Abstract
Objective
To determine if selected pro-inflammatory and anti-inflammatory cytokines/mediators of inflammation reported to be related to development of cerebral palsy predict neurodevelopmental outcome in extremely low birth weight infants.
Study design
Infants with birth weights ≤ 1000 g (n=1067) had blood samples collected at birth and on days 3±1, 7±1, 14±3, and 21±3 to examine the association between cytokines and neurodevelopmental outcomes. The analyses were focused on five cytokines (IL-1β, IL-8, TNF-α, RANTES, and IL-2) reported to be most predictive of CP in term and late preterm infants.
Results
IL-8 was higher on days 0–4 and subsequently in infants who developed CP compared with infants who did not develop CP in both unadjusted and adjusted analyses. Other cytokines (IL-12, IL-17, TNF-β, SIL-rα, MIP-1β) were found to be altered on days 0–4 in infants who developed CP.
Conclusions
CP in former preterm infants may, in part, have a late perinatal and/or early neonatal inflammatory origin.
Despite advances in perinatal care and improved survival, the incidence of neurodevelopmental handicap, including cerebral palsy (CP), mental retardation, blindness, and/or deafness, has not declined in extremely low birth weight (ELBW) infants. The etiology of neurodevelopmental morbidity, including CP, remains unclear but is thought to be multifactorial. In the past, neurodevelopmental morbidity was attributed to hypoxia and/or ischemia associated with perinatal asphyxia; however, only a small proportion of neurologically impaired children have evidence of acute perinatal stress. There is increasing evidence that intrauterine or early postnatal inflammation may play a role in the development of CP.1,2
Perinatal infection or inflammation may lead to fetal inflammatory response, premature delivery, and white matter brain injury. Occult infection or inflammation may be an important precursor of neurodevelopmental handicap. Data from a meta-analysis indicated that clinical chorioamnionitis was significantly associated with a 1.9-fold increase in CP in preterm infants and a 4.7-fold increase in CP in term infants.2 Measurement of inflammatory mediators may help clarify the role of perinatal infection/inflammation in the pathophysiology of neurodevelopmental handicap because routine bacterial cultures may be negative in the presence of a true bacterial or non bacterial infection or inflammatory process.3 It is still unclear whether infection and/or inflammatory mediators result in or exacerbate neurodevelopmental morbidity or if alterations in cytokine concentrations are simply the result of markers of the pathologic process or are associated with its development.
Increased pro-inflammatory cytokines [interleukin-1 (IL-1β), IL-8, IL-9, tumor necrosis factor-α (TNF-α), and regulated upon activation, normal T-cell expressed and secreted (RANTES)] during the first days after birth were found to have 100% sensitivity and 100% specificity in the prediction of CP in late preterm and term infants in a case controlled study (31 with CP and 65 controls).1 Anti-inflammatory cytokines (IL-2 and IL-3) were found to be decreased in the infants with CP.1 In this study, blood samples were obtained on any day between days 1 and 18 (median 2 days; mean ± SD 3.5±3.4 days). Another study of preterm infants did not confirm these findings.4 Because cytokines may be elevated at different times and have different half lives after exposure to inflammatory stimuli, future studies need to separately analyze early neonatal samples and those samples taken later in the neonatal period to determine possible timing of neurological damage. As inflammatory cytokines act in concert and may have opposite effects on the inflammatory processes, both pro-inflammatory and anti-inflammatory cytokines should be assessed. This study was designed to test the primary hypothesis that selected pro-inflammatory cytokines/mediators of inflammation and anti-inflammatory cytokines (IL-1β, IL-8, TNF-α, RANTES, and IL-2) at birth and/or up to day 3±1 are predictive of development of moderate or severe CP.
METHODS
This cohort study was performed in the 17 centers of the NICHD Neonatal Research Network from 1999–2002. Infants weighing 401–1000 g at birth were screened for eligibility. Infants were excluded if they were > 72 hours of age or if they had a major congenital anomaly that would affect neurodevelopmental outcome (e.g. trisomies, structural congenital heart defect, diaphragmatic hernia, congenital hydrocephalus, encephalocele, and holoprosencephaly). The study was approved by the institutional review boards at participating centers, and written informed consent was obtained from the parent(s). Whole blood spots were collected on filter paper (about 0.2 ml per day) on days 0 (cord blood or on day 0–1), 3±1, 7±1, 14±3, and 21±3 and frozen to −70° C. Clinical data were collected by trained research coordinators using standardized registry forms. The stored blood spots were analyzed in a batch for 25 cytokines (including IL-1β, IL-8, TNF-α, RANTES, and IL-2 because of their reported predictive ability for CP1) using a multiplex Luminex assay (Luminex Corp., Austin, TX) with established reliability.5
Details of the neurodevelopmental assessment at 18–22 months corrected age have been published previously.6 The Bayley Scales of Infant Development II and a neurological assessment7 were administered by certified examiners trained for reliability and masked to cytokine data. The Mental Development Index (MDI) and the Psychomotor Development Index (PDI) were derived. CP was defined as a non-progressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and abnormal control of movement or posture. Moderate or severe CP included children who were non-ambulatory or required an assistive device for ambulation. CP was classified by type, including diplegia, hemiplegia, and quadriplegia. Neurodevelopmental impairment was defined as a score below 70 on the MDI, a score below 70 on the PDI, moderate or severe CP, severe hearing loss (presence of bilateral hearing aids), and/or blindness (no functional vision in either eye).
The primary hypothesis was that in logistic regression analysis, pro-inflammatory cytokines/mediators of inflammation and anti-inflammatory cytokines at birth and/or up to day 3±1 are independently predictive of development of moderate or severe CP. We also hypothesized that cytokines are independently predictive of other measures of neurodevelopmental impairment in these infants.
The primary analyses were performed for CP and measures of neurodevelopmental impairment in survivors using clinical data and average cytokine levels on days 0 and 3±1. Additional analyses were performed for cytokine levels through day 21 and for other cytokines to test for a secondary hypotheses. Due to the skewness of the data, non parametric two-sample median tests were used to analyze the association of cytokine levels to CP. Initial exploratory unadjusted analysis and final confirmatory analyses, adjusted for variables selected a priori, were conducted to independently assess the association of cytokine levels with CP and other measurements of neurodevelopmental impairment. Variables included in the multivariable regression adjustment were race, sex, gestational age, birth weight, antenatal steroid therapy, and study center. A more complex model with maternal insurance, maternal hypertension, and chest compressions at birth was also tested. Because of the large number of centers relative to the number of subjects, the analyses used mixed models incorporating random intercept for center to adjust for center effects. Due to the skewness of the data, non parametric tests were used for unadjusted analyses, and cytokine levels were log-transformed in adjusted analyses. Cytokine concentrations over time in survivors without CP and in infants who developed CP were explored graphically by plotting population median values over time, in order to evaluate temporal profiles of the cytokines over the first three postnatal weeks. All statistical analyses were performed by the data coordinating center (RTI International).
RESULTS
Of 1067 ELBW infants enrolled in the study, 855 (80%) infants survived to 18–22 months corrected age, and 755 of the survivors (88%) had neurodevelopmental assessment completed. Several of the demographic variables and hospital outcomes differed between the infants who developed CP (n = 102) and those who did not develop CP (n = 653) (Table I). Infants with CP were more likely to be males, of lower gestation, of lower birth weight, outborn, resuscitated, treated with a full course of antibiotics soon after birth, and to have more hospital morbidities, especially neurological ones (Table I). Mothers of infants with CP were more likely to be African American and have Medicaid, and were less likely to have hypertension. 281 infants had neurodevelopmental impairment; 37 had neurodevelopmental impairment due to moderate-severe CP and one or more other measures, 8 for CP only, 124 for MDI < 70 only, 31 for PDI < 70 only, 5 for deafness only, and none for blindness only. The remaining 76 had MDI < 70 and one or more other measures. Demographic variables and hospital outcomes for surviving infants followed to 18–22 months corrected age and those lost to follow-up were comparable, except that infants who completed the follow up were more likely female (54 vs. 42%, P = 0.03), had lower birth weights (777±133 vs. 808±141 grams, P = 0.03), and had more antenatal steroid exposure.
Table 1.
Maternal and Infant Characteristics of the Cerebral Palsy and No Cerebral Palsy Subjects
| Cerebral Palsy (N = 102) | No Cerebral Palsy (N = 653) | p value* | |
|---|---|---|---|
| Maternal characteristics | |||
| Age - years - Mean ± SD | 27±6 | 27±7 | 0.67 |
| Married - no. (%) | 44 (44) | 285 (44) | 1.00 |
| African American - no. (%) | 60 (59) | 293 (45) | 0.01 |
| Educational level - no. (%) | 0.79 | ||
| ≤ 9th grade | 11 (11) | 88 (14) | |
| 10th – 12th grade | 18 (19) | 110 (17) | |
| > 12th grade | 68 (70) | 442 (69) | |
| Insurance - no. (%) | 0.02 | ||
| Medicaid | 79 (81) | 432 (68) | |
| Private | 15 (15) | 153 (24) | |
| Other/none (HMO and Self-pay) | 3 (3) | 54 (8) | |
| Pregnancy complications - no. (%) | |||
| Multiple birth | 18 (18) | 147 (23) | 0.30 |
| Hypertension (incl. pre-eclampsia and eclampsia) | 19 (19) | 189 (29) | 0.03 |
| Any rupture of membranes prior to delivery | 64 (72) | 476 (80) | 0.10 |
| Rupture of membranes > 24 hours | 22 (22) | 154 (24) | 0.71 |
| Antenatal steroids | 77 (76) | 518 (79) | 0.51 |
| Maternal antibiotics | 66 (65) | 470 (72) | 0.16 |
| Antepartum hemorrhage | 17 (17) | 90 (14) | 0.44 |
| Mode of delivery | 0.91 | ||
| Vaginal | 40 (39) | 250 (38) | |
| Cesarean section | 62 (61) | 403 (62) | |
| Infant characteristics - no. (%) | |||
| Gestational age - wk, Mean ± SD | Mean 25.5±1.9 | Mean 26.2±1.9 | < 0.001 |
| Birth weight - g, Mean ± SD | Mean 735±136 | Mean 784±132 | < 0.001 |
| Male | 56 (55) | 288 (44) | 0.04 |
| Inborn | 89 (87) | 619 (95) | 0.007 |
| Chest compressions and/or drugs at birth | 16 (16) | 52 (8) | 0.02 |
| Early onset sepsis | 1 (1) | 7 (1) | 1.00 |
| Late onset sepsis | 48 (47) | 251 (39) | 0.10 |
| Full antibiotic course at birth (≥ 5 days) | 61 (60) | 298 (46) | 0.01 |
| Prophylactic indomethacin | 25 (25) | 148 (23) | 0.70 |
| Periventricular leukomalacia | 13 (13) | 18 (3) | < 0.001 |
| Porencephalic cyst at 36 weeks | 6 (6) | 2 (0.3) | < 0.001 |
| No intraventricular hemorrhage | 49 (48) | 477 (74) | < 0.001 |
| Intraventricular hemorrhage - grade 1 | 15 (15) | 64 (10) | |
| Intraventricular hemorrhage - grade 2 | 7 (7) | 49 (8) | |
| Intraventricular hemorrhage - grade 3 | 11 (11) | 33 (5) | |
| Intraventricular hemorrhage - grade 4 | 20 (20) | 24 (4) | |
| Meningitis | 12 (12) | 26 (4) | 0.003 |
| Neonatal seizures | 24 (24) | 33 (5) | < 0.001 |
| Bronchopulmonary dysplasia at 36 weeks | 68 (67) | 303 (46) | < 0.001 |
| Steroids for bronchopulmonary dysplasia | 40 (39) | 152 (23) | < 0.001 |
| Necrotizing enterocolitis ≥ Stage II | 12 (12) | 46 (7) | 0.11 |
| Retinopathy of prematurity | 86 (86) | 421 (67) | < 0.001 |
| Retinopathy of prematurity with surgery | 33 (33) | 70 (11) | < 0.001 |
p values are from Fisher exact test for categorical variables and from Wilcoxon two-sample test (t approximation) for continuous variables. Chi-square is used for mothers’ education level, mothers’ insurance, and intraventricular hemorrhage.
Cytokine blood levels and outcomes – primary hypothesis
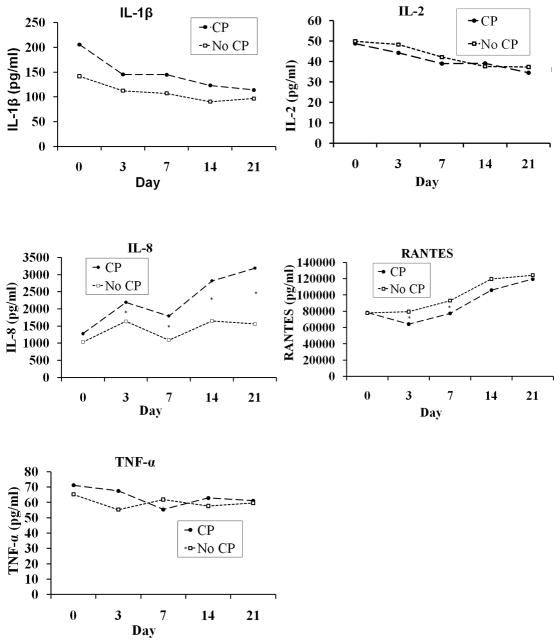
IL-8 was elevated on days 0–4 in infants who developed moderate to severe CP, as well as in those who developed any CP (Table II). IL-8 increased between day 0 and day 3 in both infants with CP and without CP (Figure and Table III; available at www.jpeds.com). After adjustment using multivariable logistic regression analysis, the differences in IL-8 remained significant for day 3 samples (Table IV; available at www.jpeds.com). A more complex model adding maternal insurance, maternal hypertension, and chest compression yielded similar results (data not shown). A unit increase in log IL-8 (measured in pg/ml) on day 3 was associated with a 54% increase (p < 0.001) in the odds of any CP and a 73% increase (p = 0.002) in the odds of moderate-severe CP. Race and gestational age were statistically significant covariates for any CP and gestational age was a significant covariate for moderate-severe CP. IL-8 levels were also elevated in infants who subsequently had a neurodevelopmental impairment and a PDI < 70 (Table V). IL-1β, TNF-α, RANTES, and IL-2 did not differ consistently between infants with or without CP. IL-1β, IL-8, TNF-α, RANTES, and IL-2 levels were not associated with the type of CP (data not shown). TNF-α was decreased at birth in infants who subsequently had a MDI < 70.
Table 2.
Unadjusted comparison of average cytokine levels in pg/ml on days 0–4 in infants with moderate-severe cerebral palsy and in infants with any degree of cerebral palsy.
| Moderate-Severe Cerebral Palsy | No Moderate-Severe Cerebral Palsy | p value* | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| IL-1β | 203 | 335 | 120 | 336 | 0.06 |
| IL-8 | 2363 | 3301 | 1652 | 2449 | 0.04 |
| TNF-α | 76 | 78 | 58 | 80 | 0.34 |
| RANTES | 59772 | 89680 | 80059 | 70707 | 0.20 |
| IL-2 | 46 | 48 | 50 | 64 | 0.53 |
| Any Cerebral Palsy | No Cerebral Palsy | p value* | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| IL-1β | 163 | 351 | 119 | 332 | 0.06 |
| IL-8 | 2340 | 3732 | 1635 | 2273 | 0.01 |
| TNF-α | 69 | 89 | 58 | 76 | 0.32 |
| RANTES | 65875 | 87966 | 80413 | 69480 | 0.15 |
| IL-2 | 45 | 56 | 50 | 65 | 0.44 |
IQR = interquartile range;
TNF-α = Tumor necrosis factor
RANTES = Regulated upon activation, normal T-cell expressed, and secreted
p values are from non-parametric two-sample median test
Figure.
Cytokine levels (median values) in infants with CP and in those without CP. Overall, IL-8 but not IL-1β, TNF-α, RANTES, or IL-2 differed on days 0–21 between the infants who went on to develop CP and those without CP. Statistical results of the comparison of cytokine levels at each time point are identified. (*p < 0.05 for comparison of infants with versus those without CP)
Table 5.
Multivariable regression analysis for neurodevelopmental impairment, Mental Developmental Index < 70, and Psychomotor Developmental Index < 70. Cytokine were averaged among samples from days 0–4 and then log-transformed due to skewness.
| Neurodevelopmental Impairment | ||||
|---|---|---|---|---|
| OR | CI | p value | Significant covariates | |
| IL-1β | 0.92 | 0.80 – 1.06 | 0.27 | Race, gender, birth weight |
| IL-8 | 1.17 | 1.00 – 1.37 | 0.049 | Gender, birth weight |
| TNF-α | 0.85 | 0.72 – 1.01 | 0.07 | Race, gender, birth weight |
| RANTES | 0.97 | 0.78 – 1.21 | 0.79 | Race, gender, birth weight |
| IL-2 | 1.08 | 0.92 – 1.28 | 0.34 | Race, gender, birth weight |
| Mental Developmental Index < 70 | ||||
|---|---|---|---|---|
| OR | CI | p value | Significant covariates | |
| IL-1β | 0.89 | 0.79 – 1.01 | 0.08 | Race, gender, birth weight |
| IL-8 | 1.16 | 0.98 – 1.36 | 0.08 | Race, gender, birth weight |
| TNF-α | 0.84 | 0.71 – 0.98 | 0.03 | Race, gender, birth weight |
| RANTES | 0.98 | 0.78 – 1.23 | 0.84 | Race, gender, birth weight |
| IL-2 | 1.05 | 0.89 – 1.24 | 0.58 | Race, gender, birth weight |
| Psychomotor Developmental Index < 70 | ||||
|---|---|---|---|---|
| OR | CI | p value | Significant covariates | |
| IL-1β | 0.94 | 0.79 – 1.12 | 0.48 | Gender, birth weight |
| IL-8 | 1.21 | 1.00 – 1.46 | 0.047 | Gender, birth weight |
| TNF-α | 0.90 | 0.73 – 1.11 | 0.32 | Gender, birth weight |
| RANTES | 0.94 | 0.71 – 1.23 | 0.63 | Gender, birth weight |
| IL-2 | 0.99 | 0.81 – 1.21 | 0.91 | Gender, birth weight |
Secondary hypothesis
IL-8 but not IL-1β, TNF-α, RANTES, or IL-2 differed on days 0–21 between infants who went on to develop CP and those without CP (Figure). Levels of other analyzed cytokines and proteins showed significant differences between the infants with and without CP (Table VI; available at www.jpeds.com).
DISCUSSION
This study demonstrates that levels of selected cytokines and proteins are altered soon after birth in ELBW infants who subsequently develop CP. Of cytokines previously reported to have a high prognostic ability for CP in term and near term infants,1 IL-8 level was significantly increased on days 0–4 as well as on each of the three sampling dates through day 21 in the group of ELBW infants who subsequently developed CP. Furthermore, IL-8 gene polymorphisms indicate that over production of IL-8 is associated with CP and other motor deficits. In addition, other cytokines and proteins also differed between infants with CP and those without CP on days 0–4, so this adds to the limited data on their potential association with neurodevelopmental impairment in infants. These data suggest that perinatal (i.e. late prenatal and/or early neonatal) inflammation, as reflected by the increased pro-inflammatory cytokines early after birth, is associated with increased risk for CP and other measures of motor deficits.
The strengths of this study include validated laboratory testing,5 comprehensive neurodevelopmental assessment6 performed by trained/certified study personnel, prospective data collection using standardized forms and definitions, and a large sample size of ELBW infants. Although infants were only followed until 18–22 months corrected age when the diagnosis of CP in some children may not be able to be confirmed, a previous large cohort study found that 86% of the infants with severe disability (including CP) at 30 months still had moderate to severe disability at six years of age.8 CP occurs predominately in the most immature infants, and this study included the largest cohort of ELBW infants so far studied for cytokine analysis. However, it is possible that the contribution of inflammation to CP is smaller in extremely preterm infants who are at high risk for other neurological insults, such as intracranial hemorrhage and periventricular leukomalacia, compared with more mature infants. A weakness of the study is the lack of clinical or histological data on chorionamnionitis.
The interpretation of the results should be restricted as support of an association only and not necessarily a causal relationship. These findings suggest that a late prenatal and/or early neonatal inflammation accounts for only a relatively small part of the variance of CP and other motor impairments in ELBW infants. Furthermore, IL-8 was the only one of five cytokines tested as part of the primary hypothesis that was found to consistently have abnormal levels in the infants with CP. Further research is necessary to confirm or refute the findings.
Several cohort studies, a meta-analysis of these studies,2 and a large cohort study9 suggest that maternal and/or neonatal inflammation or infection may lead to long-term neurodevelopmental handicap and in particular, CP.2 These associations have been observed in preterm as well as in term infants. Inflammatory cytokines and other mediators may play an important role in normal and abnormal brain development,1,2,10–15 and therefore, may be involved in the development of permanent brain damage and serious neurodevelopmental handicap. Mechanisms that may mediate the putative role of infection/inflammation remote to the brain on brain damage have been proposed.16,17 Gestational age and birth weight were found to be covariates in the association of cytokine levels and CP and other neurodevelopmental deficits in the current study. When controlling for prematurity, at least two studies have shown that the association between inflammation/infection and CP disappears.18,19 Other associations with increased risk of CP noted in the current study included Medicaid insurance, maternal hypertension, male sex, African American race, and outborn status.
Single nucleotide polymorphisms have been associated with CP.20–22 Variants for cytokine genes including IL-8 were associated with CP in preterm infants. The variants of IL-8 reported are associated with increased production of IL-8. Variants of several genes were associated with CP in girls but not in boys. However, there was minimal overlap (IL-8, TNF-β) between the single-nucleotide polymorphisms tested in that study and the cytokines measured in the current study. A recent systematic review identified other promising candidate genes.22 However, cohorts were usually small, without controls, and not ethnically diverse. The finding of altered IL-8 and other cytokines observed in the ELBW infants in the current study suggest that these and other related genes may be involved with the pathophysiology and/or predisposition to CP.
Existing clinical and experimental evidence emphasizes the pivotal role of IL-8 in neutrophil infiltration and tissue damage during cerebral injury caused by ischemia-reperfusion, anoxia, and traumas.23–25 Early elevations of IL-8 is associated with neonatal disorders including bronchopulmonary dysplasia, retinopathy of prematurity, and death.26–29 High levels of IL-8 support the accumulation of activated neutrophils in the cerebral blood vessels, which contributes to the injury by physically obstructing microvascular perfusion and by releasing various bioactive mediators.30 In vitro, neutrophils, unlike lymphocytes, can cause direct neuronal injury by exacerbating kainic acid-induced excitotoxicity or oxygen glucose deprivation.31 In rodent models, inhibition of IL-8 effects using monoclonal anti-IL-8 antibodies or pharmacological inhibitors of its cognate receptors is neuroprotective during cerebral ischemia.32,33 In contrast, other experiments have found IL-8 to be neuroprotective, including in neonatal models of asphyxia.34
In summary, most cytokines that have been previously shown to have high prognostic ability for CP in term and near term infants during the first days after birth did not differ between preterm infants with and without CP. Only IL-8 levels differed significantly between infants with and without CP after covariate adjustment. Race, sex, and gestational age were significant covariates found to be associated with differences in risk for CP. Additional cytokines from exploratory analyses differed between infants with and without CP. This study provides further support that a late prenatal and/or early neonatal exposure to inflammation may predispose infants to neurodevelopmental impairment, the marked overlap of the cytokine values limits the prognostic ability of this testing in ELBW infants. In addition, hospitalization findings, including a full course of antibiotics at birth, periventricular leukomalacia, porencephalic cyst, intraventricular hemorrhage, meningitis, seizures, retinopathy of prematurity, and bronchopulmonary dysplasia, were associated with CP. Investigations of genetic polymorphisms and other genetic variations of cytokine and other gene expression during this vulnerable developmental window may uncover novel mechanisms or associations that link cytokine specific genes to an increased risk for CP.
Supplementary Material
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Department of Health and Human Services (U10 HD21385, U10 HD40689, U10 HD27871, U10 HD21373, U01 HD36790, U10 HD40498, U10 HD40461, U10 HD34216, U10 HD21397, U10 HD27904, U10 HD40492, U10 HD27856, U10 HD40521, U10 HD27853, U10 HD27880, U10 HD27851, and R03 HD054420) and from the National Institutes of Health (GCRC M01 RR 08084, M01 RR 00125, M01 RR 00750, M01 RR 00070, M01 RR 0039–43, M01 RR 00039, and 5 M01 RR00044). The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Centers for Disease Control and Prevention provided grant support for recruitment for 1999–2001 and data analysis for the Neonatal Research Network’s Cytokines Study. The funding agencies provided overall oversight for study conduct, but all data analyses and interpretation were independent of the funding agencies.
Grant support information available at www.jpeds.com (Appendix 2). L.W. and R.H. are employees of the Eunice Kennedy Shriver NICHD, and assisted with the study design; analysis and interpretation of data; writing of the report; and decision to submit for publication.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. Data collected at participating Neonatal Research Network sites were transmitted to RTI International, the data coordinating center (DCC) for the Neonatal Research Network, which stored, managed, and analyzed the data for this study. On behalf of the network, Dr. Abhik Das (DCC PI) and Mr. Scott A. McDonald (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Abbreviations
- CP
cerebral palsy
- ELBW
extremely low birth weight
- MDI
Mental Development Index
- PDI
Psychomotor Development Index
The following investigators are part of the Eunice Kennedy Shriver NICHD Neonatal Research Network:
Neonatal Research Network Steering Committee Chair: Alan Jobe, MD PhD, University of Cincinnati.
Centers for Disease Control and Prevention (IAA Y1-HD-5000-01) – Diana Schendel, PhD.
Cincinnati Children’s Hospital Medical Center University of Cincinnati Hospital and Good Samaritan Hospital (GCRC M01 RR8084, U10 HD27853) – Edward F. Donovan, MD; Vivek Narendran MD, MRCP; Barb Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Mersmann, RN; Holly Mincey, RN; Jody Shively, RN.
Duke University University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (GCRC M01 RR30, U10 HD40492) – Ronald N. Goldberg, MD; C. Michael Cotten, MD; Kathy Auten, BS.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (GCRC M01 RR39, U10 HD27851) – Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Ellen Hale, RN BS.
Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (GCRC M01 RR750, U10 HD27856) – James A. Lemons, MD; Brenda B. Poindexter, MD MS; Diana D. Appel, RN BSN; Dianne Herron, RN; Leslie D. Wilson, RN BSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Rosemary D. Higgins, MD; Sumner J. Yaffe, MD; Elizabeth M. McClure, MEd.
Rainbow Babies & Children’s Hospital (GCRC M01 RR80, U10 HD21364) – Avroy A. Fanaroff, MD; Michele C. Walsh, MD MS; Nancy S. Newman, BA RN; Bonnie S. Siner, RN.
RTI International (U01 HD36790) – W. Kenneth Poole, PhD; Abhik Das, PhD; Betty Hastings; Kristin Zaterka-Baxter, RN; Jeanette O’Donnell Auman, BS.
Stanford University Lucile Packard Children’s Hospital (GCRC M01 RR70, U10 HD27880) – David K. Stevenson, MD; Krisa P. Van Meurs, MD; M. Bethany Ball, BS CCRC.
University of Aarhus Department of Epidemiology and Social Medicine, Denmark – Poul Thorsen, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (GCRC M01 RR32, U10 HD34216) – Namasivayam Ambalavanan, MD; Waldemar A. Carlo, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) – Neil N. Finer, MD; Maynard R. Rasmussen MD; David Kaegi, MD; Kathy Arnell, RN; Clarence Demetrio, RN; Wade Rich, BSHS, RRT.
University of Miami Holtz Children’s Hospital (GCRC M01 RR16587, U10 HD21397) – Charles R. Bauer, MD; Shahnaz Duara, MD; Ruth Everett-Thomas, RN BSN.
University of New Mexico Health Sciences Center (GCRC M01 RR997, U10 HD27881) – Lu-Ann Papile, MD; Conra Backstrom Lacy, RN.
University of Tennessee (U10 HD21415) – Sheldon B. Korones, MD; Henrietta S. Bada, MD; Tina Hudson, RN BSN.
University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas (GCRC M01 RR633, U10 HD40689) – Abbot R. Laptook, MD; Walid A. Salhab, MD; R. Sue Broyles, MD; Susie Madison, RN; Jackie F. Hickman, RN; Sally Adams, PNP; Linda Madden, PNP; Elizabeth Heyne, PA; Cristin Dooley, MS.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon B. Johnson General Hospital (U10 HD21373) – Jon E. Tyson, MD MPH; Kathleen Kennedy, MD, MPH; Brenda H. Morris, MD; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia Y. Franco, RN BSN MSN NNP; Anna E. Lis, RN BSN; Georgia McDavid, RN; Patti Tate, RRT.
Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (GCRC M01 RR7122, U10 HD40498) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN BSN; Nancy Peters, RN.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Ganesh Konduri, MD; Geraldine Muran, RN BSN; Rebecca Bara, RN BSN.
Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Lewis P. Rubin, MD; Angelita Hensman, BSN RNC.
Yale University Yale-New Haven Children’s Hospital (GCRC M01 RR6022, U10 HD27871) – Richard A. Ehrenkranz, MD; Patricia Gettner, RN; Monica Konstantino, RN BSN; Elaine Romano, RN MSN.
Footnotes
The other authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 2.Wu YW, Colford JM. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 3.Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics. 1994;93:54–58. [PubMed] [Google Scholar]
- 4.Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana GS, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–607. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- 5.Skogstrand K, Thorsen P, Nørgaard-Pedersen P, Schendel DE, Miranda M, Sørensen LC, et al. Simultaneous determination of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay xMAP technology. Clin Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 6.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 7.Amiel-Tison C. Neuromotor status. In: Taeusch HW, Yogman MW, editors. Follow-up management of the high-risk infant. Boston, MA: Little, Brown & Company; 1987. pp. 115–126. [Google Scholar]
- 8.Marlow N, Wolke D, Bracewell MA, Samara M EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:71–72. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2730–2732. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 10.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1439. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 11.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1130. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110:124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 13.Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 14.Kaukola T, Satyaraj E, Patel DD, Tchernev VT, Grimwade BG, Kingsmore SF, et al. Cerebral palsy is characterized by protein mediatros in cord serum. Ann Neurol. 2004;55:186–194. doi: 10.1002/ana.10809. [DOI] [PubMed] [Google Scholar]
- 15.Hansen-Pupp I, Hallin AL, Hellstrom-Westas L, Cilio C, Berg AC, Stjernqvist, et al. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr Res. 2008;64:183–188. doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- 16.Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35:643–663. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan WC, Vohr B, Makuch RW, Katz KH, Ment LR. Antecedents of cerebral palsy in a multicenter trial of indomethacin for intraventricular hemorrhage. Arch Pediatr Adolesc Med. 1997;151:580–585. doi: 10.1001/archpedi.1997.02170430046010. [DOI] [PubMed] [Google Scholar]
- 19.Baud O, Foix-L’helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341:1190–1196. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- 20.Nelson KB, Dambrosia JM, Iovannisch DM, Cheng S, Grether JK, Lammer E. Genetic polymorphisms and cerebral palsy in very preterm infants. Pediatr Res. 2005;57:494–500. doi: 10.1203/01.PDR.0000156477.00386.E7. [DOI] [PubMed] [Google Scholar]
- 21.Gibson CS, MacLennan AH, Dekker GA, Goldwater PN, Sullivan TR, Munroe DJ, et al. Candidate genes and cerebral palsy: a population-based study. Pediatrics. 2009;122:1079–1085. doi: 10.1542/peds.2007-3758. [DOI] [PubMed] [Google Scholar]
- 22.O’Callaghan ME, MacLennan AH, Haan EA, Dekker G the South Australian Cerebral Palsy Research Group. The genomic basis of cerebral palsy: a HuGE systematic literature review. Hum Genet. 2009;126:149–172. doi: 10.1007/s00439-009-0638-5. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood ER, Prough DS. Interleukin-8, neuroinflammation, and secondary brain injury. Crit Care Med. 2000;28:1221–1223. doi: 10.1097/00003246-200004000-00054. [DOI] [PubMed] [Google Scholar]
- 24.Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Yokoi K, Mukaida N, Harada A, Yamashita J, Watanabe Y, et al. Pivotal role of interleukin-8 in the acute respiratory distress syndrome and cerebral reperfusion injury. J Leukoc Biol. 1997;62:581–587. doi: 10.1002/jlb.62.5.581. [DOI] [PubMed] [Google Scholar]
- 26.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi N, Uehara R, Kobayashi M, Yada Y, Koike Y, Kawamata R, et al. Cytokine profiles of seventeen cytokines, growth factors and chemokines in cord blood and its relation to perinatal clinical findings. Cytokine. 2010;49:331–337. doi: 10.1016/j.cyto.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Najati N, Rafeey M, Melekian T. Comparison of umbilical cord interlukin-8 in low birth weight infants with premature rupture of membranes and intact membranes. Pak J Biol Sci. 2009;12:1094–1097. doi: 10.3923/pjbs.2009.1094.1097. [DOI] [PubMed] [Google Scholar]
- 30.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, et al. Cerebral protection in homozygous null icam-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci USA. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki Y, Matsuo Y, Zagorski J, Matsuura N, Onodera H, Itoyama Y, et al. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759:103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- 34.Hussein MH, Daoud GA, Kakita H, Kato S, Goto T, Kamei M, et al. High cerebrospinal fluid antioxidants and interleukin 8 are protective of hypoxic brain damage in newborns. Free Radic Res. 2010;44:422–429. doi: 10.3109/10715760903548245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.