Abstract
Vibrio vulnificus, a highly virulent marine bacterium, is the causative agent of both serious wound infections and fatal septicemia in many areas of the world. To identify the genes required for resistance to human serum, we constructed a library of transposon mutants of V. vulnificus and screened them for hypersensitivity to human serum. Here we report that one of the isolated serum-susceptible mutants had a mutation in an open reading frame identified as trkA, a gene encoding an amino acid sequence showing high identity to that of TrkA of Vibrio alginolyticus, a protein required for the uptake of potassium. A trkA isogenic mutant was constructed via insertional inactivation, and it was significantly more easily killed by human serum, protamine, or polymyxin B than was the wild type. At K+ concentrations of 1 to 20 mM, this isogenic mutant showed attenuated growth compared to the wild-type strain. In addition, infection experiments demonstrated virulence attenuation when this mutant was administered intraperitoneally or subcutaneously to both normal and iron-treated mice, indicating that TrkA may modulate the transport of potassium and resistance to host innate defenses and that it is important for virulence in mice.
Vibrio vulnificus is a halophilic gram-negative bacterium that has emerged as an increasingly important pathogen capable of causing both serious wound infections and fatal septicemia in humans (3, 7, 27, 36). Primary septicemia, with a mortality rate exceeding 50%, may be acquired by consuming seafood containing this organism. Infections are associated with the exposure of wounds to seawater, with a mortality rate of about 25% (4, 7, 18). Although the pathogenic mechanism of V. vulnificus infection has not been fully delineated, several potential virulence factors, such as capsule polysaccharide (CPS) (37, 42, 43), iron-sequestering systems (20), and type IV leader peptidase-N-methyltransferase (28), have been described. In addition, a correlation between the presence of two exotoxins, hemolysin and metalloprotease, and the virulence of V. vulnificus strains has been reported (13, 19), although these two exotoxins were not confirmed as virulence factors by genetic analysis (35, 41).
The bactericidal effect of serum is an important defense by the host against invading microorganisms. In response to this host defense, V. vulnificus, like many pathogenic bacteria, may evolve strategies, including encapsulating itself with CPS, to counter the bactericidal effect of serum. In addition, clinical isolates of V. vulnificus have been reported to exhibit a strong tropism for blood vessels and often spread intravascularly (4). To identify bacterial factors of V. vulnificus that are required for serum resistance, we have undertaken the isolation and characterization of mutants of V. vulnificus with defects in resistance to serum. This paper reports the isolation of these serum-susceptible mutants (designated SS mutants) and the characterization of one of them by genetic analysis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used for this study are listed in Table 1. V. vulnificus CKM-1 is clinical isolate from the blood of a septicemic patient (6). All strains were routinely grown in minimal medium (12.8 g of Na2HPO4 · 7H2O/liter, 3 g of KH2PO4/liter, 10 g of NaCl/liter, 1 g of NH4Cl/liter, 2 mM MgSO4, 0.2 mM CaCl2, 4 g of glucose/liter) or Luria-Bertani (LB) medium at 37°C, with aeration. Antibiotics were used as follows: ampicillin at 100 μg/ml, chloramphenicol at 34 μg/ml, and kanamycin at 100 μg/ml for Escherichia coli and ampicillin at 100 μg/ml, chloramphenicol at 3 μg/ml, kanamycin at 100 μg/ml, and rifampin at 50 μg/ml for V. vulnificus.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype or commentsa | Reference or source |
|---|---|---|
| Strains | ||
| V. vulnificus | ||
| CKM-1 | Wild types clinical isolate | Hospital center of Cheng Kung University |
| SSM-1 | CKM-1 trkA::Tn5 | This study |
| AKK-1 | CKM-1 trkA::pYC3 | This study |
| TRK-1 | AKK-1 strain with plasmid pYC2 | This study |
| E. coli | ||
| BL21(DE3) | F−ompT hsdSB (rB−mB−) gal dcm (DE3) | Novagen |
| S17-1λpir | Tpr SmrrecA thi pro hsdR−M,+ RP4:2Tc:Mu:Km T7, λpir | 15 |
| XL1Blue | F′::Tn10 proA+B+ laclq Δ(lacZ) M15 recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rk− mk+) glnV44 relA1 lac | Stratagene |
| Plasmids | ||
| pBC | Standard cloning vector; Cmr | Stratagene |
| pSJ1 | Positive clone from V. vulnificus CKM-1 genomic library; ∼8.0-kbp chromosomal fragment in pBR322; Apr | This study |
| pCVD422 | Vector for insertional disruption Apr | 9 |
| pET21b | Expression vector; Apr | Novagen |
| pYC1 | trkA structure gene cloned into pET21b; Apr | This study |
| pYC2 | trkA structure gene cloned into pBC; Cmr | This study |
| pYC3 | 612-bp internal fragment from V. vulnificus chromosome trkA gene cloned into pCVD422; Apr | This study |
| pUC4K | Source of kanamycin resistance cassette; Apr | Pharmacia |
| pUC19 | Standard cloning vector; Apr | New England Biolabs |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance.
Molecular techniques.
Standard techniques were used to construct recombinant plasmids (32). DNA fragments used in cloning were extracted from agarose gels by use of the Qiaex II kit (Qiagen, Mississauga, Ontario, Canada). PCR was carried out according to the manufacturer's recommendations by use of the Taq DNA polymerase kit (Amersham Pharmacia Biotech, Inc., Uppsala, Sweden). Nucleotide sequences were determined with an autosequencer (ABI Prism 373 DNA sequencer; Applied Biosystems).
Colony and Southern hybridization.
Colony hybridization and Southern hybridization were performed as described by Sambrook et al. (32). DNA probes were labeled with [α-32P]dCTP by use of a random priming kit (Megaprime DNA labeling system; Amersham Pharmacia Biotech), using either the PCR products or fragments excised from the recombinant plasmids as the templates. The nylon membrane with DNA was prehybridized with hybridization buffer (ExpressHyb hybridization solution; Clontech Laboratories, Inc.) for 30 min at 65°C, hybridized for 2 h at 65°C, washed, and visualized by autoradiography.
Construction of transposon mutant bank.
The transposon insertional library of V. vulnificus strain CKM-1 was generated by the method described by Hensel et al. (15), with minor modifications. E. coli S17-1 λpir carrying a transposon plasmid (15) was delivered to rifampin-resistant V. vulnificus strain CKM-1 by conjugation. The transconjugants were selected by growth with kanamycin and rifampin and were tested for ampicillin sensitivity. The resultant mutants were further screened by the serum sensitivity assay as described below.
Serum sensitivity assay.
Strains were grown in 96-well microtiter dishes containing LB broth at 37°C for 4 h. Bacteria were harvested, washed, and resuspended to 2 × 104 CFU/ml with phosphate-buffered saline (PBS). The bacterial suspensions (50 μl) were mixed with 50 μl of fresh serum or heat-inactivated serum (56°C, 30 min) in 96-well microtiter dishes and incubated at 37°C for 1 h. The numbers of viable bacteria in serial 10-fold dilutions before and after incubation were counted on LB agar after overnight incubation at 37°C. Results were presented as percent survival relative to the original inocula. Human serum samples were obtained from individual donors, and sera from 10 different healthy donors (from the hospital center of the College of Medicine at National Cheng-Kung University) were combined.
Analysis of transposon insertion sites.
Chromosomal DNAs from each Tn5 mutant were digested individually with BglII, EcoRI, KpnI, PstI, and SalI (there are no recognition sites for these five restriction enzymes within the transposon). The presence of the Tn5 mutant was screened with an α-32P-labeled kanamycin gene by Southern hybridization. The kanamycin probe was generated by excision from plasmid pUC4K to generate a 1.2-kb SalI fragment which was then used as a template for the random priming kit.
Cloning of V. vulnificus trkA gene.
Chromosomal DNA from strain SSM-1 was digested with EcoRI and inserted into identically digested pUC19. The ligation reaction mixtures were transformed into E. coli XL1B, and cells were selected by kanamycin resistance. Plasmid DNA was extracted, and the chromosomal DNA sequence flanking the transposon was obtained by DNA sequencing using primers P6 and P7 (15). The complete coding sequence of trkA was cloned from the genomic library of the CKM-1 strain (6) by colony hybridization with an α-32P-labeled DNA fragment of the trkA gene. One positive clone was selected for DNA sequencing.
Construction of the trkA mutant.
A 612-bp internal fragment of the trkA gene from pSJ1 was generated by PCR with primers AF3 (5′-GCTCGCATGCGTTCGCCACA-3′) and AR3 (5′-GACGTCGACCTGGTCGATGTT-3′). The PCR product was digested with SalI and SphI and inserted into identically digested pCVD442 (9). The resultant plasmid, pYC3, was transformed into E. coli S17-1 λpir and subsequently transferred into V. vulnificus CKM-1 via conjugation according to a previously described method (15). Transconjugants were selected by use of ampicillin and rifampin. The resultant strain was further confirmed by PCR and Southern blot analysis using the trkA probe.
Complementation analysis.
A 1,529-bp fragment of the trkA gene was amplified from pSJ1 by PCR with the primers AF1 (5′-GATGAGCTCTACTATGCCGT-3′) and AR1 (5′-AGTCTAGAAAGCACTAGCCC-3′). The PCR product was digested with SacI and XbaI and inserted into identically digested pBC. The resultant plasmid, pYC2, was introduced into AKK-1 via electroporation by using the method described by McDougald et al. (21). Transformants were selected by use of ampicillin and chloramphenicol. The resultant strain was further verified by plasmid extraction and PCR.
RT-PCR.
An SV total RNA isolation kit (Promega, Madison, Wis.) was used to extract RNA from wild-type CKM-1 that had been grown in LB broth for 5 h at 37°C. Primer HR1 (5′-CAGATTCATGCCGGTCAGC-3′), which is complementary to trkH mRNA, was annealed to purified RNA (1 μg) for first-strain cDNA synthesis with Superscript II RNase H reverse transcriptase (Invitrogen, Inc., Carlsbad, Calif.) according to the manufacturer's instructions. PCR amplifications were performed with Taq DNA polymerase (Invitrogen), using an aliquot (1/10) of the reverse transcription (RT) reaction mixture as the template and one of three pairs of primers designed to amplify DNA fragments corresponding to the trkA coding region (AF3 [5′-GCTCGCATGCGTTCGCCACA-3′] and AR1 [5′-AGTCTAGAAAGCACTAGCCC-3′]), the trkH coding region (HF1 [5′-CTTTAAACTCAGTGTGCGCG-3′] and HR1), and the trkA-trkH intergenic coding region (AF3 and HR1). Thirty cycles of amplification were carried out, with denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min. RNA that was subjected to PCR without prior RT was used as a negative control. One-quarter of each amplification reaction was electrophoresed on a 2% agarose gel and photographed under UV transillumination. The DNA sequences of these amplified products were confirmed by DNA sequencing.
Overexpression of TrkA in E. coli.
A 1,388-bp fragment of the trkA gene was amplified from plasmid pSJ1 by PCR with primers AF2 (5′-AGGTTGGATCCGAGCTTGGTTTAT-3′) and AR2 (5′-GGCCTCTCGAGCTTTGTAGTTTTC-3′). PCR products were digested with BamHI and XhoI and inserted into identically digested pET21(b) (Novagen, Madison, Wis.) to generate plasmid pYC1. His6-tagged TrkA was expressed in E. coli BL21(DE3) and purified under denaturing conditions by using a nickel affinity column as instructed by the manufacturer (Novagen).
Preparation of polyclonal antisera.
One hundred micrograms of purified TrkA per milliliter of saline was mixed with 1 ml of Freund's incomplete adjuvant. This mixture was then injected subcutaneously into an Elite New Zealand White rabbit. Two booster doses were administered at 2-week intervals, and the antiserum was collected after 6 weeks. The antiserum was purified through a protein A column according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Western blot analysis.
Proteins separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis were electroblotted onto a nitrocellulose membrane (Amersham Pharmacia Biotech) and then incubated with a TrkA-specific rabbit polyclonal antibody as the primary antibody. The secondary antibody was a 1:4,000 dilution of goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Sigma, St. Louis, Mo.). The proteins were visualized by using a chemiluminescence kit according to the manufacturer's protocol (ECL Western blotting detection reagent; Amersham Pharmacia Biotech).
Effect of K+ on the growth of V. vulnificus trkA.
Strains were grown at 37°C in LB broth and harvested at the mid-log phase of growth. Bacteria were centrifuged for 5 min at 3,000 × g, washed twice with K+-free medium (12.8 g of Na2HPO4 · 7H2O/liter, 3 g of NaH2PO4/liter, 10 g of NaCl/liter, 1 g of NH4Cl/liter, 2 mM MgSO4, 0.2 mM CaCl2, 4 g of glucose/liter), and resuspended in K+-free medium to an optical density at 600 nm (OD600) of 0.1. Bacterial suspensions were added at 1/100 the volume of the medium to K+-free medium containing different concentrations of KCl (0.01, 0.1, 1, 5, 10, 20, and 30 mM) and were incubated at 37°C. Samples were shaken gently, and aliquots removed at specified times were assayed for bacterial counts by plating of serial dilutions on LB plates. The doubling times (g) for the number of bacteria in K+ medium were calculated with the following equation: g = t/n = 0.301t/(logNt − logN0), where N0 is the initial population number, Nt is the population number at time t, and n is the number of generations in time t.
Protamine and polymyxin B sensitivity assay.
Strains were grown at 37°C in LB broth and harvested at the mid-log phase of growth. Bacteria were centrifuged for 5 min at 3,000 × g and resuspended in LB broth at an OD600 of 0.33, and protamine (5 to 15 μg/ml) (Sigma) or polymyxin B (10 to 20 μg/ml) (Gibco-BRL, Gaithersburg, Md.) was added. The suspensions were incubated at 37°C, and bacterial lysis was monitored at specified times by the decrease in OD600.
Virulence assay.
BALB/c mice (8 to 10 weeks old) purchased from the animal center of the College of Medicine at National Cheng-Kung University were challenged by intraperitoneal (i.p.) or subcutaneous (s.c.) injection of the bacterial suspension. For experiments involving pretreatment of mice with iron dextran, mice were injected i.p. with 5 mg of iron dextran (Sigma) per mouse at 2 h preinfection. A group of eight mice was given 0.2 ml of a 10-fold serially diluted (in PBS) bacterial suspension per mouse, and mortality was recorded at 5 days postinfection. The 50% lethal dose (LD50) for each strain was calculated by the method of Reed and Muench (30).
Enumeration of complemented strain TRK-1 in the spleen.
Mice were sacrificed by cardiac exsanguination under anesthesia at 24 h postinfection. Spleens were removed aseptically and homogenized in 1 ml of PBS by use of a grinder. The numbers of viable bacteria in homogenates of spleen were monitored by plating serial dilutions on LB plates containing ampicillin and on LB plates containing ampicillin and chloramphenicol and by counting bacterial colonies after 24 h of incubation at 37°C. The percentage of the TRK-1 strain was calculated as 100 × (ampicillin- and chloramphenicol-resistant CFU/ampicillin-resistant CFU).
Nucleotide sequence accession number.
The nucleotide sequences of trkA and trkH of V. vulnificus have been deposited in the EMBL database under accession no. AY293743.
RESULTS
Production and classification of transposon mutants.
Mutagenesis with the conjugative kanamycin resistance transposon Tn5 has been successfully utilized in V. vulnificus (42). Therefore, we constructed a bank of Tn5 mutants of a clinical strain of V. vulnificus, CKM-1, and screened them for susceptibility to serum by a serum sensitivity assay. Of the 3,000 Tn5 mutants screened, 15 SS mutants were isolated. Compared to the wild-type strain, which had an ∼48% survival rate relative to the original inocula after incubation with 50% (vol/vol) serum for 1 h, the SS mutants had a 10- to 1,000-fold lower survival rate. Of the 15 SS mutants, 3 were of particular interest. Compared to the remaining 12 SS mutants, which exhibited either a translucent colony morphology or a marked deficit in growth on a minimal medium, these 3 mutants had an opaque colony morphology and could grow on a minimal medium, suggesting that they probably were neither the unencapsulated mutants nor the auxotrophic mutants.
Southern blot analysis with the kanamycin gene as the probe revealed that of these three SS mutants, two (designated SSM-1 and SSM-2) contained a unique site of transposon insertion (data not shown). The DNA sequences immediately flanking the Tn5 integration sites in the SSM-1 and SSM-2 mutants were obtained as described in Materials and Methods. The resultant plasmids were sequenced, and through sequence analysis, 354 bp of SSM-1 and 456 bp of SSM-2 chromosomal DNAs beyond the transposon were located and tentatively named sequences A and B, respectively. Database searches by the BLAST algorithm (1) for homology of the two sequences to known gene sequences were performed, and several relevant sequences were identified. The A segment encoded a peptide with a high degree of sequence homology to TrkA, an NAD+ binding protein that was part of a low-affinity potassium uptake system of E. coli (33). The B segment encoded a peptide that showed sequence homology to a large group of σ54-dependent response regulators that modulate cellular response to environmental signals. Cloning of the intact gene of the B segment and characterization of its gene product will be reported elsewhere.
Characterization of the genetic loci.
For isolation of the complete coding sequence of the A segment, a genomic library of V. vulnificus CKM-1 DNA was subjected to colony hybridization, and a plasmid, pSJ1, obtained from a probe-reactive clone was selected for analysis. Two complete and two partial open reading frames (ORFs) were identified (Fig. 1A). The first complete ORF encompassed the insertion site of the Tn5 mutant strain SSM-1 and was identified as trkA. It was preceded by a putative ribosome binding site (GAGA) 7 bp upstream of the initiation codon and was predicted to encode a protein of 458 amino acids with a molecular mass of ∼50 kDa. The putative amino acid sequence of TrkA of V. vulnificus showed high identities to NAD+ binding proteins of many other bacteria implicated in K+ transport, including Vibrio alginolyticus TrkA (96%) (23), E. coli TrkA (78%) (33), and Salmonella enterica serovar Typhimurium SapG (78%) (29). The second ORF was located 9 bp downstream of trkA and was predicted to encode a protein of 481 amino acids with a molecular mass of ∼53 kDa. This amino acid sequence shared 92% sequence identity to TrkH from V. alginolyticus (25) and 33% sequence identity to the trkH-trkG gene products from E. coli (34) and thus was identified as TrkH of V. vulnificus. TrkA is a peripheral membrane protein bound to the inner side of the cytoplasmic membrane and is absolutely required for Trk activity in the K+ transport complex (5, 10, 11), whereas TrkH is an integral membrane protein and probably forms the K+-translocating subunit of the complex (34). In addition to those in V. vulnificus, the trkA and trkH genes clustered together on the chromosome have also been found in Archaeoglobus fulgidus (17) and V. alginolyticus (25).
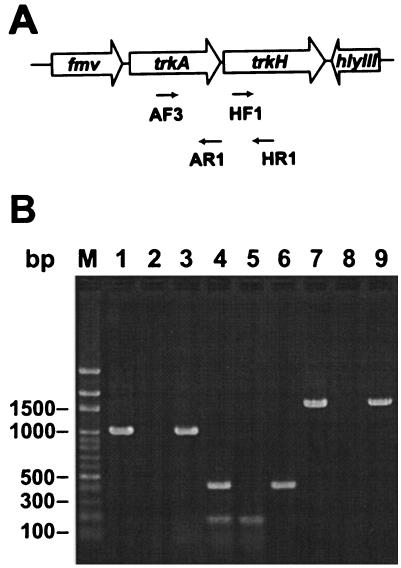
FIG. 1.
Organization of trkA-trkH and RT-PCR analysis. (A) Chromosome organization of trkA-trkH and the flanking region in V. vulnificus CKM-1. Open arrows represent the direction of transcription and the ORF of trkA-trkH. The locations of the primers used for RT-PCR are depicted by arrows below the trkA and trkH genes. (B) Cotranscription of trkA and trkH demonstrated by RT-PCR. RNA isolated from V. vulnificus CKM-1 was used as a template to generate cDNAs. The cDNAs were then used as templates in PCR with trkA-specific primers AF3 and AR1 (lane 1), with trkH-specific primers HF1 and HR1 (lane 4), and with trkA-trkH-specific primers AF3 and HR1 (lane 7). Plasmid pSJ1 was used as a positive control with the same primers (lane 3, AF3-AR1; lane 6, HF1-HR1; lane 9, AF3-HR1). RNA subjected to PCR without prior RT was used as the negative controls (lanes 2, 5, and 8).
Although only partially sequenced, an incomplete ORF upstream of trkA of V. vulnificus was homologous to the fmv gene which is analogously positioned upstream of trkA-trkH in V. alginolyticus (25). The other incomplete ORF lay downstream from V. vulnificus trkH and potentially encoded the C-terminal 91 amino acids of a protein that shared significant (46%) homology to hemolysin III (hlyIII) of Bacillus cereus (2). In this region, the chromosome organization of V. vulnificus differs from that of V. alginolyticus. In V. alginolyticus, the gene (orf1) is immediately downstream of trkH (25). The complete genome sequence of V. vulnificus CMCP6 has recently been submitted (http://www.ncbi.nlm.nih.gov/genomes/MICROBES/Complete.html). A comparison of strain CKM-1 trkA and trkH with those of strain CMCP6 revealed that there are very few nucleotide differences between these two strains. The only points at which there are differences in the predicted trkA and trkH amino acid sequences are as follows: strain CKM-1 trkA has Asn instead of Asp at position 32, Lys instead of Glu at position 176, and His instead of Asn at position 391, and strain CKM-1 trkH has Phe instead of Ser at position 9 and Ile instead of Val at position 53.
Cotranscription of trkA and trkH.
Since the trkA and trkH genes were located adjacent to each other, with the putative ribosome binding site of trkH overlapping with the stop codon of trkA, it is very likely that these two genes are transcriptionally linked and form an operon. Thus, RT-PCR analysis was used to assess whether trkA and trkH are transcribed as a single RNA transcript. As shown in Fig. 1B, the results of RT-PCR yielded products of the expected sizes (∼1,100, 500, and 1,700 bp) (lanes 1, 4, and 7) from the RNA of CKM-1. DNA sequencing confirmed that these amplified products were indeed from trkA and trkH. This indicates that the trkA and trkH genes are organized as a single bicistronic operon.
Isolation of V. vulnificus trkA mutant.
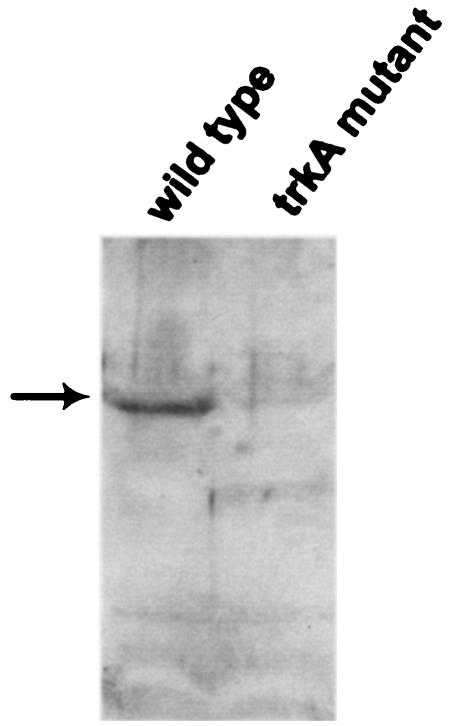
To demonstrate that the observed serum-sensitive phenotype was reproducibly linked to the transposon insertion at the trkA locus, we reconstructed a trkA isogenic mutant, AKK-1. Inactivation of the wild-type trkA with the insertional disruption of the trkA gene in AKK-1 was checked by PCR using a pair of primers complementary to sequences located in the trkA and ampicillin resistance genes (data not shown). For confirmation of the lack of TrkA protein in the AKK-1 strain, Western blot analysis of the parental and AKK-1 strains was performed. The results of the Western blot analysis demonstrated that no anti-TrkA-reactive protein was produced by the AKK-1 mutant (Fig. 2).
FIG. 2.
Confirmation of V. vulnificus trkA mutant by Western blotting. Proteins (40 μg) were isolated from the wild-type and trkA mutant strains, separated by electrophoresis, and transferred to a nitrocellulose membrane. Immunodetection of the TrkA product was performed with a rabbit polyclonal anti-TrkA antibody. Goat anti-rabbit immunoglobulin G conjugated to peroxidase was used to amplify the signals, and the reacting bands were visualized by using enhanced chemiluminescence reagents.
Effect of K+ on the growth of V. vulnificus trkA.
Since TrkA showed a high degree of sequence homology to an NAD+ binding protein that was part of a low-affinity potassium uptake system of E. coli (33), the growth of the wild-type strain CKM-1 and the mutant strain AKK-1 in a K+-free medium and in medium with different levels of added potassium was investigated. The results showed that at a very low concentration of potassium (<0.01 mM), neither the wild type nor the mutant grew, and that at relatively high concentrations of potassium (30 mM and above), both strains grew and had approximately identical growth rates (data not shown). However, at intermediate potassium concentrations, the mutant showed attenuated growth compared to the wild type. For example, in medium containing 1, 5, 10, or 20 mM K+, the doubling times for the mutant were 39, 43, 40, and 34 min, respectively, whereas those for the wild type were 17, 21, 23, and 21 min, respectively. To confirm that the attenuated growth of the AKK-1 mutant at intermediate potassium concentrations was indeed due to inactivation of the trkA gene, we cloned the gene into plasmid pYC2, which was then introduced into the AKK-1 mutant. The results revealed that complementation of the AKK-1 mutant with pYC2 caused it to regain the ability to exhibit wild-type growth rates at intermediate potassium concentrations (data not shown).
TrkA is required for serum resistance.
The AKK-1 strain was examined for sensitivity to serum, and the results revealed that AKK-1 had an ∼0.6% survival rate relative to the original inocula after incubation with 50% serum at 37°C for 1 h; this survival rate was identical to that of the Tn5 mutant SSM-1. However, the serum sensitivity of AKK-1 and SSM-1 was abolished by heat treating of the serum at 56°C for 30 min, indicating that complement is the sensitizing factor. The introduction of pYC2 into the AKK-1 mutant increased the level of serum resistance to be similar to that of the wild type (Table 2).
TABLE 2.
Percentage of survival upon exposure of V. vulnificus to heat-inactivated and non-heat-inactivated 50% normal human serum
| Strain | % Survivala
|
|
|---|---|---|
| HHSb | HSb | |
| Wild type | 79.2 ± 3.4 | 48.0 ± 2.4 |
| AKK-1 | 68.1 ± 2.4 | 0.60 ± 0.4 |
| TRK-1 | 83.2 ± 2.8 | 55.2 ± 3.1 |
The data correspond to a representative experiment performed in duplicate. Data are given as means of survival relative to the original inocula ± standard errors of the means.
HHS, heat-inactivated human serum; HS, non-heat-inactivated human serum.
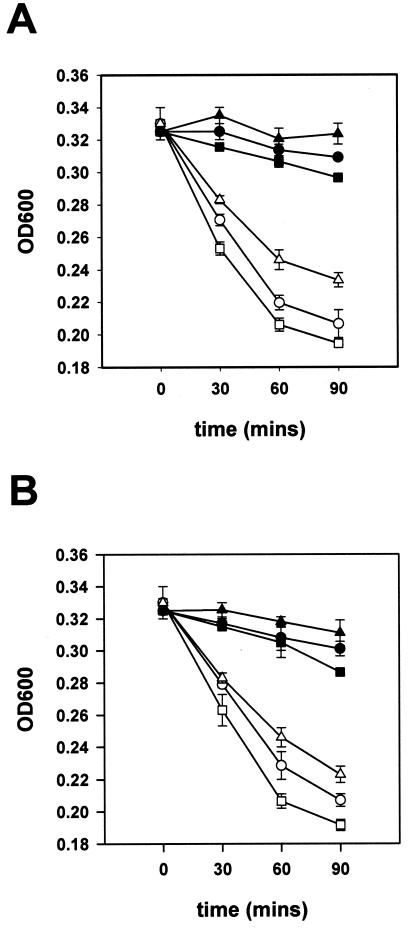
TrkA is required for protamine and polymyxin B resistance.
Since TrkA shared striking homology to SapG of S. enterica serovar Typhimurium and since an S. enterica serovar Typhimurium sapG isogenic mutant has been reported to be highly susceptible to the antimicrobial peptide protamine and attenuated for virulence in mice (29), we examined the sensitivity of the AKK-1 mutant to protamine and polymyxin B. As revealed in Fig. 3, the AKK-1 mutant showed an increased sensitivity to polymyxin B and protamine compared with the wild-type strain for the concentration ranges of 5 to 15 μg/ml and 10 to 20 μg/ml, respectively. Again, resistance to protamine and polymyxin B was restored in AKK-1 containing pYC2 (data not shown).
FIG. 3.
Bacterial killing kinetics of the wild-type and trkA mutant strains by polymyxin B (A) and protamine (B). Wild-type CKM-1 (closed symbols) and trkA mutant strain AKK-1 (open symbols) were incubated in LB broth containing different concentrations of polymyxin B (squares, 15 μg/ml; circles, 10 μg/ml; triangles, 5 μg/ml) or protamine (squares, 20 μg/ml; circles, 15 μg/ml; triangles, 10 μg/ml) at 37°C. Bacteria lysis was monitored by measuring the OD600 value at the indicated times. The data correspond to a representative experiment performed in duplicate. Data are given as the means of the OD600 values ± the standard errors of the means.
Reduced virulence is associated with TrkA.
The virulence of the trkA isogenic mutant AKK-1 and the wild-type strain CKM-1 was studied by infecting BALB/c mice by either the i.p. or s.c. route. Mice were either untreated or were pretreated with iron dextran. As shown in Table 3, the LD50 values for the mutant AKK-1 strain in normal mice were 4.0 × 107 and 6.0 × 105 at 72 h for infection via the i.p. and s.c. routes, respectively. These were 80- and 7-fold increases compared to the LD50 values of 5.0 × 105 and 9.0 × 104 for wild-type infection in normal mice via the i.p. and s.c. routes, respectively. For iron-treated mice, the LD50 values of the mutant AKK-1 were 1.2 × 103 and 2.4 × 103 for infection via the i.p. and s.c. routes, respectively. These values represented 150- and 300-fold increases compared to the LD50 value of 8 for infection by the wild-type strain via the i.p. and s.c. routes (Table 3). Similar results were obtained when the experiment was repeated (Table 3). For the repeated experiment, the virulence of the mutant AKK-1 strain containing pYC2 was also determined, and the results showed that the LD50 values of this complemented mutant were approximately 10- to 15-fold less than those of the uncomplemented mutant but approximately 25- to 19-fold higher than those of the wild-type strain. To investigate whether the complemented mutants were able to maintain their plasmids during the in vivo experiment, we recovered bacteria at 24 h postinfection from spleens of mice that were inoculated with the complemented or uncomplemented mutant, and their antibiotic phenotypes were determined. The results revealed that approximately 95% of the complemented mutants were unable to grow in medium containing chloramphenicol, indicating that the plasmid pYC2 was not maintained in these strains in the absence of antibiotic selective pressures and was rapidly lost within 24 h postinfection. This may explain why the level of virulence was partially restored in complemented mutants in animal infection experiments.
TABLE 3.
Virulence of V. vulnificus strains in untreated or iron-treated mice
| Expt no. or strain | LD50 (CFU/mouse) for mice infected by indicated route
|
|||
|---|---|---|---|---|
| Iron-treated mice
|
Untreated micea
|
|||
| i.p. | s.c. | i.p. | s.c. | |
| Expt 1 | ||||
| Wild type | ∼8 | ∼8 | 5.0 × 105 | 9.0 × 104 |
| AKK-1 | 1.2 × 103 | 2.4 × 103 | 4.0 × 107 | 6.0 × 105 |
| Expt 2 | ||||
| Wild type | ∼8 | ∼10 | ND | ND |
| AKK-1 | 1.9 × 103 | 2.8 × 103 | ND | ND |
| TRK-1 | 2.0 × 102 | 1.9 × 102 | ND | ND |
ND, not done.
DISCUSSION
Resistance to the bactericidal effect of serum is an important property of pathogenic enteric bacterium. The ability of V. vulnificus to resist the killing action of serum and to spread intravascularly is clearly important in the pathogenesis of this infection. In this study, we isolated the SS mutants of V. vulnificus and identified a genetic locus, trkA, involved in the serum resistance of the wild-type strain. The trkA mutant was highly susceptible to serum and this serum hypersensitivity was abolished by heat treating the serum at 56°C, suggesting that trkA contributes to serum resistance by avoiding the complement attack during V. vulnificus infections.
CPS has been shown to play a role in serum resistance in V. vulnificus (42, 43). Opaque-to-translucent colony variations are associated with CPS production. Opaque colonies are encapsulated, while translucent colonies have little or no capsule production (43). Since the trkA mutant exhibited opaque colony phenotypes on LB agar, it is likely that the serum complement hypersensitivity of the trkA mutant was due to the loss of TrkA, but not CPS. However, because it is known that some V. vulnificus isolates that express less CPS still form opaque colonies on LB agar (40), at present we cannot exclude the possibility that the trkA mutant produces less CPS than the wild-type strain does; this remains to be determined.
Bacteria contain multiple K+ uptake systems, and in E. coli K+ is taken up by two major systems, Trk and Kdp (11, 12, 39), and one minor system, Kup (11, 31). The products of four nonlinked genes, namely trkA, trkE, trkG, and trkH, have been implicated as key components of the constitutive Trk system (11, 12). Of these four proteins, TrkA is absolutely required for Trk activity. Either TrkG or TrkH is necessary for activity; only when both are mutated is Trk activity abolished (10, 11). The Kdp system is inducible and transports K+ at a high affinity (16). Trk transports K+ with an ∼103-fold lower affinity but severalfold larger capacity than Kdp does (31) and is therefore responsible for K+ uptake under most conditions of growth. Since the putative amino acid sequence of V. vulnificus TrkA showed high identities to TrkA of V. alginolyticus and E. coli and to SapG of S. enterica serovar Typhimurium, it is reasonable to conjecture that V. vulnificus has a Trk-like system for K+ transport. On the other hand, although a Kdp-like K+ transport system has been detected in many gram-negative bacteria (39), the results of our database research for genes homologous to kdp genes of E. coli revealed that no kdp-like genes were found in the V. vulnificus CMCP6 genome, indicating that V. vulnificus CKM-1 may not have a Kdp-type K+ transport system. It has been reported that V. alginolyticus does not have an inducible high-affinity Kdp-type K+ transport but possesses an inducible middle-affinity K+ transport system (24). Recently, a KtrAB-type system, which was proposed to be a new type of bacterial K+ uptake system, was found in V. alginolyticus and several bacterial genomes (26). We therefore examined whether genes homologous to ktrAB could be found in the V. vulnificus CMCP6 genome. The results showed that KtrA-like (accession number NP_761917.1) and KtrB-like (NP_761918.1) proteins from V. vulnificus CMCP6 exhibit 85% (with 95% similarity) and 88% (with 95% similarity) amino acid sequence identity to V. alginolyticus KtrA (BBA31234.1) and KtrB (BAA32063.1), respectively. Since PCR products of ktrA and ktrB have been detected for V. vulnificus CKM-1 (data not shown), we therefore presume that V. vulnificus CKM-1 may have a KtrAB-like K+ transport system in addition to a Trk-like K+ transport system. Hence, the fact that the trkA mutant AKK-1 exhibited attenuated growth at intermediate potassium concentrations may be due to a slower rate of K+ uptake through a second, KtrAB-like system and to repression of an inducible K+ transport system which is different from the Kdp-type system, if V. vulnificus CKM-1 possesses such a system. In addition, because the AKK-1 mutant showed increased sensitivity to serum, polymyxin B, and protamine compared to the wild-type strain, we can also speculate that either the KtrAB-like system in the mutant may not be expressed to a significant extent under our experimental conditions or Ktr activity may not contribute to serum, polymyxin B, and protamine resistance in V. vulnificus CKM-1. The impaired growth rate of an S. enterica serovar Typhimurium sapG (trkA) mutant in a minimal medium containing 10 mM K+ has been reported (29). This sapG mutant also exhibited protamine hypersensitivity, although it was presumed that this mutant still possessed both an inducible Kdp protein and a constitutive Kup (formerly called TrkD) protein (29).
In addition to identifying the trkA gene of V. vulnificus, we found that trkH was located immediately downstream of trkA and presented evidence that trkA and trkH are transcribed as a single mRNA. Based on this finding, the insertional inactivation of the trkA gene should cause a polar effect leading to the lack of expression of TrkH. Nevertheless, the serum resistance, protamine and polymyxin B resistance, and growth rates at intermediate potassium concentrations could be restored to approximately wild-type levels and mouse virulence could be partially restored when the trkA mutant was complemented with an intact trkA gene supplied in trans. There are two hypotheses to explain this: (i) the role of TrkH may be minor with regard to these in vitro phenotypic measurements but may be more important in vivo and (ii) V. vulnificus may contain another protein(s) that can compensate for the loss of TrkH. The results of our database research revealed that there are two trkH-like genes, one (NP_760004.1) located downstream of trkA and the other (NP_759942.1) located downstream of protoporphyrinogen oxidase, but only one trkA-like gene, in the V. vulnificus CMCP6 genome. We therefore favor the second hypothesis based on this finding. In addition, we also speculate that most of the complemented mutants were unable to maintain plasmid pYC2 in the absence of antibiotic selection during replication in vivo, which may be responsible for the fact that mouse virulence could only be partially restored in these complemented mutants.
In summary, we have isolated the SS mutants of V. vulnificus and identified one of these transposon insertion mutants is a trkA mutant. An isogenic insertionally inactivated trkA mutant was constructed. This trkA mutant exhibited attenuated growth at intermediate potassium concentrations and was 80-fold more sensitive to human serum than was the wild type. It also showed increased sensitivity to protamine and polymyxin B and became attenuated for virulence in mice compared to the wild-type strain. Both polymyxin B and protamine are cationic peptides and are predicated to act by inserting into the membrane, creating a large pore in the membrane, and causing an increase in the ability of ions to permeate the membrane (14). The membrane attack complex of serum complement is also predicted to form ion-permeable channels (22) and causes leakage of the inner membrane potential (8). By examining the susceptibility of a variety of E. coli K+ transport mutants to protamine, Stumpe and Bakker proposed that protamine forms channels in the E. coli cell membrane, through which K+ leaves the cells, and that a high-rate K+ uptake system rescues the cells by mediating the reaccumulation of K+ until protamine becomes detoxified by the cells (38). Our results seem to be consistent with their hypothesis and suggest that TrkA may function by rapidly accumulating K+ while antibacterial peptides and the membrane attack complex form ion-permeable channels in the V. vulnificus cell membrane to prevent this bacterium from death, thus conferring virulence for normal and iron-treated mice. This is the first study to report the relationship between TrkA and the susceptibility of V. vulnificus to killing by serum.
Acknowledgments
We gratefully acknowledge M. S. Donnenberg and J. B. Kaper for providing their plasmid and D. W. Holden for providing the transposon system.
This work was supported by a grant (NSC 91-3112-B-384-001) from the National Science Council of Taiwan.
Editor: F. C. Fang
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baida, G. E., and N. P. Kuzmin. 1995. Cloning and primary structure of a new hemolysin gene from Bacillus cereus. Biochim. Biophys. Acta 1264:151-154. [DOI] [PubMed] [Google Scholar]
- 3.Bisharat, N., V. Agmon, R. Finkelstein, R. Raz, G. Ben-Dror, L. Lerner, S. Soboh, and R. Colodner. 1999. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354:1421-1424. [DOI] [PubMed] [Google Scholar]
- 4.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine vibrio: clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Bossemeyer, D., A. Borchard, D. C. Dosch, G. C. Helmer, W. Epstein, I. R. Booth, and E. P. Bakker. 1989. K+ transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J. Biol. Chem. 264:16403-16410. [PubMed] [Google Scholar]
- 6.Chuang, Y. C., T. M. Chang, and M. C. Chang. 1997. Cloning and characterization of the gene (empV) encoding extracellular metalloprotease from Vibrio vulnificus. Gene 189:163-168. [DOI] [PubMed] [Google Scholar]
- 7.Chuang, Y. C., C. Y. Yuan, C. Y. Liu, C. K. Lan, and A. H. Huang. 1992. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 15:271-276. [DOI] [PubMed] [Google Scholar]
- 8.Dankert, J. R. 1991. Resistance of Escherichia coli to osmotically introduced complement component 9. Infect. Immun. 59:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg, M. S., and J. B. Kaper. 1988. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dosch, D. C. 1985. A study of the Trk transport system of Escherichia coli. Ph.D. thesis. University of Chicago, Chicago, Ill.
- 11.Dosch, D. C., G. L. Helmer, S. H. Sutton, F. F. Salvacion, and W. Epstein. 1991. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J. Bacteriol. 173:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein, W., and B. S. Kim. 1971. Potassium loci in Escherichia coli. J. Bacteriol. 108:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray, L. D., and A. S. Kreger. 1987. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J. Infect. Dis. 155:236-241. [DOI] [PubMed] [Google Scholar]
- 14.Hankcock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 16.Hesse, J. E., L. Wieczorek, K. Altendorf, A. S. Reicin, E. Dorus, and W. Epstein. 1984. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 81:4746-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klenk, H. P., R. A. Clayton, J.-F. Tomb, et al. 1997. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. [DOI] [PubMed] [Google Scholar]
- 18.Klontz, K. C., S. Lieb, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections: clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 19.Kothary, M. H., and A. S. Kreger. 1987. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J. Gen. Microbiol. 133:1783-1791. [DOI] [PubMed] [Google Scholar]
- 20.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 64:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDougald, D., L. M. Simpson, J. D. Oliver, and M. C. Hudson. 1994. Transformation of Vibrio vulnificus by electroporation. Curr. Microbiol. 28:289-291. [Google Scholar]
- 22.Müller-Eberhard, H. J. 1986. The membrane attack complex of complement. Annu. Rev. Immunol. 4:503-528. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura, T., Y. Matsuba, N. Yamamuro, I. R. Booth, and T. Unemoto. 1994. Cloning and sequencing of a K+ transport gene (trkA) from the marine bacterium Vibrio alginolyticus. Biochim. Biophys. Acta 1219:701-705. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura, T., F. Suzuki, M. Abe, Y. Matsuba, and T. Unemoto. 1994. K+ transport in Vibrio alginolyticus: isolation of a mutant defective in an inducible K+ transport system. Microbiology 140:1781-1785. [Google Scholar]
- 25.Nakamura, T., N. Yamamuro, S. Stumpe, T. Unemoto, and E. P. Bakker. 1998. Cloning of the trkAH gene cluster and characterization of the Trk K+-uptake system of Vibrio alginolyticus. Microbiology 144:2281-2289. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, T., R. Yuda, T. Unemoto, and E. P. Bakker. 1998. KtrAB, a new type of bacterial K+-uptake system from Vibrio alginolyticus. J. Bacteriol. 180:3491-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik, K. W., B. Moon, C. W. Park, K. T. Kim, M. S. Ji, S. K. Choi, J. S. Rew, and C. M. Yoon. 1995. Clinical characteristics of ninety-two cases of Vibrio vulnificus infections. Kor. J. Infect. Dis. 27:355-365. [Google Scholar]
- 28.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to Hep-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parra-Lopez, C., R. Lin, A. Aspedon, and E. A. Groisman. 1994. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 13:3964-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed, L. J., and H. Muench. 1938. A simple method of estimating the 50% endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 31.Rhoads, D. B., F. B. Waters, and W. Epstein. 1976. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J. Gen. Physiol. 67:325-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schlosser, A., A. Hamann, D. Bossemeyer, E. Schneider, and E. P. Bakker. 1993. NAD+ binding to the Escherichia coli K(+)-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol. Microbiol. 9:533-543. [DOI] [PubMed] [Google Scholar]
- 34.Schlosser, A., M. Meldorf, S. Stumpe, E. P. Bakker, and W. Epstein. 1995. TrkH and its homolog, TrkG, determine the specificity and kinetics of cation transport by the Trk system of Escherichia coli. J. Bacteriol. 177:1908-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao, C. P., and L. I. Hor. 2000. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect. Immun. 68:3569-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro, R. L., S. Altekruse, L. Hutwagner, R. Bishop, R. Hammond, S. Wilson, B. Ray, S. Thompson, R. V. Tauxe, P. M. Griffin, and Vibrio Working Group. 1998. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988-1996. J. Infect. Dis. 178:752-759. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correction between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stumpe, S., and E. P. Bakker. 1997. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 167:126-136. [PubMed] [Google Scholar]
- 39.Walderhaug, M. O., E. D. Litwack, and W. Epstein. 1989. Wild distribution of homologues of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J. Bacteriol. 171:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright, A. C., J. L. Powell, M. K. Tanner, L. A. Ensor, A. B. Karpas, J. G. Morris, Jr., and M. B. Sztein. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright, A. C., and J. G. Morris, Jr. 1991. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect. Immun. 59:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida, S., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]