Abstract
Type IV pili (Tfp) of gram-negative species share many characteristics, including a common architecture and conserved biogenesis pathway. Much less is known about the regulation of Tfp expression in response to changing environmental conditions. We investigated the diversity of Tfp regulatory systems by searching for the molecular basis of the reported variable expression of the Tfp gene cluster of the pathogen Actinobacillus pleuropneumoniae. Despite the presence of an intact Tfp gene cluster consisting of four genes, apfABCD, no Tfp were formed under standard growth conditions. Sequence analysis of the predicted major subunit protein ApfA showed an atypical alanine residue at position −1 from the prepilin peptidase cleavage site in 42 strains. This alanine deviates from the consensus glycine at this position in Tfp from other species. Yet, cloning of the apfABCD genes under a constitutive promoter in A. pleuropneumoniae resulted in pilin and Tfp assembly. Tfp promoter-luxAB reporter gene fusions demonstrated that the Tfp promoter was intact but tightly regulated. Promoter activity varied with bacterial growth phase and was detected only when bacteria were grown in chemically defined medium. Infection experiments with cultured epithelial cells demonstrated that Tfp promoter activity was upregulated upon adherence of the pathogen to primary cultures of lung epithelial cells. Nonadherent bacteria in the culture supernatant exhibited virtually no promoter activity. A similar upregulation of Tfp promoter activity was observed in vivo during experimental infection of pigs. The host cell contact-induced and in vivo-upregulated Tfp promoter activity in A. pleuropneumoniae adds a new dimension to the diversity of Tfp regulation.
Fimbriae or pili are filamentous polymeric structures that protrude from the bacterial cell surface (48). Type IV pili (Tfp) form a unique class of multifunctional fimbriae defined by shared structural features and a conserved biogenesis pathway. They are typically composed of thousands of core subunits with masses of 15 to 20 kDa that are polymerized into a fiber. During Tfp biogenesis, the major subunit is formed as a prepilin that is processed into mature pilin by a type IV prepilin peptidase. This enzyme removes the unique amino-terminal leader peptide and methylates the newly formed N-terminal amino acid residue prior to assembly of the subunits into pili. The genes and gene products required for Tfp biogenesis are remarkably conserved among the extremely diverse groups of gram-negative species that can produce Tfp (37). Tfp can display a diverse set of functions and may be involved in DNA uptake (16, 25, 47), adherence (10, 27, 28, 34, 37), protein export (9, 13, 16, 29, 30), twitching motility (23), and phage infection (44).
Besides the apparent conservation in biogenesis, architecture, and function, Tfp from different species can exhibit unique properties. The plasticity of Tfp ranges from variable length of the leader peptide to noted differences in the genetic regulation of Tfp expression among species (37, 46). The best-understood regulatory systems involve transcriptional modulation of the major subunit gene. In Pseudomonas aeruginosa, the PilS/R sensor-response regulator pair (12) and the alternative sigma factor σ54 (15) are essential for pilA transcription. In contrast, Neisseria meningitidis pilE utilizes a σ70 promoter (4) and is down-regulated upon cell contact by CrgA (5). Knowledge of the regulation of Tfp expression is of obvious importance in the dissection of the functions of Tfp in bacterial pathogenesis and their potential as a target for future infection intervention strategies.
In order to further explore the boundaries set to the plasticity of the Tfp system, we investigated the Tfp of Actinobacillus pleuropneumoniae. The Tfp of this respiratory pathogen may possess unique properties because of its high host specificity for pigs. A. pleuropneumoniae has been demonstrated to express fimbrial structures and to possess a 17-kDa protein that, based on its immunological cross-reactivity with Tfp of M. bovis and N-terminal amino acid sequence homology, was classified as belonging to the type IV family of pilus proteins. The potential to produce Tfp was further supported by the recent demonstration of a gene cluster that consists of four genes (apfABCD) that share homology at the deduced amino acid level with pilABCD of the Tfp gene family, although gene transcription was not demonstrated (33). Here we report the successful constitutive expression of fimbria subunits and of intact Tfp in A. pleuropneumoniae after placement of the cloned Tfp gene cluster behind a constitutive promoter. Additional experiments with promoter-reporter gene fusion constructs indicated that the Tfp cluster is preceded by an intact but tightly regulated promoter. Activation of native Tfp promoter activity required specific environmental conditions and was induced during the adherence of the pathogen to host epithelial cells and during experimental infection in pigs.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Strains and plasmids used in this study are listed in Tables 1 and 2. A. pleuropneumoniae strains were grown on sheep blood agar plates containing 0.1% NAD (Calbiochem, La Jolla, Calif.) or in brain heart infusion medium (BHI) (Gibco BRL, Paisley, United Kingdom) containing 0.008% NAD (BHI-NAD) with or without 1.5% Bacto Agar (Becton Dickinson, Alphen aan den Rijn, The Netherlands). To study fimbria expression, A. pleuropneumoniae was grown on Luria-Bertani (LB) agar plates containing 0.008% NAD (LB-NAD) or in 5 ml of chemically defined medium (CDM) (11) in air, in CDM under microaerophilic conditions (composition: 6% O2, 7% CO2, 7% H2, and 80% N2, obtained with an Anoxomat WS8000 [Mart Microbiology, Lichtenvoorde, The Netherlands]), in tryptic soy broth (TSB) (Biotrading Benelux, Mijdrecht, The Netherlands) plus 0.008% NAD, or in LB medium plus 0.008% NAD. All Escherichia coli strains were routinely grown in LB with or without 1.5% Bacto Agar (Becton Dickinson). When appropriate, ampicillin (AMP) was added to the growth medium at a concentration of 100 μg/ml (E. coli) or 5 μg/ml (A. pleuropneumoniae). E. coli M15(pREP4) was grown in the presence of kanamycin at a concentration of 25 μg/ml. Bacteria were grown at 37°C unless indicated otherwise.
FIG. 1.
Arrangement of the type IV fimbria operon in A. pleuropneumoniae. The region between accolades was completely sequenced. Open arrows represent type IV fimbria genes, while filled arrows represent genes that are not involved in fimbria biogenesis. Small black arrows with numbers indicate the positions of primers.
FIG. 2.
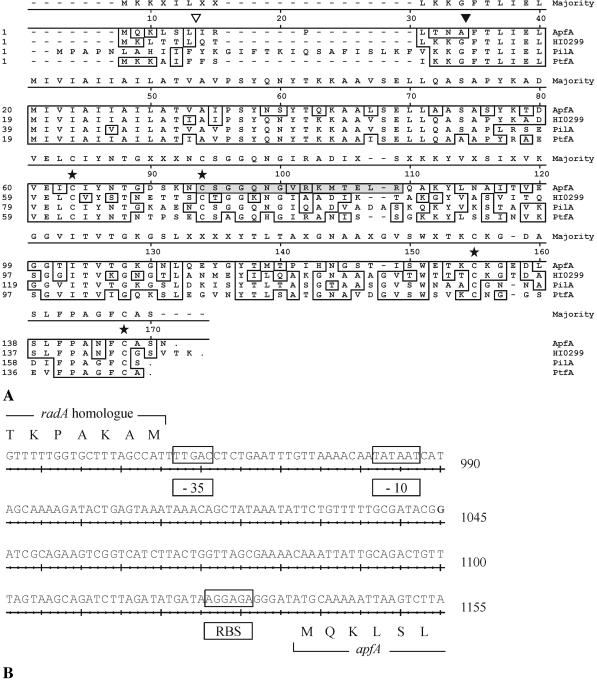
(A) Alignment of amino acid sequences of type IV fimbria subunits of A. pleuropneumoniae strain S4074 of serotype 1 and of three other Pasteurellaceae. Residues that are identical to the consensus are boxed. The putative cleavage site is indicated by a filled triangle. The position of the fusion of ApfA to the His tag in pQE-ApfA is indicated by an open triangle. Conserved cysteine residues are indicated by stars. A synthetic peptide with the amino acid sequence of residues 73 to 87 (CSGGQNGVRKMTELR [shaded]) of ApfA was used to immunize mice. GenBank accession numbers are as follows: for A. pleuropneumoniae ApfA, AY235718; for H. influenzae Rd HI0299, AAC21963.1; for A. actinomycetemcomitans PilA, AAF89188.1; for P. multocida PtfA, AAF61196.1. (B) Nucleotide sequence of the promoter region of the fimbria gene cluster from A. pleuropneumoniae strain S4074 of serotype 1. A putative σ70 promoter sequence (−35 and −10 box) and ribosome binding site (RBS) are boxed.
Preparation of inocula.
For preparation of inocula, A. pleuropneumoniae strains were grown in 5 ml of BHI-0.008% NAD-AMP for 16 h. Bacteria were washed with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 2.8 mM K2HPO4, pH 7.2) and diluted to approximately 2 × 106 CFU/ml in PBS. The number of CFU before and after inoculation was determined by plating 10-fold dilutions in triplicate on BHI-NAD-AMP agar plates.
DNA transformation.
For use in electro-transformation, A. pleuropneumoniae reference strain S4074 (serotype 1) was grown in 5 ml of TSB with 0.008% NAD (TSB-NAD) at 37°C with shaking at 120 rpm. After overnight growth, the culture was diluted 10-fold in TSB-NAD and incubated for 90 min at 37°C with shaking. Then, the bacteria were collected by centrifugation (5,500 × g, 10 min, 4°C), washed with 25 ml of chilled 274 mM sucrose-15% glycerol, and resuspended in 274 mM sucrose-15% glycerol to an optical density at 600 nm (OD600) of 6.0. Fifty microliters of this cell suspension (which was kept on ice) was mixed with plasmid DNA (1 μg) and transferred to a prechilled electroporation cuvette (Bio-Rad, Richmond, Calif.) with an electrode distance of 2 mm. Electrical charges (2,500 V; capacitance, 25 μF; resistance of parallel resistor, 200 Ω) were delivered to ice-cold samples using a Gene-Pulser (Bio-Rad). Immediately after the electrical charge 900 μl of SOC medium (31) supplemented with 0.008% NAD was added, and the cells were allowed to recover at 37°C for 3 h with shaking. The cell suspension was plated onto BHI-NAD agar plates containing AMP (5 μg/ml) (BHI-NAD-AMP). Transformants were grown overnight in 5 ml of BHI-NAD-AMP and stored at −70°C in 50% glycerol in BHI. Transformation to E. coli was done according to the instructions supplied by the manufacturer.
PCRs.
Oligonucleotides used for PCR and DNA sequencing were obtained from Isogen Biosciences (Maarsen, The Netherlands) or Gibco. Relevant oligonucleotides are listed in Table 3. Touch down PCR was carried out by using the AmpliTaq DNA polymerase kit reagents (Roche Molecular Systems, Inc., Branchburg, N.J.) according to the supplied protocol using primers 1024 and 1025. Each 50-μl PCR mixture contained 50 ng of template DNA, 15 pmol of (each) primer, 200 μM deoxynucleoside triphosphate mix, 1× PCR buffer, and 1.25 U of enzyme. Each sample was amplified using the following conditions: 10 min at 94°C; 10 cycles of 15 s at 94°C, 15 s at 55°C increased by 0.5°C per cycle, and 10 s at 72°C; 30 cycles of 15 s at 94°C, 15 s at 50°C, and 1 min at 72°C; and 7 min at 72°C.
TABLE 3.
Oligonucleotides used in this study
| Oligonucleotide (restriction site) | Location (nt)a | Use | Sequence (5′ to 3′)b |
|---|---|---|---|
| 1024 | 1167-1198 | Touch down PCR on fimbrial subunit | AAAAAAGGGTTTACATTAATCG |
| 1025 | 1379-1354 | Touch down PCR on fimbrial subunit | GCTIIAATICCITTTTGTCCICCIITAC |
| FwG | 1742-1768c | Insert PCR on pGH432 and pGH433 | CGGCCAAGCTTACTCCCCATCCCC |
| RevG | 1947-1921c | Insert PCR on pGH432 and pGH433 | CCACTCCCTGCCTCTGTCATCACG |
| 8 | 135-156 | Sequence analysis of cleavage site | TGTTCGGTCATGGCAAATACGC |
| 9 (BamHI) | 1155-1175 | Cloning of apfA | CGGGATCCCGTATTCGACCGCTTACTAACGCG |
| 10 (SphI) | 1642-1664 | Cloning of apfA | ACATGCATGCATGTGCCACTGTTCCTCGGAAATCCGG |
| 25 (XbaI) | 1037-1059 | Cloning of apfABCD | GCTCTAGAGCGATACGGATCGCAGAAGTCGG |
| 26 (BamHI) | 4882-4902 | Cloning of apfABCD | CGGGATCCCGCCGATTCCACCGGTTAAACCG |
| 29 (BamHI) | 1177-1158 | Cloning of promoter region | CGGGATCCCGAAACGCGTTAGTAAGCGGTCG |
| 30 (BamHI) | 791-813 | Cloning of promoter region | CGGGATCCCGCATATCCGCTGAAGCGGTCGC |
According to the operon sequence of A. pleuropneumoniae strain AP76 determined for this work (accession number AY235719).
Inosine (I) was incorporated to reduce specificity. Underlined nucleotides are not exact matches to the sequence and were altered to add restriction enzyme sites.
Location in plasmids pGH432 and pGH433, used for sequence analysis of inserts of A. pleuropneumoniae genomic DNA.
Amplification of the complete fimbria operon was done by using the Expand High Fidelity kit (Roche) according to the supplied protocol using primers 25 and 26. Each 50-μl PCR mixture contained 50 ng of template DNA, 15 pmol of (each) primer, 200 μM deoxynucleoside triphosphate mix, 1× buffer, and 2.6 U of enzyme mix. Each sample was amplified using the following conditions: 2 min at 95°C; 10 cycles of 20 s at 94°C, 30 s at 55°C, and 270 s at 68°C; 20 cycles of 20 s at 94°C, 30 s at 55°C, and 270 s plus 5 s per cycle at 68°C; and 10 min at 72°C.
Standard PCR was carried out by using the Takara ExTaq kit reagents (Takara Shuzo Co., Ltd., Otsu, Shiga, Japan) according to the supplied protocol. Each 50-μl PCR mixture contained 50 ng of template DNA, 15 pmol of primer, 200 μM deoxynucleoside triphosphate mix, 1× PCR buffer, and 1.25 U of enzyme. Each sample was amplified using the following conditions: 10 min at 94°C; 30 cycles of 15 s at 94°C, 30 s at 60°C, and 30 s at 72°C; and 7 min at 72°C. All PCRs were performed on a Primus 96 apparatus (MWG Biotech AG, Ebersberg, Germany).
DNA manipulations, Southern blotting, and hybridization.
Plasmid DNA was isolated by using the Miniprep or Midiprep Wizard kit (Promega Corporation, Madison, Wis.). Genomic DNA was isolated as described by Sambrook et al. (31). DNA ligations were done by using the rapid ligation kit (Roche Diagnostics GmbH, Roche Molecular Biochemicals, Mannheim, Germany). For use in Southern or spot blot hybridization, PCR products were labeled with [α-32P]CTP via random-primed labeling (Boehringer Mannheim). For spot blotting, 3 μl of plasmid DNA or 3 μl of culture was spotted on Genescreen Plus (NEN Life Science Products, Boston, Mass.), denatured with 0.4 M NaOH-1 M NaCl (two times 5 min), and neutralized in 2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate). For Southern blotting, approximately 20 μg of bacterial genomic DNA was digested with EcoRI, subjected to electrophoresis in a 0.8% agarose gel, and transferred to Genescreen Plus by standard procedures (31). Radioactive labeled amplicons were boiled for 10 min, chilled in ice, and used as probes. Blots were incubated with the labeled probes for 16 h at 65°C in hybridization solution (342 mM Na2HPO4, 158 mM NaH2PO4, 1 mM EDTA, 7% [wt/vol] sodium dodecyl sulfate [SDS]). The membranes were washed twice (30 min, 65°C) with washing solution (27 mM Na2HPO4, 13 mM NaH2PO4, 1 mM EDTA) containing 5% SDS and twice (30 min, 65°C) with the same solution containing 1% SDS.
Cloning.
In order to verify the specificity of the ApfA peptide antiserum, the apfA gene was PCR amplified with primers 9 and 10 (Table 3; Fig. 1) and cloned in frame with a His tag at the amino terminus (Fig. 2A) in the expression plasmid pQE30, generating pQE-ApfA. pQE-ApfA was used to transform E. coli M15(pREP4). Expression was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside).
The entire fimbria operon of A. pleuropneumoniae serotype 1 containing the putative ribosome binding site but lacking its own putative promoter sequence was amplified with primers 25 and 26 (Table 3; Fig. 1) and the High Fidelity kit. The resulting PCR product was cloned in pUC18 with XbaI and BamHI generating pUC-ApfABCD. An EcoRI fragment from pSD2 containing the transcription terminator T4 and constitutive A. pleuropneumoniae promoter SD2 was subcloned in pGEM7, and a 600-bp fragment containing T4/SD2 was cloned with HindIII and XbaI upstream of the fimbria operon in pUC-ApfABCD, generating pUC-SD2-ApfABCD. The fragment containing T4/SD2 and the fimbria operon was subsequently cloned in pGZRS19 with HindIII and BamHI, generating pGZRS-F1. pGZRS19 and pGZRS-F1 were used to transform E. coli XL2-blue as well as A. pleuropneumoniae S4074.
A PCR product with primers 29 and 30 (Table 3; Fig. 1) containing the fimbria promoter region of A. pleuropneumoniae S4074 was cloned in pKUN with BamHI, generating pKUN-F. A 320-bp BamHI fragment from pKUN-F was cloned in front of the promoterless luxAB genes in pTF86 generating pTF-F (orientation for the fimbria promoter) and pTF-R (orientation for the radA promoter). The orientations of the inserts in pTF86 were confirmed by restriction analysis with EcoRI and BglII.
Sequence analysis was performed on inserts in plasmids pQE-ApfA, pGZRS-F1, pTF-F, and pTF-R.
DNA sequencing and analysis.
DNA sequences were determined by using the Dye Terminator cycle sequencing ready reaction kit (PE Biosystems, Warrington, United Kingdom) in an ABI 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). Reaction mixtures contained 500 ng of template plasmid DNA or 20 ng of PCR product, 8 μl of reaction mixture, and 3.2 pmol of primer in a 20-μl volume. Alternatively, DNA sequences were determined by Plant Research International (Wageningen, The Netherlands) by using the BigDye Terminator mix (version 2.0; Applied Biosystems). Reactions contained 500 ng of template plasmid DNA, 4 μl of reaction mix, and 10 pmol of primer in a 10-μl volume. Cycle sequencing reactions were performed on a Primus 96 apparatus (MWG Biotech). In all cases, both strands were sequenced. Primers FwG and RevG (Table 3) were used for sequence analysis of inserts in plasmids pGH432 and pGH433. Sequence analysis was performed using the DNAstar software package (DNAstar Inc., Madison, Wis.). To search for homologies, the nucleotide and amino acid sequences were compared with sequences in the GenBank databases by using BLAST (1).
SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Production of fimbria subunits was analyzed by SDS-polyacrylamide gel electrophoresis (17.5% polyacrylamide) and Western blotting. Blots were immunostained with six-His-tagged monoclonal antibody (anti-His; Clontech Laboratories, Palo Alto, Calif.) or polyclonal antifimbria peptide serum (Eurogentec, Seraing, Belgium). The antifimbria peptide serum was raised in mice against a short synthetic peptide with amino acid sequence CSGGQNGVRKMTELR from ApfA (Eurogentec).
Lux analysis.
Quantitative analysis of Lux expression was performed on a Victor 1420 multilabel counter (Wallac, Turku, Finland). N-decyl aldehyde (Sigma Chemical Co., St. Louis, Mo.) substrate was made by dissolving a 20-mg/ml concentration of Essentially Fatty Acid Free bovine serum albumin (Sigma) in 1 ml of H2O with N-decyl aldehyde (1 μl/ml). This mixture was incubated in a glass screw-cap test tube for 30 min in a sonicating water bath at room temperature to disperse the N-decyl aldehyde into micelles. For Lux analysis, 20 μl of bacterial lysate was mixed with 20 μl of substrate in white Polysorb luminescence plates (Nunc GmbH & Co. KG, Wiesbaden, Germany). This mixture was then read with normal emission aperture, a delay of 5 s, and a counting time of 10 s. Luminometer readings (counts per second [CPS]) were normalized to the number of bacteria in the sample as determined by plate counts on selective media (μCPS per CFU) or to the OD600 for pure cultures of bacteria. An OD600 of 1.0 equals approximately 109 CFU/ml.
Promoter activity in vitro.
To investigate promoter activity in vitro, overnight cultures of A. pleuropneumoniae strains grown in BHI-0.008% NAD-AMP were diluted 10 times in 5 ml of BHI-0.008% NAD-AMP and incubated for 3 h at 37°C without shaking. Bacteria were washed once with test medium, resuspended, and incubated in test medium for 2 h at 37°C without shaking. Test media included BHI-0.008% NAD, CDM, CDM-20 μM FeSO4, and CDM-0.03% NAD, and all media were supplemented with AMP at a concentration of 5 μg/ml. OD600 was determined and 2.5 ml of culture was centrifuged for 10 min at 5,500 × g at 4°C, and pellets were resuspended in 40 μl of lysis buffer (50 mM KCl, 2.5 mM MgCl2, 1.8 μM SDS, 15 mM Tris-HCl) and directly used for Lux quantitation.
Promoter activity in the presence of LEC.
The isolation and culture of porcine lung epithelial cells (LEC) is described elsewhere (3). Overnight bacterial cultures were centrifuged and the pellets were resuspended in Dulbecco's modified Eagle's medium (Gibco). Cell monolayers of LEC were infected at a multiplicity of infection of 1,000 (with approximately 108 CFU/ml) in the presence of AMP (5 μg/ml) and were incubated at 37°C in a 5% CO2 atmosphere. After 2 h, supernatant medium with nonadherent bacteria was removed and kept at 4°C. LEC were washed four times with 3 ml of PBS. Adherent bacteria were released by treating the cell monolayers with 1% Triton X-100 in PBS (for 1 min). Controls consisted of bacteria incubated with medium alone. For additional controls, bacteria that were incubated with medium alone were centrifuged for 10 min at 5,500 × g at 4°C and were resuspended in 1% Triton X-100 in PBS (for 1 min). The numbers of CFU in supernatant medium and medium alone and of Triton X-100-treated bacteria and adherent bacteria were determined by plating 10-fold dilutions in triplicate on BHI-NAD-AMP agar plates. One milliliter of each suspension was centrifuged for 10 min at 5,500 × g at 4°C. The pellets were resuspended in 20 μl of lysis buffer and directly used for Lux quantitation.
Promoter activity in vivo.
Animal experiments were performed in three similar, consecutive trials in pigs in good health free of A. pleuropneumoniae. The pigs were about 5 weeks of age and were housed in sterile stainless steel isolators. For endobronchial infection, pigs were anesthetized with a combination of azaperone (Stresnil; Jansen Pharmaceutica B.V., Tilburg, The Netherlands) and ketamine hydrochloride (Ketamine; Kombivet B.V., Etten-Leur, The Netherlands). Inoculation was performed as previously described (42). Briefly, a catheter with an outer diameter of 2.2 mm was advanced through the trachea deep into the bronchi and 5 ml of bacterial suspension was slowly administered. Three pigs per group were inoculated with approximately 107 CFU of A. pleuropneumoniae S4074 containing plasmids pTF86, pTF-F, or pSD2. The average inoculum contained 8.54 × 106 CFU. Two hours postinfection, pigs were anesthetized by intravenous injection of pentobarbital and exsanguinated. The lungs were excised, and three tissue specimens of approximately 1 cm3 were taken from both distal caudal lung lobes for Lux analysis. Tissues were minced with scalpels, and 1.5 ml of PBS was added. Tissue suspensions were transferred to 5-ml tubes, mixed for 5 s, and centrifuged for 5 min at 200 × g to remove large clumps of tissue. Bacterial concentrations of the supernatant were determined by plating 10-fold dilutions on BHI-NAD-AMP agar plates. One milliliter of supernatant was centrifuged for 5 min at 10,000 × g. The pellets were resuspended in 100 μl of lysis buffer and directly used for Lux quantitation. For Lux analysis, the bacterial lysate was mixed with 100 μl of N-decyl aldehyde. All animal experiments were approved by the ethical committee of ID-Lelystad.
Electron microscopy.
Cultures were examined for the presence of fimbriae by negative staining. Bacteria were absorbed on carbon-coated collodion nickel grids from agar plates or suspensions. The grids were then floated three times for 5 s on a solution of 1% methylamine tungstate (Bio-Rad). After the staining procedure, the specimens were viewed in a Philips CM10 electron microscope.
Statistics.
Student's t test was used for statistical analyses. P values of ≤0.05 were considered significant.
Nucleotide sequence accession numbers.
The nucleotide sequences of the Tfp gene clusters of strains S4074 and AP76 are available at GenBank under accession numbers AY235718 and AY235719.
RESULTS
Cloning of the A. pleuropneumoniae fimbrial gene cluster.
The A. pleuropneumoniae Tfp gene cluster was amplified in two steps. First, part of the major subunit gene was PCR amplified with primers based on a conserved fimbria subunit sequence of Haemophilus influenzae and Actinobacillus actinomycetemcomitans (primer 1025) and deduced from the previously determined N-terminal amino acid sequence of an A. pleuropneumoniae subunit (primer 1024). Inosines were incorporated at seven positions in primer 1025 to reduce its specificity. Touch down PCR on genomic DNA from A. pleuropneumoniae reference strains S4074, 1536, and WF83 yielded bands of the expected size (220 bp) at annealing temperatures ranging from 35 to 40°C. DNA sequencing and subsequent analysis of the PCR fragments revealed 55% similarity at the amino acid level with the type IV fimbria subunits of A. actinomycetemcomitans, H. influenzae Rd, and Pasteurella multocida.
In order to obtain the entire A. pleuropneumoniae subunit gene (designated apfA) and possible flanking fimbrial genes, a DNA library of A. pleuropneumoniae serotype 7 was hybridized with the obtained apfA PCR fragments. Hybridizing clones were collected, and the entire DNA sequence of the inserts was determined. This procedure yielded a 5,303-bp DNA region that contained four complete and two partial open reading frames (ORFs) (Fig. 1). Similar data were obtained for reference strain S4074, serotype 1.
Properties of the major Tfp subunit gene, apfA.
Sequence analysis indicated that the first complete ORF of the identified region was the apfA gene. The gene was 444 bp long and was predicted to encode a 15.9-kDa protein (Fig. 2A). The putative protein was 75 to 92% similar to the fimbria subunits of H. influenzae, A. actinomycetemcomitans, P. multocida (Fig. 2A), and Haemophilus somnus and identical to that of the putative ApfA protein of A. pleuropneumoniae serotype 2 (GenBank accession number AF302997). The deduced protein sequence of ApfA contains many of the features shared by type IV subunits in other gram-negative bacteria, except for the Ala residue at position −1 relative to the cleavage site (37) (Fig. 2A). Most known type IV prepilin-like leader sequences contain a glycine at this position (37) (Fig. 2A). PCR with primers 8 and 10 (Fig. 1; Table 3) and sequence analysis of 42 strains of A. pleuropneumoniae including HS25 (Table 1), a strain which has been reported to produce Tfp, confirmed that the Ala residue at position −1 was an intrinsic trait of the A. pleuropneumoniae subunit gene (data not shown). This analysis also revealed a stop codon at the predicted Gly residue 68 in apfA of the A. pleuropneumoniae reference strain WF83 of serotype 7.
TABLE 1.
A. pleuropneumoniae strains used in this study
| Serotypea | Strain | Source |
|---|---|---|
| 1 | S4074 | Reference |
| 2 | 1536 | Reference |
| 3 | 1421 | Reference |
| 4 | M62 | Reference |
| 5a | K17 | Reference |
| 5b | L20 | Reference |
| 6 | Femø | Reference |
| 7 | WF83 | Reference |
| 8 | 405 | Reference |
| 9 | CVI13261 | Reference |
| 10 | D13039 | Reference |
| 11 | 56153 | Reference |
| 12 | 8329 | Reference |
| 1 | N273 | ID-Lelystad |
| 2 | N282 | ID-Lelystad |
| 1 | HS25 | Blackallb |
| 1 | HS57 | Blackall |
| 2 | 126023-1 | ID-Lelystad |
| 3 | 117559-5 | ID-Lelystad |
| 3 | 16169 | ID-Lelystad |
| 3 | HS77 | Blackall |
| 3 | 126023-3 | ID-Lelystad |
| 5 | J45 | Inzanac |
| 6 | 125739 | ID-Lelystad |
| 7 | 2827 | ID-Lelystad |
| 7 | 25535-2578 | ID-Lelystad |
| 7 | HS30 | Blackall |
| 7 | 212:89-32159 | Hilbinkd |
| 7 | 22:91-895 | Hilbink |
| 7 | 126398-165 | ID-Lelystad |
| 8 | 20044 | ID-Lelystad |
| 8 | 896 | ID-Lelystad |
| 9 | HS17 | Blackall |
| 9 | 125943-191 | ID-Lelystad |
| 10 | 3177/89 | Nielsene |
| 11 | 117559-1 | ID-Lelystad |
| 11 | 111290 | ID-Lelystad |
| 11 | 20492 | ID-Lelystad |
| 11 | 126219-2 | ID-Lelystad |
| 12 | 6807/90 | Nielsen |
| 2 | 118126G | ID-Lelystad |
| 2 | 118126K | ID-Lelystad |
All listed strains are of biotype 1, except strains N273, N282, 118126G, and 118126K (biotype 2).
P. Blackall, Animal Research Institute, Brisbane, Australia.
J. Inzana, Virginia Polytechnic Institute and State University, Blacksburg.
F. Hilbink, Central Animal Health Laboratory, Upper Hutt, New Zealand.
R. Nielsen, State Veterinary Serum Laboratory, Copenhagen, Denmark.
Organization and characterization of the remaining of the Tfp gene cluster.
Downstream of the A. pleuropneumoniae apfA gene three ORFs were identified which were designated apfB, apfC, and apfD (Fig. 1). These ORFs encode proteins with similarities to PilB, PilC, or PilD analogues of A. actinomycetemcomitans, P. multocida, H. influenzae, and H. somnus which are involved in fimbria assembly (17, 29, 40, 41). The predicted protein ApfD showed 45% similarity to PilD of A. actinomycetemcomitans but showed very low similarities to P. multocida and H. influenzae sequences. apfD putatively encodes a prepilin peptidase that cleaves the positively charged N-terminal signal peptide of ApfA and methylates the exposed phenylalanine residue (22, 38). ApfD contains two Asp residues (at positions 89 and 147) thought to be involved in the active site of prepilin peptidases (20) but lacks a cluster of four Cys residues found in other prepilin peptidases (35).
Analysis of the ORFs flanking apfABCD revealed a partial ORF at 181 bp upstream of apfA on the opposite strand that showed similarity to radA. This gene is involved in DNA repair and has no known relation with fimbria biogenesis (32). Downstream of apfD, a partial ORF was found that showed similarity to yacE. This gene encodes the enzyme dephosphocoenzyme A kinase, which catalyzes the final step in coenzyme A biosynthesis, the phosphorylation of the 3′-hydroxy group of the ribose sugar moiety (26). This gene also has no known relation with fimbria biogenesis.
Analysis of the intergenic sequences indicated that the apfA gene was preceded at 6 bp upstream of the putative start codon by the sequence AGGAGA (Fig. 2B), which resembles the AGGAGG consensus ribosomal binding sequence for A. pleuropneumoniae (6). A putative promoter with the sequence TTGAC (−35) and TATAAT (−10) with a spacing of 19 bp was identified at 180 bp from the ATG start codon (Fig. 2B). This promoter structure is similar to the consensus σ70 promoter structure TT(G/A)AA (−35) and TATAAT (−10) in A. pleuropneumoniae (6). None of the different fimbria genes was followed by a transcriptional terminator. This in conjunction with the spacing of the apfABCD genes suggests that the genes are arranged in an operon and may be cotranscribed.
A. pleuropneumoniae carries a single type IV fimbria operon.
To ascertain the presence of a single copy of apfA in the A. pleuropneumoniae genome, Southern blot hybridization was performed. Genomic DNA, isolated from A. pleuropneumoniae reference strains of serotypes 1 and 7 (S4074 and WF83) and field isolates HS25 and HS77, was digested with EcoRI, separated on agarose gel, and blotted. The blot was hybridized with a PCR product containing the first half of apfA as a probe. In all four strains, only one band hybridized with the probe (data not shown), indicating that only a single copy of apfA is present in the A. pleuropneumoniae genome of serotypes 1, 3, and 7. This was confirmed by homology searches using the complete ApfA or the signal peptide sequence of ApfA and the unfinished genome sequences of A. pleuropneumoniae serotypes 1, 5b, and 7 (available from GenBank).
Expression of the recombinant A. pleuropneumoniae Tfp.
Electron microscopy on A. pleuropneumoniae strains S4074, WF83, HS77, and HS25 grown on LB-NAD agar plates yielded no fimbria-like structures protruding from the cell surface. Similar negative results were obtained for ApfA in Western blots when lysates of strains grown in a diverse set of media (including CDM) were probed with an antiserum raised against a synthetic ApfA peptide with the amino acid sequence CSGGQNGVRKMTELR (Fig. 2A). These data indicate that Tfp expression is either tightly regulated and/or that the identified operon is not functional.
To distinguish between these possibilities, a PCR product (obtained with primers 25 and 26 [Fig. 1]) containing the entire Tfp gene cluster of A. pleuropneumoniae S4074 but lacking its own promoter sequence was cloned in plasmid pGZRS19 downstream of the constitutive SD2 promoter. The resulting plasmid pGZRS-F1 was used to transform E. coli XL2-blue. Western blot analysis on whole-cell lysates of XL2-blue(pGZRS-F1) with the ApfA-specific antiserum demonstrated the presence of an approximately 15-kDa protein (Fig. 3, lane 5) that was absent from E. coli carrying the empty plasmid pGZRS19 (Fig. 3, lane 4). Western blot analysis with His-tagged ApfA and anti-His antibody (data not shown) confirmed that it was ApfA that was recognized by the peptide antiserum (Fig. 3, lanes 1 and 2). Similar results were obtained for A. pleuropneumoniae strain S4074 carrying plasmid pGZRS-F1 (Fig. 3, lane 8), indicating that Tfp subunits were produced. Electron microscopy demonstrated straight fimbriae protruding from the bacterial cell surface from the recombinant strain carrying the Tfp operon but not from the parent A. pleuropneumoniae S4074 (Fig. 4) or from A. pleuropneumoniae S4074(pGZRS19) (data not shown). Together, the data indicate that A. pleuropneumoniae carries an intact Tfp operon but that, at least under the laboratory growth conditions employed, the promoter activity may be insufficient to stimulate the formation of intact fimbriae.
FIG. 3.
Western blot analysis of fimbria subunit ApfA expressed in E. coli XL2-blue or A. pleuropneumoniae S4074 containing no plasmid, pGZRS19, or pGZRS-F1. Results for samples of E. coli M15(pQE-ApfA) are shown in lanes 1 and 2 (2 and 0.2 μl). The blot was stained with antifimbria peptide serum. The arrow indicates the position of the fimbria subunit ApfA (±15 kDa). Molecular size markers are indicated on the right (in kilodaltons).
FIG. 4.
Electron micrographs of A. pleuropneumoniae reference strain S4074 (A) and S4074(pGZRS-F1) (B). Bacteria were stained with methylamine tungstate and examined by electron microscopy as described in Materials and Methods. Bars represent 200 nm.
Assessment of the Tfp promoter activity using luxAB gene reporter fusions.
The molecular basis for the apparent absence of Tfp in A. pleuropneumoniae was further investigated with the use of promoter-reporter gene fusions. Sequence analysis of the putative Tfp promoter region suggested that a promoter is located between the apfA gene and the adjacent oppositely oriented radA gene (Fig. 2B). To determine possible promoter activity in this region, a PCR fragment containing this entire region of A. pleuropneumoniae S4074 was cloned in both orientations into reporter vector pTF86 in front of the promoterless luxAB genes, generating pTF-F (orientation for the fimbria promoter) and pTF-R (orientation for the radA promoter), respectively. As a positive control, plasmid pSD2, in which the luxAB genes are placed behind the constitutive A. pleuropneumoniae promoter SD2, was used (6). All plasmids (pTF86, pTF-F, pTF-R, and pSD2) were used to transform A. pleuropneumoniae S4074, and the level of expression of the luxAB genes was determined by measurement of Lux activity.
Growth of the various strains in different media (BHI-NAD-AMP or CDM-AMP) for 16 h or 10-fold dilutions of these cultures for an additional 1 to 4 h yielded no reproducible Lux activity for strain S4074 carrying the pTF-F plasmid or pTF86 (negative control). Under these conditions, strong positive signals were obtained for S4074 carrying pTF-R that carried the promoter region in the opposite (radA) orientation and S4074 carrying pSD2 (positive control) (data not shown). However, when bacteria at 3 h of exponential growth in BHI were collected by centrifugation, washed, and grown in CDM-AMP for an additional 2 h, S4074 carrying pTF-F did exhibit a luciferase activity of 4,678 μCPS/CFU, which was 26 times higher than that of the negative control strain S4074 carrying pTF86 (P < 0.05 [Table 4]). Similar experiments but with the strains grown in the final 2 h of incubation in BHI-NAD-AMP instead of in CDM indicated virtually no activity for the strain carrying the putative Tfp promoter (pTF-F), although good activity was observed for strains carrying pSD2 and pTF-R (Table 4). Extensive variation in the concentrations of potential regulatory compounds such as Fe2+ or NAD (between 0.0004 and 0.03%) in the media (43), or in growth temperature (33 versus 37°C), either had no effect or caused a minor increase (by a factor of 1.6 to 1.8) in Lux activity (data not shown). Together, these results strongly suggest that the A. pleuropneumoniae Tfp promoter is intact but active only under distinct and strictly defined environmental conditions.
TABLE 4.
In vitro promoter activity in A. pleuropneumoniae S4074
| Plasmid | BHI-NAD-AMP
|
CDM-AMP
|
||
|---|---|---|---|---|
| Mean Lux activitya ± SEM (μCPS/CFU) | Relative activityb | Mean Lux activity ± SEM (μCPS/CFU) | Relative activity | |
| pTF86 | 90 ± 17 | 1 | 179 ± 15 | 1 |
| pSD2 | 7,307 ± 6,651c | 81.12 | 16,690 ± 986c | 93.41 |
| pTF-R | 5,426 ± 659c | 60.24 | 11,590 ± 723c | 64.85 |
| pTF-F | 77 ± 14 | 0.85 | 4,678 ± 959c | 26.18 |
The results of three experiments in triplicate are shown.
Relative Lux activity compared to pTF86.
Significantly different from pTF86 in the same medium (P < 0.05).
Host cell contact-induced activation of the Tfp promoter.
The apparent strict bacterial growth conditions required for activation of the Tfp promoter led us to assess its activity during infection of primary cultures of porcine LEC. The cells were inoculated with A. pleuropneumoniae strain S4074 carrying pTF86, pSD2, pTF-R, or pTF-F for a 2-h period. At this point, nonadherent bacteria, collected from the culture supernatant, and adherent bacteria, released from the host cells with 1% Triton X-100, were assayed for luciferase activity. Under these conditions, adherent S4074 carrying pTF-F demonstrated a luciferase activity of 1,523 μCPS/CFU (Table 5). This was substantially higher than the activity measured for the nonadherent bacteria isolated from the culture supernatant (221 μCPS/CFU, P < 0.05) and those from the adherent and nonadherent negative control S4074 carrying pTF86 with the promoterless luxAB genes (Table 5). Lux activities of the adherent and nonadherent positive controls S4074(pSD2) and S4074(pTF-R) were high under all conditions and ranged from 4,851 to 17,432 μCPS/CFU (Table 5). Treatment of bacteria with 1% Triton X-100 slightly reduced Lux activities of all four strains (data not shown). Together, these results clearly indicate that the Tfp promoter is active when bacteria adhere to the cell surface but not when they are present in the culture supernatant.
TABLE 5.
In vitro promoter activity in A. pleuropneumoniae S4074 in the presence of LEC
| Plasmid | Bacteria treated with medium alone
|
Bacteria in supernatant of LEC
|
Bacteria binding to LEC
|
|||
|---|---|---|---|---|---|---|
| Mean Lux activitya ± SEM (μCPS/CFU) | Relative activityb | Mean Lux activity ± SEM (μCPS/CFU) | Relative activity | Mean Lux activity ± SEM (μCPS/CFU) | Relative activity | |
| pTF86 | 318 ± 74 | 1 | 296 ± 73 | 1 | 93 ± 18 | 1 |
| pSD2 | 15,890 ± 3,673c | 50.06 | 15,940 ± 2,735c | 53.95 | 17,430 ± 3,539c | 188.33 |
| pTF-R | 9,230 ± 2,660c | 29.07 | 4,851 ± 1,333c | 16.42 | 5,970 ± 943c | 64.49 |
| pTF-F | 214 ± 54 | 0.67 | 221 ± 58 | 0.75 | 1,523 ± 414c | 16.45 |
Results of six experiments are shown.
Relative Lux activity compared to pTF86.
Significantly different from pTF86 under the same conditions (P < 0.05).
In vivo activity of the Tfp promoter.
To validate our in vitro observations, the in vivo activity of the Tfp promoter was measured in an A. pleuropneumoniae pig infection model. A. pleuropneumoniae S4074 containing plasmids pTF86, pSD2, or pTF-F was used for endobronchial inoculation of pigs. Two hours after inoculation, pigs were sacrificed and the Lux activity of minced lung tissue was determined and related to the number of CFU (Table 6). As expected, the in vivo Lux activity of the strain with the promoterless luxAB genes, S4074(pTF86), was low (326 μCPS/CFU [Table 6]), and the in vivo Lux activity of the strain with the constitutive expressed lux genes in S4074(pSD2) was very high (22,601 μCPS/CFU [Table 6]). The in vivo Lux activity of S4074(pTF-F) carrying the Tfp promoter in the correct orientation was 1,176 μCPS/CFU. This was substantially higher than that of the negative control (P < 0.05) (Table 6) and of the activity determined after growth in BHI medium (Table 4). These data strongly suggest that the Tfp promoter is active in vivo during infection of lung tissue.
TABLE 6.
In vivo promoter activity in A. pleuropneumoniae S4074
| Plasmid | Lux activitya ± SEM (μCPS/CFU) | Relative activityb | In vivo/in vitro ratio (BHI) |
|---|---|---|---|
| pTF86 | 326 ± 60 | 1 | 3.62 |
| pTF-F | 1,176 ± 305c | 3.61 | 15.27 |
| pSD2 | 22,601 ± 2,814c | 69.29 | 3.09 |
Results of three tissue specimens of three pigs are shown.
Relative Lux activity compared to pTF86.
Significantly different from pTF86 (P < 0.05).
DISCUSSION
Tfp are important multifunctional bacterial surface organelles expressed by most gram-negative bacterial pathogens. Awareness is growing that regulation of Tfp expression is an essential quality enabling Tfp to function at the appropriate time. The environmental cues that control Tfp expression in the various species, however, are generally still poorly understood. In the present study, we investigated Tfp expression for the porcine respiratory pathogen A. pleuropneumoniae. This species carries a set of genes that shares features with the type IV pilin gene family, but this appears to result only rarely in the formation of Tfp (33, 43). We characterized the Tfp gene cluster and demonstrated that Tfp are formed when the cluster is placed behind a constitutive promoter. Promoter-reporter gene fusions showed that the A. pleuropneumoniae Tfp promoter is intact but tightly controlled by environmental conditions. Promoter activity was demonstrated to be induced upon contact of the bacteria with epithelial cells and in vivo during experimental infection of pigs.
The Tfp clusters of two different A. pleuropneumoniae strains consisted of four genes (apfABCD) separated by no or only small intergenic sequences and lacked apparent transcriptional terminator sequences. The overall organization of the gene cluster resembled that of the related bacterial pathogens H. influenzae, A. actinomycetemcomitans, and P. multocida (7, 8, 24) (unfinished genome of A. actinomycetemcomitans available from GenBank). It was remarkable that the apfA gene was preceded by radA, whereas in A. actinomycetemcomitans, P. multocida, and H. influenzae the major Tfp subunit gene is preceded by ampD (unfinished genome of A. actinomycetemcomitans available from GenBank) (7, 8, 24). The frequent clustering of the type IV subunit gene with ampD in other species and the fact that in other species radA is located elsewhere in the genome suggest that in A. pleuropneumoniae genomic rearrangements may have occurred that may have changed the characteristics of the Tfp promoter region and influenced the regulation of Tfp promoter activity.
Initially, the striking finding of an Ala residue at position −1 relative to the ApfA cleavage site, which was found to be a conserved feature among all 42 A. pleuropneumoniae isolates, was considered as a possible explanation for the apparent rare presence of Tfp at the surface of this pathogen. The consensus cleavage site of Tfp subunits consists of the residues Gly (−1), Phe (+1), and Glu (+5) (37). In P. aeruginosa, all but one mutation at residue −1 resulted in lack of processing of the major subunit PilA (36). Partial processing of PilA was observed with a mutation to Ala (−1), but this did not result in production of intact Tfp (36). A spontaneous mutant of Neisseria gonorrhoeae encoding a subunit containing Ser (−1) instead of Gly (−1) was also unable to assemble pili (19). Another type IV fimbrial subunit with an Ala (−1) is PilEL of Legionella pneumophila, which can be assembled in intact fimbriae (34). Thus, the consequence for Tfp expression of the presence of an Ala (−1) in the ApfA protein is difficult to predict. In our hands, cloning of the Tfp cluster into an expression vector in A. pleuropneumoniae resulted in the expression of ApfA and Tfp formation. This suggests that, at least with a strong promoter used, the Ala (−1) in ApfA does not preclude Tfp biogenesis. At this time we do not know whether the supposed prepilin peptidase ApfD of A. pleuropneumoniae has unique characteristics with respect to cleavage activity in comparison with other (PilD) prepilin peptidases or whether ApfA is cleaved at a reduced efficiency. It can be imagined that the latter may affect Tfp assembly when promoter activity is less strong. Putative prepilin peptidases of Pasteurellaceae appear to lack a cluster of Cys residues in the N-terminal half of the protein. Mutational analysis showed that the Cys residues are required for both cleavage and methylation activity of PilD (35). However, the role of these Cys residues in the activity of prepilin peptidases has been recently questioned. Some of the pilD mutants exhibited partial activity, and naturally occurring leader peptidases lacking the Cys residues can be fully functional (14, 35). Mutational analysis showed that two highly conserved Asp residues are absolutely required for protease activity, suggesting that type IV prepilin peptidases comprise a novel family of aspartic acid proteases (20).
Evidence that the Tfp promoter activity was subject to regulation was obtained when the putative Tfp promoter region of A. pleuropneumoniae S4074 was cloned into a promoter trap vector carrying the luxAB reporter genes. This strategy, which allowed direct monitoring of promoter activity, demonstrated that the DNA region preceding the Tfp operon carried two promoters: the Tfp promoter that turned out to have variable activity dependent on the environmental conditions and, on the opposite strand, the radA promoter that appeared to be constitutively active. The changes in luciferase activity observed with strains carrying this construct clearly indicated that promoter activity varied with the bacterial growth phase and the type of growth medium that was employed. Tfp promoter activity was found in cultures grown to mid- to late log phase in CDM but not when grown in BHI. These data likely provide the molecular basis for the reported variable presence of Tfp at the surface of A. pleuropneumoniae when these bacteria are grown in standard medium or in a CDM under microaerophilic conditions. It has been reported that in certain A. pleuropneumoniae serotypes (5a, 9, and 10) but not in others (serotype 2) NAD restriction is a critical factor for Tfp production (43). In our hands, variation in the concentration of NAD did not influence the activity of our (serotype 1) Tfp promoter. These data suggest the existence of serotype specific differences in the regulation of Tfp promoter activity. The exact signals that drive Tfp promoter activity in serotype 1 are unknown. We noticed that changes in temperature—which influence Tfp expression in, among others, L. pneumophila (21)—or the availability of iron had minor effects. These effects are probably not very specific and may well be related to concomitant changes in growth phase, which appear to influence Tfp promoter activity.
A key topic in the assessment of regulation of Tfp expression is the status of the system in the natural setting of an infection, i.e., during the adherence of the pathogenic bacteria to mucosal epithelial cells and during experimental infection in the legitimate host. A. pleuropneumoniae turned out to be an ideal model system to address this topic. The strong Tfp promoter activity measured for A. pleuropneumoniae bacteria that were adherent to primary cultures of LEC compared to that for nonadherent bacteria present in the culture supernatant strongly suggests that contact with epithelial cells is a trigger for Tfp production. Furthermore, our finding that the Tfp promoter was upregulated in vivo after endobronchial inoculation of pigs indicates that this regulation does occur in the natural host environment. The in vivo Lux activity appeared less than that observed for the bacteria adherent to the cultured lung cells, but this may be explained by the fact that we measured the total Lux activity in all bacteria (both adherent and nonadherent) present in the tissue samples. The finding that Tfp promoter activity is upregulated during contact with host cells may seem bizarre considering that Tfp often confer the initial bacterial attachment to host cells. For N. meningitidis, it has been demonstrated that the transcription of the Tfp-tip-associated adhesin PilC1 is upregulated in the presence of host cells (39). On the basis of the functions of type IV fimbriae in other bacterial pathogens, that the fimbriae of A. pleuropneumoniae play a role in the adherence and, possibly, at other stages of the infection must be considered a possibility. Whether Tfp of A. pleuropneumoniae are involved in other typical functions of Tfp like twitching motility, DNA uptake, protein secretion, or phage infection remains to be investigated. The nature of the environmental signals that drive the regulation of apfABCD transcription is still unknown. The Tfp-luxAB reporter system that we have developed may provide a good basis to take up this major challenge.
TABLE 2.
E. coli strains and plasmids used in this study
| E. coli strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| DH5αF′ | Library of partially Sau3AI-digested DNA fragments of A. pleuropneumoniae strain AP76 of serotype 7 DNA in plasmids pGH432 and pGH433 | Gerald F. Gerlach, Tierärtzliche Hochschule, Hannover, Germany (2) |
| XL2-Blue | Used for plasmid construction and analysis | Stratagene, La Jolla, Calif. |
| M15(pREP4) | Used for analysis of expression vectors | Westburg, Leusden, The Netherlands |
| Plasmids | ||
| pQE30 | Expression vector | Westburg |
| pQE-ApfA | 0.5-kb BamHI-SphI fragment containing A. pleuropneumoniae apfA lacking part of signal peptide in frame with N-terminal HIS tag in pQE30 | This study |
| pGEM7 | Used for cloning | Promega |
| pUC18 | Used for cloning | Gibco |
| pKUN | Used for cloning | 18 |
| pGH432 | Vector used for construction of a library of partially Sau3AI-digested DNA fragments of A. pleuropneumoniae strain AP76 of serotype 7 | Gerald F. Gerlach (2) |
| pGH433 | Vector used for construction of a library of partially Sau3AI-digested DNA fragments of A. pleuropneumoniae strain AP76 of serotype 7 | Gerald F. Gerlach (2) |
| pTF86 | Promoter-trap vector that contains, in sequence, the T4 terminator, a unique BamHI site, and a promoterless copy of the Vibrio harveyi luxAB genes in pGZRS19 | Martha Mulks, Michigan State University, East Lansing (6) |
| pSD2 | Active SD2 promoter of A. pleuropneumoniae serotype 1 in promoter-trap vector pTF86 | Martha Mulks (6) |
| pTF-F | Promoter region in apfA orientation of A. pleuropneumoniae serotype 1 in promoter-trap vector pTF86 | This study |
| pTF-R | Promoter region in radA orientation of A. pleuropneumoniae serotype 1 in promoter-trap vector pTF86 | This study |
| pUC-ApfABCD | 3.9-kb XbaI-BamHI fragment containing A. pleuropneumoniae apfABCD operon, including RBS but lacking promoter in pUC18 | This study |
| pUC-SD2-ApfABCD | SD2 promoter upstream of fimbria operon in pUC-ApfABCD | This study |
| pGZRS19 | E. coli-A. pleuropneumoniae shuttle vector | Susan West, University of Wisconsin—Madison (45) |
| pGZRS-F1 | Fimbria operon downstream of SD2 promoter in pGZRS19 | This study |
Editor: V. J. DiRita
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Baltes, N., W. Tonpitak, I. Hennig-Pauka, A. D. Gruber, and G. F. Gerlach. 2003. Actinobacillus pleuropneumoniae serotype 7 siderophore receptor FhuA is not required for virulence. FEMS Microbiol. Lett. 220:41-48. [DOI] [PubMed] [Google Scholar]
- 3.Boekema, B. K. H. L., N. Stockhofe-Zurwieden, H. E. Smith, E. M. Kamp, J. P. van Putten, and J. H. Verheijden. 2003. Adherence of Actinobacillus pleuropneumoniae to primary cultures of porcine lung epithelial cells. Vet. Microbiol. 93:133-144. [DOI] [PubMed] [Google Scholar]
- 4.Carrick, C. S., J. A. Fyfe, and J. K. Davies. 1997. The normally silent sigma54 promoters upstream of the pilE genes of both Neisseria gonorrhoeae and Neisseria meningitidis are functional when transferred to Pseudomonas aeruginosa. Gene 198:89-97. [DOI] [PubMed] [Google Scholar]
- 5.Deghmane, A. E., D. Giorgini, M. Larribe, J. M. Alonso, and M. K. Taha. 2002. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 43:1555-1564. [DOI] [PubMed] [Google Scholar]
- 6.Doree, S. M., and M. H. Mulks. 2001. Identification of an Actinobacillus pleuropneumoniae consensus promoter structure. J. Bacteriol. 183:1983-1989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Doughty, S. W., C. G. Ruffolo, and B. Adler. 2000. The type 4 fimbrial subunit gene of Pasteurella multocida. Vet. Microbiol. 72:79-90. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 9.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192:99-108. [DOI] [PubMed] [Google Scholar]
- 11.Herriott, R. M., E. Y. Meyer, M. Vogt, and M. Modan. 1970. Defined medium for growth of Haemophilus influenzae. J. Bacteriol. 101:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs, M., E. S. Collie, P. D. Free, S. P. Livingston, and J. S. Mattick. 1993. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 7:669-682. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs, M., and J. S. Mattick. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10:233-243. [DOI] [PubMed] [Google Scholar]
- 14.Hu, N. T., P. F. Lee, and C. Chen. 1995. The type IV pre-pilin leader peptidase of Xanthomonas campestris pv. campestris is functional without conserved cysteine residues. Mol. Microbiol. 18:769-777. [DOI] [PubMed] [Google Scholar]
- 15.Ishimoto, K. S., and S. Lory. 1989. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc. Natl. Acad. Sci. USA 86:1954-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennan, R. M., O. P. Dhungyel, R. J. Whittington, J. R. Egerton, and J. I. Rood. 2001. The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J. Bacteriol. 183:4451-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga, T., K. Ishimoto, and S. Lory. 1993. Genetic and functional characterization of the gene cluster specifying expression of Pseudomonas aeruginosa pili. Infect. Immun. 61:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konings, R. N., E. J. Verhoeven, and B. P. Peeters. 1987. pKUN, vectors for the separate production of both DNA strands of recombinant plasmids. Methods Enzymol. 153:12-34. [DOI] [PubMed] [Google Scholar]
- 19.Koomey, M., S. Bergstrom, M. Blake, and J. Swanson. 1991. Pilin expression and processing in pilus mutants of Neisseria gonorrhoeae: critical role of Gly-1 in assembly. Mol. Microbiol. 5:279-287. [DOI] [PubMed] [Google Scholar]
- 20.LaPointe, C. F., and R. K. Taylor. 2000. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J. Biol. Chem. 275:1502-1510. [DOI] [PubMed] [Google Scholar]
- 21.Liles, M. R., V. K. Viswanathan, and N. P. Cianciotto. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect. Immun. 66:1776-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lory, S., and M. S. Strom. 1997. Structure-function relationship of type-IV prepilin peptidase of Pseudomonas aeruginosa—a review. Gene 192:117-121. [DOI] [PubMed] [Google Scholar]
- 23.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 24.May, B. J., Q. Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genomic sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier, P., C. Berndt, N. Weger, and W. Wackernagel. 2002. Natural transformation of Pseudomonas stutzeri by single-stranded DNA requires type IV pili, competence state and comA. FEMS Microbiol. Lett. 207:75-80. [DOI] [PubMed] [Google Scholar]
- 26.Mishra, P., P. K. Park, and D. G. Drueckhammer. 2001. Identification of yacE (coaE) as the structural gene for dephosphocoenzyme A kinase in Escherichia coli K-12. J. Bacteriol. 183:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassif, X., M. Marceau, C. Pujol, B. Pron, J. L. Beretti, and M. K. Taha. 1997. Type-4 pili and meningococcal adhesiveness. Gene 192:149-153. [DOI] [PubMed] [Google Scholar]
- 28.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepe, C. M., M. W. Eklund, and M. S. Strom. 1996. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol. Microbiol. 19:857-869. [DOI] [PubMed] [Google Scholar]
- 30.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 32.Song, Y., and N. J. Sargentini. 1996. Escherichia coli DNA repair genes radA and sms are the same gene. J. Bacteriol. 178:5045-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson, A., J. Macdonald, and M. Roberts. 2003. Cloning and characterisation of type 4 fimbrial genes from Actinobacillus pleuropneumoniae. Vet. Microbiol. 92:121-134. [DOI] [PubMed] [Google Scholar]
- 34.Stone, B. J., and Y. Abu Kwaik. 1998. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect. Immun. 66:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strom, M. S., P. Bergman, and S. Lory. 1993. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J. Biol. Chem. 268:15788-15794. [PubMed] [Google Scholar]
- 36.Strom, M. S., and S. Lory. 1991. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J. Biol. Chem. 266:1656-1664. [PubMed] [Google Scholar]
- 37.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47:565-596. [DOI] [PubMed] [Google Scholar]
- 38.Strom, M. S., D. N. Nunn, and S. Lory. 1993. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl. Acad. Sci. USA 90:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taha, M. K., P. C. Morand, Y. Pereira, E. Eugene, D. Giorgini, M. Larribe, and X. Nassif. 1998. Pilus-mediated adhesion of Neisseria meningitidis: the essential role of cell contact-dependent transcriptional upregulation of the PilC1 protein. Mol. Microbiol. 28:1153-1163. [DOI] [PubMed] [Google Scholar]
- 40.Tonjum, T., N. E. Freitag, E. Namork, and M. Koomey. 1995. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol. Microbiol. 16:451-464. [DOI] [PubMed] [Google Scholar]
- 41.Turner, L. R., J. C. Lara, D. N. Nunn, and S. Lory. 1993. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 175:4962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Leengoed, L. A., and E. M. Kamp. 1989. Endobronchial inoculation of various doses of Haemophilus (Actinobacillus) pleuropneumoniae in pigs. Am. J. Vet. Res. 50:2054-2059. [PubMed] [Google Scholar]
- 43.Van Overbeke, I., K. Chiers, G. Charlier, I. Vandenberghe, J. Van Beeumen, R. Ducatelle, and F. Haesebrouck. 2002. Characterization of the in vitro adhesion of Actinobacillus pleuropneumoniae to swine alveolar epithelial cells. Vet. Microbiol. 88:59-74. [DOI] [PubMed] [Google Scholar]
- 44.Watson, A. A., J. S. Mattick, and R. A. Alm. 1996. Functional expression of hetereologous type 4 fimbriae in Pseudomonas aeruginosa. Gene 175:143-150. [DOI] [PubMed] [Google Scholar]
- 45.West, S. E., M. J. Romero, L. B. Regassa, N. A. Zielinski, and R. A. Welch. 1995. Construction of Actinobacillus pleuropneumoniae-Escherichia coli shuttle vectors: expression of antibiotic-resistance genes. Gene 160:81-86. [DOI] [PubMed] [Google Scholar]
- 45a.Winther-Larsen, H. C., and M. Koomey. 2002. Transcriptional, chemosensory and cell-contact-dependent regulation of type IV pilus expression. Curr. Opin. Microbiol. 5:173-178. [DOI] [PubMed] [Google Scholar]
- 46.Wolfgang, M., P. Lauer, H. S. Park, L. Brossay, J. Hebert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321-330. [DOI] [PubMed] [Google Scholar]
- 47.Wu, H., and P. M. Fives-Taylor. 2001. Molecular strategies for fimbrial expression and assembly. Crit. Rev. Oral Biol. Med. 12:101-115. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y., J. M. Tennent, A. Ingham, G. Beddome, C. Prideaux, and W. P. Michalski. 2000. Identification of type 4 fimbriae in Actinobacillus pleuropneumoniae. FEMS Microbiol. Lett. 189:15-18. [DOI] [PubMed] [Google Scholar]