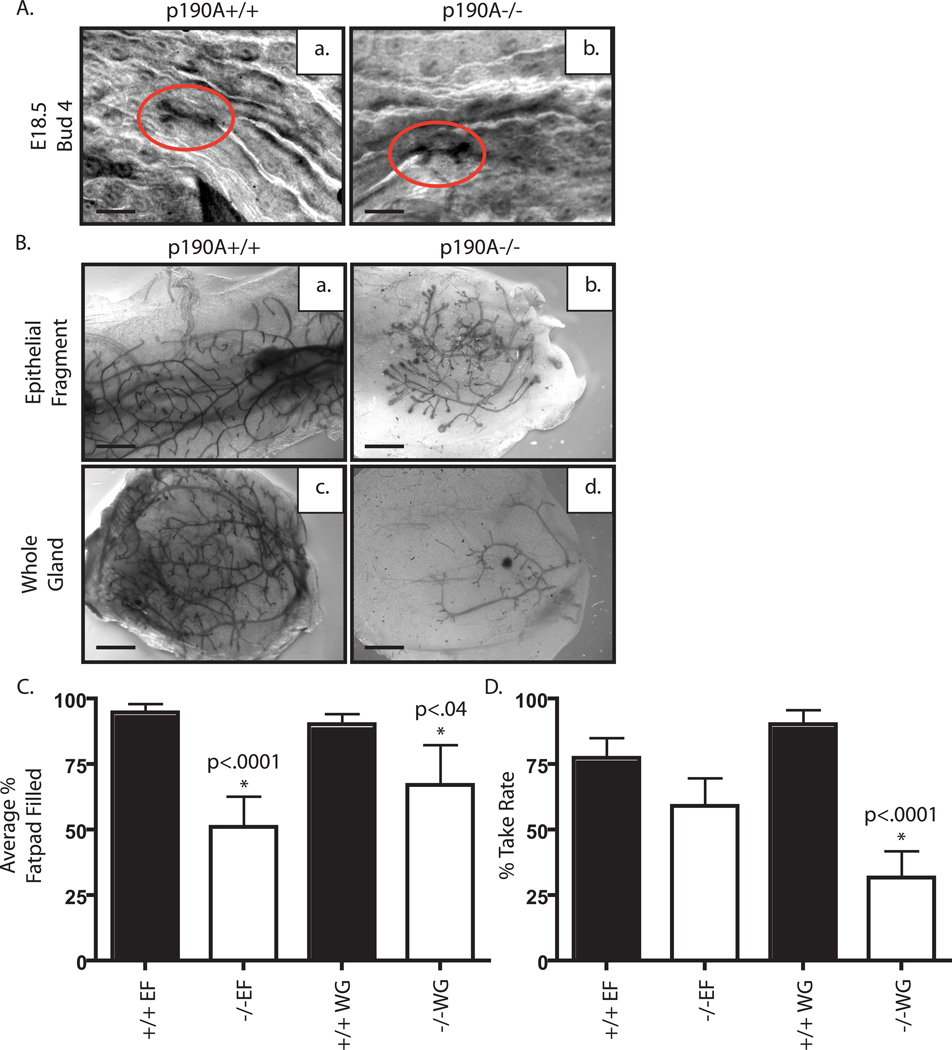
We next wanted to determine if complete loss of p190A would inhibit mammary gland development. However, because homozygous p190A deficiency in mice, similar to p190B deficiency (Sordella et al., 2002), results in perinatal lethality (Brouns et al., 2000), rescue experiments using embryonic tissue transplantation were required. Because mammary gland development is critically dependent on stromal-epithelial interactions (Parmar and Cunha, 2004; Streuli and Edwards, 1998), as well as systemic hormones, we used two types of transplantation techniques to determine if p190A was important in the epithelium, stroma, or both. Traditional epithelial fragment (EF) transplants were done to examine the importance of p190A within the epithelium, whereas whole gland (WG) transplants tested the role for p190A in the combined epithelium and stroma. For this assay, EF and WG tissues were isolated at 18.5 days of gestation and transplanted into the cleared fat pad of SCID/beige mice with opposing genotypes placed in contralateral glands, or for the whole gland transplants within the interstitial space between the contralateral endogenous thoracic and inguinal glands of the host mouse. In all p190A genotypes, a rudimentary ductal tree was present at E18.5 (Fig. 3A). After 6 weeks of outgrowth, 94% of EF p190A+/+ transplants that grew out filled the entire fat pad with normal ductal structures (Fig. 3Ba,c and Table 1). In contrast, p190A deficiency inhibited ductal outgrowth, and on average, only 51% of the fat pad was filled with ductal epithelium. These outgrowths also displayed persistent TEB-like structures and/or rudimentary ductal branching (Fig. 3Bb,d). The EF transplantation experiments revealed that p190A is required in the mammary gland epithelium for ductal outgrowth (Table 1), p<.0001. There was no significant difference in EF transplantation efficiencies (Table 1). However, p190A deficiency significantly reduced the WG transplantation efficiency (Table 1), p<.0001. The WG transplants also displayed decreased outgrowth potential (Table 1), p<.04. These results suggest that p190A is required in both the epithelium and the stroma for proper ductal outgrowth.
Figure 3. p190A is required in both the epithelium and the stroma for mammary morphogenesis.
A Whole mount analysis of mammary glands from embryonic day 18.5 (E18.5) in wildtype (a) and p190A deficient (b) embryos. Ductal epithelium is circled in red. B. Whole mount analysis of mammary glands from SCID/Beige mice harboring E18.5 mammary transplants. Representative images are shown from epithelial fragment (EF) transplants and whole gland (WG) transplants of wildtype (a,c) and p190A deficient (b,d) tissue.
Table 1. p190A deficiency in epithelium and stroma inhibits ductal outgrowth.
Chart depicting the types of transplants as well as % take rate including number of transplants and the average percentage of the fat pad filled based on successful transplants. Also included are graphical representations of the extent of each transplant’s outgrowth.
| Genotype | p190A+/+ | p190A−/− | ||
|---|---|---|---|---|
| Type of Transplant |
Epithelial Fragment |
Whole Gland |
Epithelial Fragment |
Whole Gland |
| % Take Rate |
77% 24/31 |
90% 28/31 |
59% 13/22 |
31% 7/22 |
| Average % Fatpad Filled |
94% | 90% | 51% | 67% |
| % Fatpad Filled |
 |
 |
 |
 |
P190A deficiency inhibits stromal condensation around mammary ducts
Histological analysis of the p190A EF transplants revealed a reduction in stromal condensation (black arrow) around the p190A-deficient ducts in comparison to p190A wildtype ducts (Fig. 4a–b). The stromal defect was further demonstrated by a reduction in collagen around the p190A-deficient ducts (Fig. 4f) compared to wildtype (Fig. 4e) as shown by Masson’s Trichome staining, which stains collagen fibrils blue. Disrupted TEB-like structures are also present in the p190A-deficient transplants (Fig. 4d) compared to the wildtype transplants (Fig. 4c). Although fibroblasts were observed at the neck of the p190A-deficient TEBs (Fig. 4d, red arrow), they appear not to secrete collagen to the in the same extent as the p190A wildtype fibroblasts (Fig. 4g, red arrow), as shown by the lack of blue staining (Fig. 4h, red arrow). These results suggest that p190A deficiency in the epithelium disrupts cross-talk with the stromal environment leading to impaired extracellular matrix deposition. In addition, these results are consistent with the more pronounced defects seen in the WG transplants and suggest that further impairment of extracellular matrix deposition by p190A-deficient stromal cells may contribute to this phenotype.
Figure 4. p190A deficiency inhibits stromal condensation around mammary ducts and disrupts steroid hormone receptor expression and pattern.
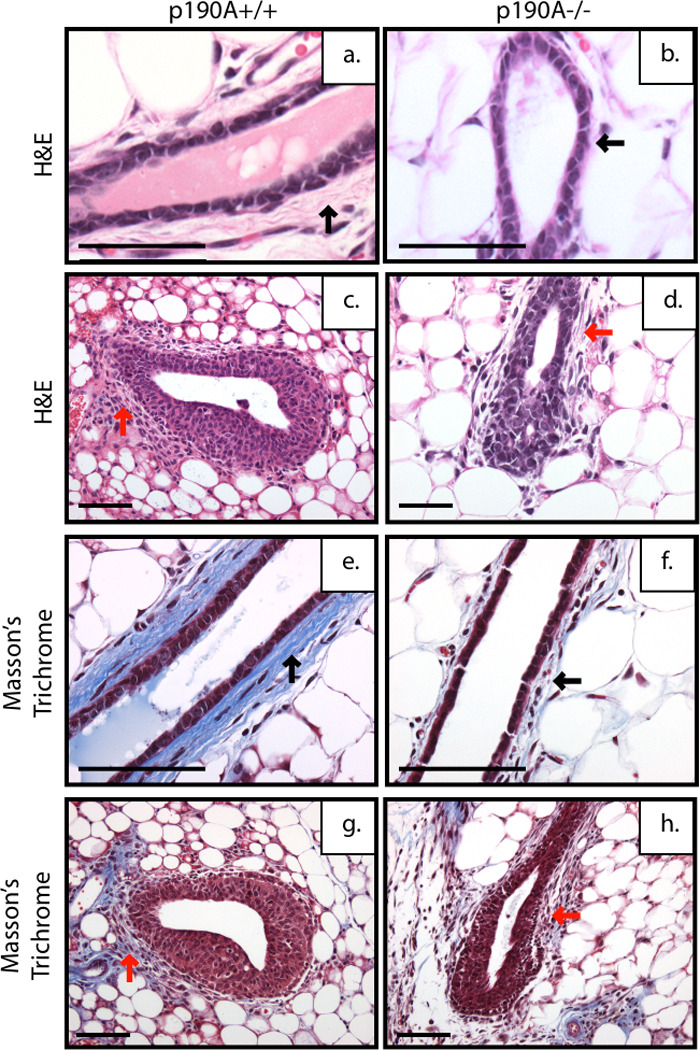
Representative images of H&E-stained ducts and TEBs from SCID/Beige mice transplanted with EF from mammary glands from wildtype (a,c) and p190A deficient (b,d) mice. P190A-deficient ducts lack stromal condensation (a,b black arrow) and have TEBs with loose stroma at the neck (c,d red arrow) compared to wildtype at 6 weeks post transplantation. Representative images of Mason’s Trichrome stain showing collagen fibrils stained in blue. Reduced collagen deposition around the ducts of p190A deficient ducts (f) compared to wildtype (e) (black arrow) and at the neck of the p190A-deficient TEB (h) compared to wildtype (g) (red arrow).
Expression of steroid hormone receptors is altered in the p190A deficient glands
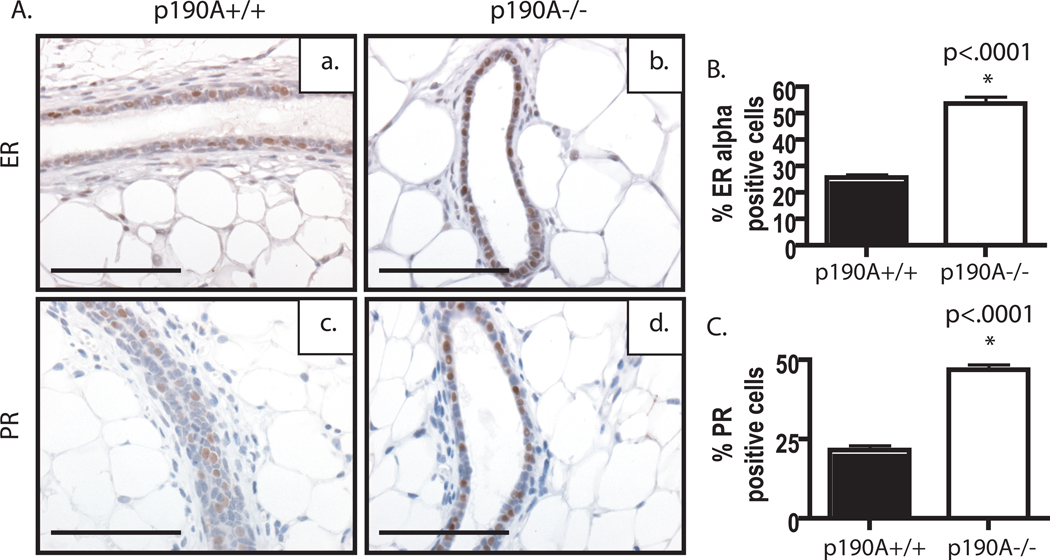
We next examined the expression of steroid receptors within the transplants to determine if the lack of outgrowth was due to an inability of the p190A-deficient ducts to respond to hormonal stimulation. Surprisingly, these studies revealed in increase the expression and number of steroid receptor-positive cells. The number of estrogen receptor α (ERα) positive cells was increased in the p190A-deficient transplants in comparison to p190A-wildtype transplants, p<.0001 (Fig. 5Aa–b, 4B). Because ERα and progesterone receptor (PR) co-localize in more than 96% of normal breast epithelial cells, we also examine the expression of PR (Clarke et al., 1997). Similar to ERα, an increase in the number of cells that express PR was detected, p<.0001 (Fig. 5Ac–d, 4C). However, no significant changes in proliferation or apoptosis were apparent within the ductal epithelium as determined by number of BrdU- and cleaved caspase 3 –positive cells, respectively. These results suggest that p190A may play a role in the differentiation of steroid hormone receptor-expressing cells.
Figure 5. Expression of steroid receptors is altered in the p190A-deficient mammary glands.
P190A-deficient ducts have increased expression of ER (b) and PR (d) in number and level, in comparison to wildtype ER (a) and PR (c) expression. B. Graphical representation of % of ER positive cells (p<.0001). C. Graphical representation of % of PR positive cells (p<.0001).
P190A deficiency disrupts mammary architecture and alters mammary gland differentiation
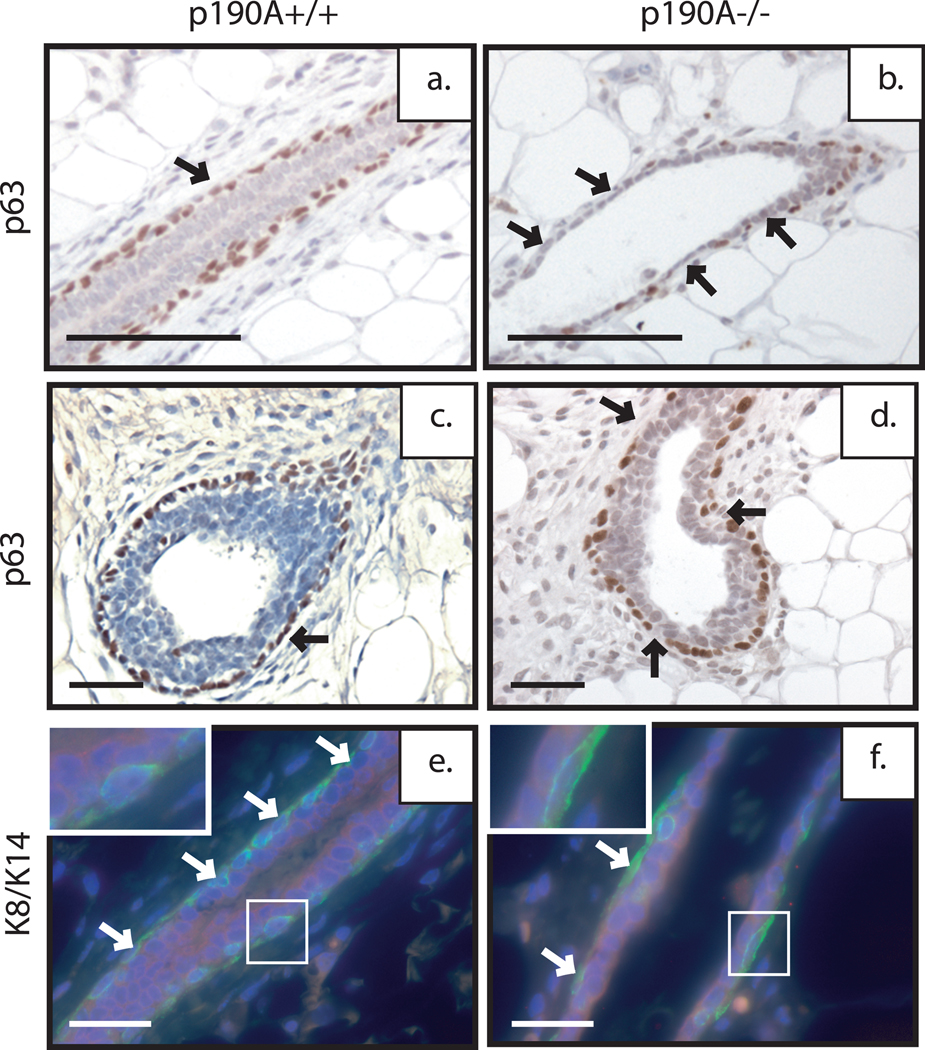
We next wanted to look at the cellular composition of the ductal structures within the p190A deficient outgrowths. We first examined the expression pattern of p63, a cap and myoepithelial cell marker. This analysis revealed that there were fewer myoepithelial cells lining the ducts in the p190A deficient transplants in comparison to the p190 wildtype transplants, which maintain a uniform layer of myoepithelial cells around the ducts (Fig. 6a–b). We next examined the expression of cytokeratin 8 (CK8) and cytokeratin 14 (CK14) by immunofluorescence, which serve as markers of epithelial and myoepithelial layers, respectively. Analysis of CK14 expression, like p63, confirmed that there are fewer myoepithelial cells underlying the luminal epithelium (Fig. 6c–d, green), whereas no change in CK8 expression was detected (Fig. 6c–d, red). We also examined expression of E-cadherin and smooth muscle actin (SMA), luminal and myoepithelial cell markers, respectively (data not shown). Interestingly, although the myoepithelial nuclei are spread further apart as shown by the p63 staining, the CK14 and SMA staining pattern in the p190A deficient transplants suggests that the cells still interact to form an intact myoepithelial cell layer (Fig. 6e–f, green). Consistent with the K8 staining, E-cadherin expression was unaltered (data not shown). These results in combination with the change in steroid hormone receptor expression suggest that p190A is required for cell-cell interactions that contribute to the differentiation of the mammary epithelium.
Figure 6. p190A deficiency disrupts mammary architecture and inhibits proper mammary gland differentiation.
Immunohistochemical analysis of epithelial/ myoepithelial cell layers within the EF transplants. P63 immunostaining demonstrated discontinuity of the myoepithelial cell layer around the ducts of the p190A deficient transplant (b) in comparison to wildtype (a). Co-immunofluorescence was performed for K14 (green)/K8 (red) to examine cell-cell connections between myoepithelial cells and epithelial cells respectively. Note the elongated shape of the myoepithelial cell layer stained by K14 with no change in K8 between the wildtype (e) and p190A-deficient (f) transplants.
Discussion
We observed that p190A, like p190B, is expressed throughout the developing mammary gland and that its function is required for proper mammary gland morphogenesis. Furthermore, our results suggest that p190A plays a role in mediating cell adhesion within the TEB and that complete loss of p190A leads to altered mammary gland differentiation. These results suggest that p190A has similar, but distinct roles from p190B within the mammary gland, as it does in the developing neural system (Matheson et al., 2006). For example, loss of one allele of p190B delayed mammary gland outgrowth between 4 and 6 weeks, while heterozygosity for p190A did not significantly delay development past 4 weeks. The increased expression of p190B within the cap cell layer may account for the difference in the outgrowth potential, as the TEB is the driving force of ductal outgrowth due to the high proliferative capacity of the cap cell layer (Chakravarty et al., 2003; Humphreys et al., 1997).
Interestingly, haploinsufficiency for p190A disrupts the TEB architecture resulting in adhesion defects within the cap cell layer and body cell layer. Reduced levels of the axon guidance molecule neogenin, that is known to play an important role in cap and body cell adhesion within TEBs (Srinivasan et al., 2003), may in part underlie the adhesion defect. Other genes involved in axonal guidance, slit2/robo1, have also been shown to play a role in adhesion with the TEB and ducts of the developing mammary gland (Srinivasan et al., 2003; Strickland et al., 2006). These proteins are members of the plexin family (Hinck, 2004). Interestingly, p190A deficient fibroblasts are blocked or greatly impaired with respect to the typical functional activities mediated by plexins, including cell collapse and inhibition of integrin-based adhesion. Re-expression of wildtype p190A, but not a mutant form specifically lacking RhoGAP activity, rescued plexin-mediated functions in these cells (Barberis et al., 2005). Both members of the p190 family have been shown to accumulate at sites of integrin crosslinking, while only p190A has been shown to function downstream of cadherin engagement suggesting a novel mechanism through which p190A may be mediating this adhesion defect (Burbelo et al., 1995; Noren et al., 2003; Sharma, 1998). Further studies are required to determine the mechanisms by which p190A regulates adhesion within the mammary gland.
P190A knockout mice die perinatally or just after birth due to defects in forebrain midline structures (Brouns et al., 2000). At E18.5 the p190A-deficient embryonic mammary glands are indistinguishable from those in wildtype littermates. We, therefore, used embryonic mammary gland transplantation techniques to examine the effects of complete loss of p190A on mammary gland development. The use of both EF and WG transplants allowed us to investigate the role of p190A in both the epithelium and stroma. These results revealed p190A was important in both compartments as EF and WG p190A-deficient transplants demonstrated reduced outgrowth potential, and WG deficient transplants also displayed reduced transplantation efficiency. Interestingly, the outgrowth potential is greater in the p190A-deficient glands than observed previously in the p190B-deficient glands (Chakravarty et al., 2003), suggesting that p190B may compensate for some functions of p190A, while p190A appears not to compensate for the loss of p190B. While these proteins normally may have some overlapping functions, a compensatory up regulation of p190B in the absence of p190A has not been previously observed (Brouns et al., 2000).
Histological examination of p190A-deficient EF transplants revealed reduced stromal condensation around the ductal epithelium as well as the persistence of TEB-like structures. This is in contrast to our studies of p190B overexpression in the mammary gland where we have observed an increase in stromal condensation (Vargo-Gogola et al., 2006). This is of interest because activity of Rho kinase (ROK), a downstream effector, is required for breast epithelial cells to sense the rigidity of their environment and down regulate Rho activity to differentiate (Wozniak et al., 2003). Thus, the lack of stromal condensation in the p190A-deficient glands may lead to aberrant differentiation.
In addition to changes in matrix deposition, a significant increase in the number of steroid receptor positive cells as well as the level of expression was detected by immunostaining. At 10–12 weeks of age, the steroid receptor expression changes from a pattern where it is present in a high percentage of cells to a restricted pattern (Silberstein et al., 1996). This change in receptor expression pattern correlates with a reduction in proliferation of the ductal epithelium (Clarke et al., 1997). No difference in the proliferative capacity of the mature ductal epithelium within the wildtype and p190A-deficient transplants was observed in our studies, although this analysis could only be conducted on those samples in which outgrowth had occurred. The approximately 50% reduction in percentage of fat pad filled in the EF transplants suggests that the p190A deficient mammary epithelium do grow at a reduced rate. In addition, Rho GTPases and their regulators have been implicated in estrogen dependent proliferation, invasion, and transcriptional activation in ER positive breast cancer cell lines (Barone et al.; Su et al., 2001; Walker et al.; Xie and Haslam, 2008). It is intriguing to speculate that Rho signaling proteins, including p190A, may also regulate ER dependent cellular processes in the developing mammary gland.
Further examination of the p190A-deficient transplants also points to p190A’s putative role as a tumor suppressor. P190A-deficient transplants have a reduced myoepithelial cell layer. Myoepithelial cells secrete fibronectin, collagen, nidogen, and laminin to form the basement membrane. The reduction of myoepithelial cells may contribute to the reduced collagen present in the stroma. Disruption of the myoepithelial cell layer is also seen following p190B overexpression, which led to hyperplastic lesions during pregnancy (Vargo-Gogola et al., 2006).
Previous studies have shown that overexpression of p190A resulted in abnormal positioning of the cleavage furrow during cytokinesis (Su et al., 2003), and that gene silencing of p190A was also detrimental to cell cycle progression. These results, in combination with data presented here implicating p190A in differentiation of the mammary epithelium, may suggest a role for p190A in symmetric versus asymmetric division of mammary progenitor cells. A defect in symmetric versus asymmetric division may also potentially underlie the aberrant differentiation of the mammary epithelium seen in the p190A-deficient outgrowths.
These studies have revealed a critical role for p190A in both the epithelial and stromal compartments in the developing mammary gland. Interestingly, three studies have implicated p190A as a tumor suppressor (Tikoo et al., 2000; Wang et al., 1997; Wolf et al., 2003). Our studies investigating p190B loss and gain of function in the MMTV-Neu induced mammary tumor model suggest that p190B functions as an oncogene during the stochastic process of mammary tumorigenesis in vivo (Heckman-Stoddard et al., 2009; McHenry PR, 2010) Future studies will be aimed at investigating the role of p190A during mammary tumorigenesis.
Highlights.
P190A RhoGAP is expressed in the epithelium and stroma of the developing embryonic mammary bud and postnatal mammary gland
P190A is required in both the epithelium and stroma for mammary ductal development.
P190A deficient terminal end buds display defects in adhesion between the cap and body cell compartments.
The proportion of ERα and PR positive mammary epithelial cells is increased in the p190A−/− ductal epithelium, suggesting that p190A may affect mammary epithelial cell differentiation.
Supplementary Material
Whole mounted mammary glands from 5 and 6-week-old wildtype (a,c) and p190A heterozygous (b,d) mice used to analyze the extent of ductal outgrowth.
Acknowledgements
These studies were supported through the CA030195-22. TV-G is supported by a Howard Temin Pathway to Independence award (R00CA127361). BMH was supported by a Department of Defense Breast Cancer Program Predoctoral Traineeship Fellowship (DAMD W81XWH-06-1-0704). CK8 antibody was obtained from the University of Iowa Developmental Hybridoma Bank. We thank Shirley Small for her help with animal husbandry and colony management, and Maria Gonzalez-Rimbau for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
BM Heckman-Stoddard, Email: heckmanbm@mail.nih.gov.
T Vargo-Gogola, Email: vargo-gogola.1@nd.edu.
MP Herrick, Email: mherrick@bcm.edu.
AP Visbal, Email: av148008@bcm.edu.
MT Lewis, Email: mtlewis@bcm.edu.
J Settleman, Email: settleman@helix.mgh.harvard.edu.
References
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- Barone I, Brusco L, Gu G, Selever J, Beyer A, Covington KR, Tsimelzon A, Wang T, Hilsenbeck SG, Chamness GC, Ando S, Fuqua SA. Loss of Rho GDIalpha and resistance to tamoxifen via effects on estrogen receptor alpha. J Natl Cancer Inst. 103:538–552. doi: 10.1093/jnci/djr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Miyamoto S, Utani A, Brill S, Yamada KM, Hall A, Yamada Y. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J Biol Chem. 1995;270:30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol. 2003;17:1054–1065. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- Chakravarty G, Roy D, Gonzales M, Gay J, Contreras A, Rosen JM. P190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ. 2000;11:343–354. [PubMed] [Google Scholar]
- Chang JH, Gill S, Settleman J, Parsons SJ. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Heckman BM, Chakravarty G, Vargo-Gogola T, Gonzales-Rimbau M, Hadsell DL, Lee AV, Settleman J, Rosen JM. Crosstalk between the p190-B RhoGAP and IGF signaling pathways is required for embryonic mammary bud development. Dev Biol. 2007;309:137–149. doi: 10.1016/j.ydbio.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman-Stoddard BM, Vargo-Gogola T, McHenry PR, Jiang V, Herrick MP, Hilsenbeck SG, Settleman J, Rosen JM. Haploinsufficiency for p190B RhoGAP inhibits MMTV-Neu tumor progression. Breast Cancer Res. 2009;11:R61. doi: 10.1186/bcr2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L. The versatile roles of "axon guidance" cues in tissue morphogenesis. Dev Cell. 2004;7:783–793. doi: 10.1016/j.devcel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Lydon J, O'Malley BW, Rosen JM. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endocrinol. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- Jiang W, Betson M, Mulloy R, Foster R, Levay M, Ligeti E, Settleman J. p190A RhoGAP is a glycogen synthase kinase-3-beta substrate required for polarized cell migration. J Biol Chem. 2008;283:20978–20988. doi: 10.1074/jbc.M802588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Sordella R, Chen GC, Hakre S, Roy AL, Settleman J. An FF domain-dependent protein interaction mediates a signaling pathway for growth factor-induced gene expression. Mol Cell. 2005;17:23–35. doi: 10.1016/j.molcel.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Landua JD, Visbal AP, Lewis MT. Methods for preparing fluorescent and neutral red-stained whole mounts of mouse mammary glands. J Mammary Gland Biol Neoplasia. 2009;14:411–415. doi: 10.1007/s10911-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson SF, Hu KQ, Brouns MR, Sordella R, VanderHeide JD, Settleman J. Distinct but overlapping functions for the closely related p190 RhoGAPs in neural development. Dev Neurosci. 2006;28:538–550. doi: 10.1159/000095116. [DOI] [PubMed] [Google Scholar]
- McHenry PR, Sears JC, Herrick MP, Chang P, Heckman-Stoddard BM, Rybarczyk M, Chodosh LA, Gunther EJ, Hilsenbeck SG, Rosen JM, Vargo-Gogola T. P190B RhoGAP has pro-tumorigenic function. s during MMTV-Neu induced mammary tumorigenesis and metastasis. Breast Cancer Research. 2010;12(5):R73. doi: 10.1186/bcr2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa M, Su L, Parsons SJ. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle. 2008;7:2003–2012. doi: 10.4161/cc.7.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- Noren NK, Arthur WT, Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem. 2003;278:13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- Pullikuth AK, Catling AD. Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP. Mol Cell Biol. 30:3233–3248. doi: 10.1128/MCB.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Wittinghofer A. GTPase-activating proteins: helping hands to complement an active site. Trends Biochem Sci. 1998;23:257–262. doi: 10.1016/s0968-0004(98)01224-9. [DOI] [PubMed] [Google Scholar]
- Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- Sharma SV. Rapid recruitment of p120RasGAP and its associated protein, p190RhoGAP, to the cytoskeleton during integrin mediated cell-substrate interaction. Oncogene. 1998;17:271–281. doi: 10.1038/sj.onc.1201921. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Van Horn K, Shyamala G, Daniel CW. Progesterone receptors in the mouse mammary duct: distribution and developmental regulation. Cell Growth Differ. 1996;7:945–952. [PubMed] [Google Scholar]
- Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Le Z, Takami H, Yamada Y, Settleman J. Modulation of CREB Activity by the Rho GTPase Regulates Cell and Organism Size during Mouse Embryonic Development. Dev Cell. 2002;2(5):553–565. doi: 10.1016/s1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM. Control of normal mammary epithelial phenotype by integrins. J Mammary Gland Biol Neoplasia. 1998;3(2):151–163. doi: 10.1023/a:1018742822565. [DOI] [PubMed] [Google Scholar]
- Strickland P, Shin GC, Plump A, Tessier-Lavigne M, Hinck L. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development. 2006;133:823–832. doi: 10.1242/dev.02261. [DOI] [PubMed] [Google Scholar]
- Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Knoblauch R, Garabedian MJ. Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem. 2001;276:3231–3237. doi: 10.1074/jbc.M005547200. [DOI] [PubMed] [Google Scholar]
- Tikoo A, Czekay S, Viars C, White S, Heath JK, Arden K, Maruta H. p190-A, a human tumor suppressor gene, maps to the chromosomal region 19q13.3 that is reportedly deleted in some gliomas. Gene. 2000;257:23–31. doi: 10.1016/s0378-1119(00)00387-5. [DOI] [PubMed] [Google Scholar]
- Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Heckman BM, Gunther EJ, Chodosh LA, Rosen JM. P190-B Rho GTPase-activating protein overexpression disrupts ductal morphogenesis and induces hyperplastic lesions in the developing mammary gland. Mol Endocrinol. 2006;20:1391–1405. doi: 10.1210/me.2005-0426. [DOI] [PubMed] [Google Scholar]
- Vincent S, Settleman J. Inhibition of RhoGAP activity is sufficient for the induction of Rho-mediated actin reorganization. Eur J Cell Biol. 1999;78:539–548. doi: 10.1016/S0171-9335(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Walker MP, Zhang M, Le TP, Wu P, Laine M, Greene GL. RAC3 is a pro-migratory co-activator of ERalpha. Oncogene. 30:1984–1994. doi: 10.1038/onc.2010.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DZ, Nur EKMS, Tikoo A, Montague W, Maruta H. The GTPase and Rho GAP domains of p190, a tumor suppressor protein that binds the M(r) 120,000 Ras GAP, independently function as anti-Ras tumor suppressors. Cancer Res. 1997;57:2478–2484. [PubMed] [Google Scholar]
- Wolf RM, Draghi N, Liang X, Dai C, Uhrbom L, Eklof C, Westermark B, Holland EC, Resh MD. p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 2003;17:476–487. doi: 10.1101/gad.1040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JW, Haslam SZ. Extracellular matrix, Rac1 signaling, and estrogen-induced proliferation in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2008;110:257–268. doi: 10.1007/s10549-007-9719-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole mounted mammary glands from 5 and 6-week-old wildtype (a,c) and p190A heterozygous (b,d) mice used to analyze the extent of ductal outgrowth.