Abstract
Background
Early detection of melanoma is the best way to improve prognosis. Digital follow up (DFU) programs of high-risk populations could be an efficient strategy for detecting early melanomas with low morbidity.
Objective
to report the added value of the use of the “two-step method” (digital total-body photography and digital dermoscopy)
Methods
Analysis of the surveillance of 618 high-risk melanoma patients included in our DFU-program from 1999 to 2008.
Results
A total of 11396 lesions were monitored (mean 18.44 per patient) during a median follow-up of 96 months (median 10 visits per patient). 1152 lesions, 1.86 per patient, were excised. Almost 70% (798) were lesions previously registered at least twice, while 356 (30%) were detected and removed in the same visit. During follow-up, 98 melanomas (8.5% of excised lesions) were diagnosed in 78 patients (12.6%). 53 melanomas were in situ (53.3%), while invasive (45) showed a Breslow index of less than 1 mm (median 0.5 mm) and none was ulcerated.
Limitations
Since there are no control groups we cannot convey if the combined use of total-body photography and digital dermoscopy is more beneficial than these techniques used separately.
Conclusion
DFU with Total-Body Photography and Dermoscopy in a selected high-risk population demonstrated the early detection of melanomas with a low rate of excisions. Long-term follow-up is required to allow the detection of slow growing melanomas. Based on our 10-year experience, melanomas can be diagnosed at any time, suggesting that in high-risk population, DFU should be maintained with time.
Keywords: malignant melanoma, dermoscopy, follow-up, imaging techniques, atypical mole syndrome, outcome
INTRODUCTION
Malignant melanoma (MM) may be clinically and dermoscopically indistinguishable from melanocytic nevi making early recognition a diagnostic challenge, especially in incipient lesions 1 Dermoscopic documentation of melanocytic lesions for the comparison of current and previous images in search of subtle changes over time, known as digital follow-up (DFU), has been shown to be helpful in the diagnosis of early melanomas in which specific criteria for MM may not yet be present 2.
The use of baseline regional photographs, namely total-body photography (TBP), might facilitate the detection of new lesions, as well as visual changes in pre-existing lesions, by providing a comparative reference point of areas of skin for subsequent examinations 3. Nevertheless, it has been suggested that a screening strategy focused solely on atypical nevi will likely misdiagnose MM presenting as new lesions or corresponding to lesions not considered adequate for digital follow-up 4.
The combined use of total-body photography and digital dermoscopy, called “The two-step method of digital follow-up (DFU)” 5, has been proposed by our group as an approach for the assessment of high-risk individuals, being potentially more accurate than the two strategies separately.
The present study aims to report our ten-year experience at the Melanoma Unit of Hospital Clinic of Barcelona, using the latter approach in the prospective follow-up of high-risk MM patients included in our specific surveillance program. Our study not only endorses findings from other working groups but also shows new and relevant data derived from the long follow-up period, which is more than twice as long as that reported in previous studies 6, 7, of a cohort of more than 600 individuals with more than 11000 lesions evaluated.
METHODOLOGY
Study population
A total of 629 patients included in the surveillance program with Total-Body photography (TBP) and digital dermoscopy at the Melanoma Unit of Hospital Clinic of Barcelona were followed-up between January 1999 and December 2008.
The criteria for patient inclusion in our follow-up program include: moderate to severe atypical mole syndrome (AMS, defined by more than 100 nevi and/or more than 10 clinically atypical according to ABCD criteria, and/or any histologically dysplastic nevi); personal and/or familial history of MM, carriers of high susceptibility for MM gene mutations, other cancer risk conditions, i.e. presence of congenital nevus of medium to giant size, immunosupression or genodermatosis (Xeroderma pigmentosum, Gorlin-Goltz Syndrome, etc) associated or not to AMS.
Patients included in the present analysis should have at least two follow-up visits with a minimum of 12 months of surveillance. A total of 11 patients were initially excluded because they did not fulfill these criteria in follow-up.
The study was conducted according to the Declaration of Helsinki and with institutional approval. Patient’s written consent was obtained for all invasive procedures.
Examination Procedure: Baseline and Follow-up Registries
In the first visit, a complete clinical history was recorded, including familial history, previous excised melanocytic lesions and other MM associated risk factors.
The baseline digital follow-up (DFU) examination consisted of two steps: the first step, total-body mapping, for clinical examination of the patient and total-body mapping with digital images; and the second step, digital dermoscopy, for clinical and dermoscopical examination in real time of all individual lesions. Digital storage of dermoscopy images of each lesion showing atypical features was performed. Total-body mapping standardized registry was made according to the “two-step method of digital follow-up” 5 published by our group.
The follow-up examination included: the first step (total-body mapping) for comparison of total-body images with previous registries to detect any changes in shape, color, or surface eventually occurring in any pigmented skin lesions, as well as for identification of new lesions, and the second step (digital dermoscopy follow-up), for dermoscopic comparison and storage of pigmented skin lesions images of lesions with atypical features, as well as for the clinical and dermoscopic examination of eventual new lesions not previously registered.
Follow-up visits performing only the second step, digital dermoscopy follow-up, with no registries of total-body mapping were eventually made in the surveillance of selected patients with low or moderate risk, or for monitoring the progress of specific lesions.
Every examination was performed by an expert in dermoscopy for a total time of 30–45 minutes per patient. Images were obtained using a standardized digital system (MoleMax, Derma Instruments; Vienna, Austria). Patients were scheduled for follow-up in 3, 6 or 12 months according to the judgment of the professional who performed the evaluation. Short-term follow-up (3 months) was considered for individual suspicious melanocytic lesions that do not satisfy the dermoscopic criteria for the diagnosis of melanoma, while medium and long-term (6 and 12) was considered for the surveillance of patients with high or moderate risk respectively according to inclusion criteria.
Inclusion Criteria for melanocytic lesions to DFU
Melanocytic lesions with atypical clinical or dermoscopic features were stored on the digital system. Lesions with clear-cut dermoscopic features of MM (as described in pattern analysis 8, the ABCD rule of dermoscopy 9 or the seven-point checklist 10) were not registered for follow-up, as well as lesions with definite dermoscopic features suggestive of benign nevi. Lesions remitted for excision just after our first examinations were excluded from this analysis since they were not part of the follow-up, sixteen MMs were detected in 14 patients in the initial visit.
Lesions considered for excision and histopathological study
Any lesion showing the following changes detected by digital dermoscopy was excised and histopathologically diagnosed: (1) asymmetric enlargement in size; (2) changes in dermoscopic structures (variation in shape; expansion or decrease of pigment network; variation in the distribution or number of dots/globules; modification of depigmented areas or regression structures; appearance of streaks, scar-like areas, blue-whitish veil, and atypical vessels) (3) increase in the number of colors (4) regression features affecting more than 50% of the lesion; and (5) focal pigment modifications. All new or not previously registered lesions observed during follow-up and exhibiting atypical features but no criteria for MM were registered and included in follow-up, lesions displaying criteria for MM were removed.
Twenty-two benign lesions were removed due to practical or aesthetic criteria according to either the patient’s or physician’s judgment. Since they were not suspicious of atypical melanocytic lesion or MM and therefore, not part of the follow-up, they were excluded from the study. All these lesions were confirmed histopathologically as benign lesions.
Histopathology Procedure
All lesions removed were step-sectioned and processed for standard histopathological examination. Conventional hematoxilin-eosin staining and immunohistochemistry (Melan A, HMB45, Ki67) were performed in lesions that were removed, and whenever it was considered necessary by two pathologists. Histology criteria of atypia were reported according to the National Institutes of Health (NIH) Consensus Conference (1992).
Genetic testing
Genetic studies were performed after informed consent and proper genetic counseling in patients with history of multiple primary and/or familial multiple MM. Exons 1alfa, 1beta, 2, 3; intronic change IVS2-105 and -34G>T at the CDKN2A promoter region and Exon 2 from CDK4 were studied by PCR-SSCP analysis and sequencing. MC1R was studied by direct sequencing as previously reported 11.
Compliance
Patient’s compliance was assessed according to the continuity in the follow-up program. Patients who were excluded from the program and continued with clinical and dermoscopic examination, left the program or died, were identified.
Statistical analysis
Bi-variate analysis was performed in order to assess differences in patients who were diagnosed with melanoma during follow-up and those who were not; the chi square test was used for the comparison of qualitative variables, applying Fisher’s correction according to the sample sizes’ need in tables of 2×2 and the T student test was used to compare means of the quantitative variables. Differences were considered to be statistically significant when p< 0.05. Multivariable logistic regression analysis was used to obtain the Odds Ratio (OR) using the forward approach, including in the model one by one those variables with a p<0.2 in the bi-variate analysis.
RESULTS
The surveillance program cohort consisted of 618 patients with a mean age of 37 years (mean SD ±13.3 years) at time of inclusion in the program; 45.5% were men. According to inclusion criteria, the vast majority of the patients (n=556) had atypical mole syndrome (AMS) and only 7.1 (n=44) had less than 50 nevi associated to other high-risk conditions. Of the patients, 277 had a personal history of MM, including 73 with a history of multiple primary MMs, prior to the beginning of the study; 8 patients with giant congenital melanocytic nevus and 3 patients affected with xeroderma pigmentosum were followed-up in our unit. Almost one third of the patients (n=178) also had a familial history of MM. Descriptive data regarding nevi count, skin phototype, eyes and hair color, lentiginosis actinic damage (lentiginosis and solar elastosis) as well as the presence of genetic mutations are shown in table 1.
Table I.
Descriptive data of population
| Age at inclusion | 37 years (mean SD ±13.3 years) |
| Gender | |
| male | 281 (45.5%) |
| female | 337 (54.5%) |
| Personal history at inclusion | |
| melanoma | 28 (4.53%) |
| melanoma & AMS | 245 (39.64%) |
| AMS | 311 (50.32%) |
| Xeroderma pigmentosum (all with previous MM) | 3 (0.5%) |
| Giant congenital nevus (one with previous MM) | 8 (1.29%) |
| Others (only familial history of MM, Gorlin-Goltz syndrome, etc) | 23 (3.72%) |
| Nevi count | |
| <50 | 44 (7.11%) |
| 50–100 | 218 (35.30%) |
| 100–200 | 241 (38.99%) |
| >200 | 115 (18.60%) |
| Phototype | |
| I | 19 (3.1%) |
| II | 249 (40.3%) |
| III | 327 (52.9%) |
| IV | 23 (3.7%) |
| V | 0 |
| VI | 0 |
| Eyes color | |
| Blue | 80 (12.9%) |
| Green | 76 (12.3%) |
| Brown | 445 (72.0%) |
| Black | 17 (2.8%) |
| Hair color | |
| Red | 26 (4.2%) |
| Blonde | 84 (13.6%) |
| Brown | 463 (74.9%) |
| Black | 45 (7.3%) |
| Lentiginoses | |
| mild | 209 (33.8%) |
| moderate | 97 (15.7%) |
| severe | 72 (11.7%) |
| no | 240 (38.8%) |
| CDKN2A mutation | 39 (11.5% of studied) |
| MC1R polymorphism | 163 (75.1% of studied) |
| V60L | 42 |
| V92M | 17 |
| R151C | 28 |
AMS: Atypical Mole Syndrome
Patients were followed-up for a median of 96 months (range 13–120 months). Over ten years of follow-up, 6,149 visits (4,155 with total-body photography and digital dermoscopy and 1,994 with digital dermoscopy only) were performed. Each patient was evaluated a median of 10 times (range 2–22) during the course of the study, a median of 7 visits (range 2–17) with total-body photography and digital dermoscopy, and a median of 3 intermediate visits (range 0–11) only with digital dermoscopy. During the study, 78,070 body maps (mean 126.3 per patient, range [9–410]) and 88,283 digital dermoscopy images (mean 142.9 per patient, range [6–726]) were stored.
A total of 11,396 lesions were followed-up, a mean of 18.44 per patient (1–60). Among those 1,152 lesions, a mean of 1.86 lesions per patient, were excised and remitted for histopathological assessment during the study. In 211 patients no excision was required and in 149 only one lesion was excised in ten years of follow-up. So, in almost 60% of the cohort, none or only one lesion required excision. In contrast, only 7 patients required ten or more excisions during surveillance, but they corresponded to patients with personal history of multiple primary MM and familial MM, CDKN2A mutations carriers, or patients affected with xeroderma pigmentosum.
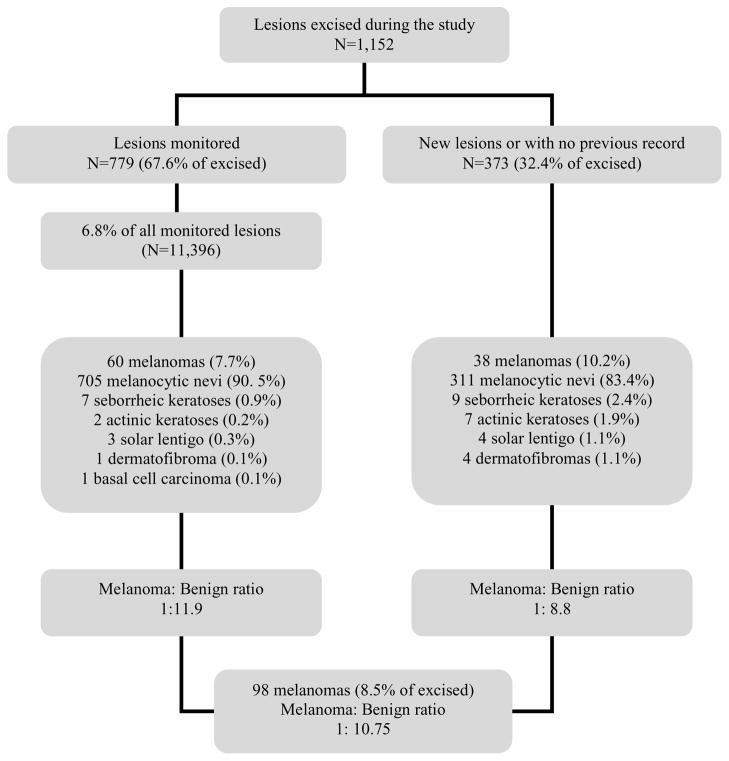
Among lesions excised during follow-up, 779 (67.6%) corresponded to lesions previously registered and under surveillance, and 373 (32.4%) corresponded to lesions detected in the visits, which were new or, being already present, were not previously counted for register in DFU. Histopathological diagnosis of melanocytic and non melanocytic lesions (initially assumed as melanocytic and thus, registered for DFU) excised in both groups is shown in Figure 1.
Figure 1.
Lesions excised during the study
* corresponded to 6.8% of all monitored lesions
During DFU, 98 melanomas (8.5% of excised lesions, benign/MM ratio 10.7:1) were detected in 78 patients; 60 MMs corresponded to monitored lesions (7.7% of registered lesions, benign/MM ratio 11.9:1; Figure 2) and 38 to lesions with no previous digital record (10.2% of new or unregistered lesions, benign/MM ratio 8.8:1; Figure 3). MMs detected due to changes in digital dermoscopy required a median of 4 (range 2–15) consecutive controls and a mean follow-up time of 23.9 months (range 1–77); of these, 16 arose in a previous nevus, but 44 did not show any evidence of a pre-existing nevus upon histopathology.
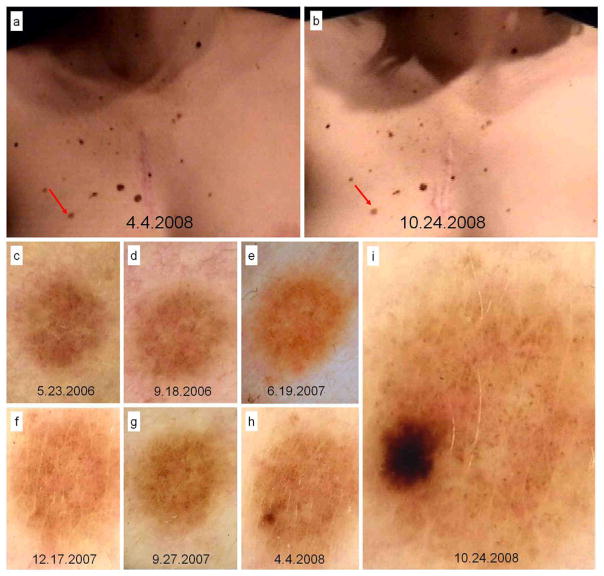
Figure 2.
In situ melanoma developed over melanocytic nevus in a 23 year-old patient, with personal and familial history of melanoma, diagnosed due to changes in digital follow-up. Body-mapping images displaying no clinical change (A and B) and dermoscopy records in chronological order until excision after 29 months and 7 visits of follow-up (C to I).
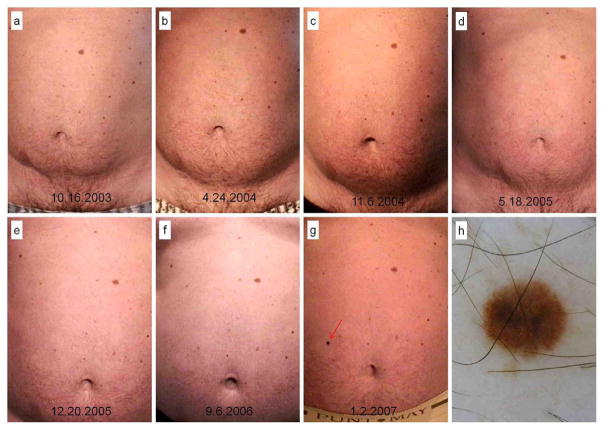
Figure 3.
Superficial spreading malignant melanoma, Breslow 0.5 mm, Clark level III detected as a new lesion during total-body mapping comparison in the abdomen of a 48 years-old male, carrier of CDKN2A mutation, with history of personal melanoma and familial melanoma and atypical mole syndrome. Body-mapping records showing the appearance of the lesion (A to G), clinically symmetric and with regular borders. Dermoscopy image (H) showing atypical pigment network, inverted pigment network and bluish hue.
Histopathologically, 53 MMs were in situ (53.3%), among invasive MMs, the median Breslow’s index was 0.5 mm (mean 0.62 mm) and no MM detected during follow-up was thicker than 1 mm or ulcerated, that is, all invasive MMs were staged in IA (AJCC 2009).
A total of 1,015 melanocytic nevi were excised during the study, almost half with some degree of histological atypia (18.7% mild, 23.8% moderate and 6% severe). On histological examination, 45.4% exhibited regression, inflammatory changes, Sutton phenomenon or fibrosis that could explain dermoscopic changes during monitoring.
During follow-up, 78 patients, 12.6% of the cohort, were diagnosed with MM. Patients diagnosed with MM during DFU were more frequently men (p=0.02), who were older at the beginning of the study (p<0.001), with a higher number of lesions monitored (p<0.001) and a higher number of lesions excised during DFU than those who were not diagnosed with MM; no significant differences in length of follow-up between the two groups were observed. History of previous MM and multiple MM were more frequent among patients diagnosed with MM during surveillance (p <0.001 and 0.003, respectively), but no significant differences were found regarding the number of MM prior to the start. No statistically significant differences were found considering the nevi count in the four pre-established categories (<50, 50–100, 100–200 and >200), but patients with > 100 nevus were more frequently diagnosed with MM than those with < 100 nevus (p 0.007). As expected, patients with AMS had more MM during follow-up than those without AMS, but differences were not significant (p=0.636). No significant differences were found regarding skin phototype, presence and degree of lentiginosis and presence of CDKN2A mutation between the two groups (table II).
Table II.
Differences between patients who were and were not diagnosed with MM during FU
| MM during follow-up | p-value | OR | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| No (N=540) | Yes (N=78) | ||||||
| n | % | n | % | ||||
| Sex | 0.020 | ||||||
| Female | 304 | 56.3 | 33 | 42.3 | 1.00 | (Reference) | |
| Male | 236 | 43.7 | 45 | 57.7 | 1.76 | (1.09–2.84) | |
| Age at inclusion | 0.001 | ||||||
| 0–20 | 51 | 9.4 | 5 | 6.4 | 1.00 | (Reference) | |
| 21–40 | 295 | 54.6 | 31 | 39.7 | 1.07 | (0.40–2.89) | |
| 41–60 | 171 | 31.7 | 31 | 39.7 | 1.85 | (0.68–5.00) | |
| >60 | 23 | 4.3 | 11 | 14.1 | 4.88 | (1.52–15.66) | |
| AMS | 0.636 | ||||||
| No | 53 | 9.8 | 9 | 11.5 | 1.00 | (Reference) | |
| Yes | 487 | 90.2 | 69 | 88.5 | 0.83 | (0.39–1.77) | |
| Previous melanoma | <0.001 | ||||||
| No | 317 | 58.7 | 24 | 30.8 | 1.00 | (Reference) | |
| Yes | 223 | 41.3 | 54 | 69.2 | 3.20 | (1.92–5.33) | |
| Previous multiple melanoma | 0.003 | ||||||
| No | 484 | 89.6 | 61 | 78.2 | 1.00 | (Reference) | |
| Yes | 56 | 10.4 | 17 | 21.8 | 2.41 | (1.32–4.41) | |
| Number of melanoma previous to beginning | 0.070 | ||||||
| 1 | 165 | 74.3 | 37 | 68.5 | 1.00 | (Reference) | |
| 2 | 49 | 22.1 | 10 | 18.5 | 0.91 | (0.42–1.96) | |
| 3 | 5 | 2.3 | 3 | 5.6 | 2.68 | (0.61–11.70) | |
| 4 | 2 | 0.9 | 2 | 3.7 | 4.46 | (0.61–32.69) | |
| 5 | 1 | 0.5 | 2 | 3.7 | 8.92 | (0.79–100.98) | |
| Nevi count | 0.058 | ||||||
| < 50 | 40 | 7.4 | 4 | 5.1 | 1.00 | (Reference) | |
| 50–100 | 200 | 37.0 | 18 | 23.1 | 0.90 | (0.29–2.80) | |
| 100–200 | 204 | 37.8 | 37 | 47.4 | 1.81 | (0.61–5.37) | |
| > 200 | 96 | 17.8 | 19 | 24.4 | 1.98 | (0.63–6.19) | |
| More than 100 nevus | 0.007 | ||||||
| No | 240 | 44.4 | 22 | 28.2 | 1.00 | (Reference) | |
| Yes | 300 | 55.6 | 56 | 71.8 | 2.04 | (1.21–3.43) | |
| Phototype | 0.422 | ||||||
| I | 15 | 2.8 | 4 | 5.1 | 1.00 | (Reference) | |
| II | 219 | 40.6 | 30 | 38.5 | 0.51 | (0.16–1.65) | |
| III | 284 | 52.6 | 43 | 55.1 | 0.57 | (0.18–1.79) | |
| IV | 22 | 4.1 | 1 | 1.3 | 0.17 | (0.02–1.68) | |
| Phototype | 0.966 | ||||||
| I–II | 234 | 43.3 | 34 | 43.6 | 1.00 | (Reference) | |
| III–IV | 306 | 56.7 | 44 | 56.4 | 0.99 | (0.61–1.60) | |
| Lentigines | 0.286 | ||||||
| No | 214 | 39.6 | 26 | 33.3 | 1.00 | (Reference) | |
| Yes | 326 | 60.4 | 52 | 66.7 | 1.31 | (0.80–2.17) | |
| Excised lesions | <0.001 | ||||||
| 0 | 211 | 39.1 | 0 | 0.0 | - | - | |
| 1 | 135 | 25.0 | 14 | 18.0 | 1,00 | (Reference) | |
| 2 | 70 | 13.0 | 14 | 18.0 | 1,93 | (0.87–4.27) | |
| 3 | 50 | 9.3 | 14 | 18.0 | 2,70 | (1.20–6.06) | |
| 4 | 35 | 6.5 | 9 | 11.5 | 2,48 | (0.99–6.20) | |
| 5 | 15 | 2.8 | 5 | 6.4 | 3,21 | (1.02–10.17) | |
| 6 | 10 | 1.9 | 7 | 9.0 | 6,75 | (2.22–20.52) | |
| >=7 | 14 | 2.6 | 15 | 19.2 | 10,33 | (4.15–25.74) | |
| CDKN2A | <0.001 | ||||||
| Negative | 239 | 44.3 | 61 | 78.2 | 1.00 | (Reference) | |
| not performed | 272 | 50.4 | 7 | 9.0 | 0.10 | (0.05–0.22) | |
| Positive | 29 | 5.4 | 10 | 12.8 | 1.35 | (0.62–2.92) | |
| mean | (SD) | mean | (SD) | p-value | OR | 95% CI | |
| Age at inclusion | 36.2 | (12.8) | 42.4 | (15.5) | <0.001 | 1.03 | (1.02–1.05) |
| Number of controlled lesions | 17.6 | (8.2) | 24.2 | (13.0) | <0.001 | 1.07 | (1.04–1.09) |
| number of excised lesions | 1.5 | (1.9) | 4.3 | (3.5) | <0.001 | 1.50 | (1.35–1.66) |
| Length of follow-up (months) | 85.3 | (29.9) | 88.8 | (31.0) | 0.348 | 1.00 | (1.00–1.01) |
AMS: Atypical Mole Syndrome
In the multivariable logistic regression analysis (table III), older age at inclusion and higher number of lesions excised during follow-up, were the variables more associated with melanoma diagnosis during DFU (p 0.003 and <0.001, respectively); male gender, previous melanoma or the presence of CDKN2A mutation, were also associated with melanoma during follow-up but differences were not statistically significant. Skin phototype IV and no indication of CDKN2A mutation analysis were associated with a lower risk of melanoma during follow-up (p=0.033 and <0.001 respectively); skin phototype II and III were associated with a lower risk of melanoma than type I, but no statistically significant differences were observed (p=0.123 and 0.423 respectively).
Table III.
Multivariable logistic regression analysis
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age at inclusion | 1.04 | (1.01–1.06) | 0.003 |
| Gender | |||
| Female | 1.00 | (Reference) | |
| Male | 1.23 | (0.68–2.22) | 0.500 |
| Previous Melanoma | |||
| No | 1.00 | (Reference) | |
| Yes | 1.55 | (0.81–2.97) | 0.181 |
| More than 100 nevus | |||
| No | 1.00 | (Reference) | |
| Yes | 1.37 | (0.72–2.60) | 0.342 |
| Nº of lesions excised | 1.55 | (1.37–1.75) | <0.001 |
| Skin phototype | |||
| I | 1.00 | (Reference) | |
| II | 0.33 | (0.08–1.35) | 0.123 |
| III | 0.57 | (0.14–2.26) | 0.423 |
| IV | 0.03 | (0.00–0.76) | 0.033 |
| CDKN2A mutation | |||
| No | 1.00 | (Reference) | |
| Not performed | 0.15 | (0.06–0.37) | <0.001 |
| Yes | 1.39 | (0.53–3.68) | 0.505 |
Regarding DFU compliance, 519 (84.1 %) patients continue under surveillance in the follow-up program, 47 (7.6%) were excluded from the program and continue clinical and dermoscopical examinations in our Unit, 38 patients (6.1%) left the program or were referred to dermatological follow-up at another centre, and 14 patients (2.2%) died, 12 because of MM progression, one as a consequence of a heart-attack and one related to Duchenne’s muscular dystrophy progression.
DISCUSSION
Various strategies have been suggested for MM detection in high risk patients, such as skin self-examination 12,13, total cutaneous examination 14, and the use of TBP 3,15–19, and dermoscopy 20,21. It has been well demonstrated that clinical examination is inaccurate for the diagnosis of incipient MM 22 while dermoscopy has been shown to improve the diagnostic accuracy of nearly all cutaneous tumors including melanoma20,21,23.
Over the last few years, increasing evidence has accumulated in favor of digital dermoscopy for the follow-up of atypical melanocytic lesions 2, 6, 7, 24–30. Digital Follow-up (DFU) has proven to be useful in the surveillance of high-risk populations by providing the double benefit of not overlooking MM with few dermoscopic criteria while minimizing the excision of benign lesions 2.
Since dermoscopy is not 100% accurate, a certain percentage of suspicious but benign lesions have to be excised in order to not miss MM. In our study, less than 2 lesions per patient were excised during a median of 8 years of surveillance, with a global MM/benign ratio of 1:10.7 and a MM detection rate of 8.5%, endorsing the fact that DFU is both an efficient and effective strategy for early MM detection in high-risk patients (table III).
The detection of new or clinically changing melanocytic lesions in a high-risk population is difficult and almost impossible in patients with a high nevi count unless total body photography (TBP) is available for comparison. Furthermore, it is well known that MM often develops de novo in clinically normal skin rather than in pre-existing melanocytic nevus 31.
The “Two-step Method of DFU”, routinely used in our unit in the surveillance of high risk MM patients, consists of the combined performance of TBP and digital dermoscopy in every visit 5 We believe that our protocol represents a more complete surveillance approach than those from other working groups, in which DFU is solely focused on digital dermoscopy of registered lesions. On the other hand, in protocols of digital dermoscopy in which TBP are performed, body-maps are only registered in the first visits, and in subsequent controls body surface is simply compared with overview images. Already in 2007, Fuller et al 4 highlighted that it is unclear in most previous studies whether any MM was missed because they either presented as new lesions or arose from nevi that were not monitored by dermoscopy, since the total number of MM occurring in those patients was not reported. In the latter study, only one MM was detected by DFU out of 6 MMs detected during a median of 22 months; with a MM/benign lesion ratio of 1:94 and 1:34.4 among lesions with and without previous dermoscopy record respectively. In our study, nearly 40% of MMs detected during follow-up corresponded to lesions that were not previously recorded, either because they were newly assessed by TBP or, being already present, they were not atypical, and hence not included for follow-up. In this MM subgroup, MM/benign ratio was, as in Fullers’ study, lower among lesions with no previous dermoscopy record (1:8.8 vs. 1:11.9).
The ten-year experience in follow-up of patients at increased risk for MM reported by Haenssle et al 6, 7 deserves special attention. As seen in table III, general data concerning number of patients, lesions monitored, percentage of lesions excised, malignant/benign ratio, and patients diagnosed with MM during the study, are remarkably similar to our study. Nevertheless, some differences are clear: first, our median follow-up of 96 months (8 years) is more than twice as long, providing more consistent data in terms of long-term follow-up; and second, unlike their study, we decided not to include lesions excised in the first visit examinations, as they were not part of the follow-up, leaving 16 MMs out of the present analysis. Haenssle et al. found a higher number of MMs in their study (127); if we exclude 40 MMs, which they report to have diagnosed after the first examination, that would leave 87 MMs detected during follow-up, which is more similar to our experience. Another interesting difference is the percentage of MMs detected due to dynamic changes during DFU, which is 36.7% (32/87) in their experience but 61.2% (60/98) in ours. No further conclusion can be made since the populations are not equivalent.
Recently, Argenziano et al 32 reported that MM may grow slowly and thus changes can only be seen after long-term follow-up. According to this, we report follow-up as long as 77 months until excision, and being almost half of the MM followed-up for more than 2 years until showing some significant change in initially featureless lesions. Two findings require special attention; first, 75% of MM with more than 2 years of follow-up before excision were in situ, and second, almost 65% of MM that required more than 2 years of follow-up showed no pre-existing nevus upon histopathological examination (data not shown). These findings may support the current evidence of the existence of a subgroup of slow-growing MM.
It is well known that the DFU procedure is not only time consuming but also a technique that requires training, experience and specific equipment. Chances of success in DFU depend basically on the proper selection of patients 33. In our study population, with 90% of the patients displaying atypical mole syndrome and almost 45% with previous melanoma, 1 out of 8 developed MM during surveillance, which is more than 1500 times higher than expected in our general population. Not unexpectedly, the percentage of patients diagnosed with MM during follow-up rose from 7% among patients with no personal history of MM, to 18% and 23% in patients with one primary MM and multiple primary MM prior to the inclusion in follow-up, respectively.
The duration of the DFU or the possibility to exclude a patient included in the program after a period with no excisions required have been a matter of debate. According to our results, MM can be diagnosed at any time once a patient is included in the DFU program, and not just at the beginning within the first follow-up examinations. Furthermore, the risk of diagnosing more than one MM during follow-up is relatively high among high risk populations. In light of these findings, maintained surveillance may be required in high risk individuals.
There is no consensus regarding the most effective melanoma screening strategy in high risk individuals. Since there are no control groups we cannot convey whether the combined use of TBP and digital dermoscopy is more beneficial than the TBP, dermoscopy examination or DFU separately. Recently, Goodson et al. 18 compared their results using TBP and digital dermoscopy monitoring of nevi in a similar patient population at risk for melanoma and they found that monitoring patients at risk for melanoma using TBP was associated with a lower biopsy rates and lower benign/melanoma ratios than using digital dermoscopy and facilitated detection of new and changing lesions with a higher MM detection rate during follow-up (4.4% vs. 1.9% respectively). With the use of the “Two-step method of DFU” we achieved a higher melanoma detection rate (8.5%) and a lower nevus:melanoma ratio (9.3 vs. 53 with DFU and 22 with TBP). In our study biopsy rate was higher, but this finding may be due to the fact that our median follow-up period is 4 times longer and our population could be considered of higher risk, since incidence of melanoma per patient during follow-up was six times higher.
In conclusion, TBP and digital dermoscopy (“two-step method of digital follow-up”) in a selected high-risk population was shown to allow the detection of melanomas in early stages with a low rate of excisions. This dual modality is useful not only for the detection of MM with few dermoscopic criteria by DFU of dermoscopy records, but also for the detection of melanoma either presented as new lesions or arising from nevi that were not monitored by dermoscopy. Long-term follow-up is required to allow the detection of slow growing melanomas. Based on our 10-year experience, melanomas can be diagnosed at any time, and not just at the beginning of follow-up, suggesting that in this kind of high-risk population, DFU should be maintained with time.
Table IV.
Comparison of clinical outcomes of our study and those from other working groups
| Authors | Lesions - Patients (number) | Mean lesions/patient | Median follow up (months) | Excisions (%) out of lesions registered | Ratio MM/No MM | MM (%)out of excisions | Patients diagnosed with MM during DFU (%) |
|---|---|---|---|---|---|---|---|
| Haenssle6,72010, Germany | 11137-688 | 16.18 | 46 | 10.9 | 1:8.5 | 10.4 | 11.4 |
| Argenziano292008, Italy | 600 – 405 | 1.48 | 23 | 9 | 1: 3.4 | 22.2 | 3 |
| Fuller42007, USA | 5945 – 297 | 20 | 22m | 5.4 | 1:53 | 1.9 | 2 |
| PRL1:95/NPRL 1:34.4 |
PRL 1.1/NPRL 2.75 |
||||||
| Haenssle252006, Germany | 7001 – 530 | 13.2 | 32.2 | 9.1 | 1:12 | 8.3 | 10 |
| Bauer262003, Germany (EPL) | 2015 – 196 | 10.28 | 25m | 1.6 | 1: 15.5 | 6.1 | 1 |
| Robinson272004, USA | 3482 - 100 | 34.82 | 36.2 | 5.5 | 1: 47.3 | 2.1 | 4 |
| Malvehy52002, Barcelona | 3170 – 290 | 10.93 | 17.2 | 1.3 | 1: 4.2 | 19 | 2.8 |
| Menzies302001, Australia | 318 -245 | 1.29 | 3m | 19.2 | 1: 7.7 | 11.5 | 2.9 |
| Kittler282000, Austria | 1862 – 202 | 9.21 | 12.6 | 4 | 1: 8.4 | 10.7 | 4 |
| Present study | 11396-618 | 18.44 | 96 | 10.1 | 1:10.7 | 8.5 | 12.6 |
| PRL 1:11.9/NPRL 1:8.8 |
PRL 7.7/NPRL 10.1 |
PRL: previously registered lesions; NPRL: non previously registered lesions
Capsule summary
Digital dermoscopy follow-up is the most reliable and efficient approach in order to detect incipient melanoma.
The combined use of total-body photography and digital dermoscopy ("two-step method of digital follow-up") allow the detection of melanomas in early stages with a significant reduction of excisions
Long-term follow-up is required to allow the detection of slow growing melanomas. In high-risk population, DFU should be maintained with time
Acknowledgments
Funding/Support
The research at the Melanoma Unit in Barcelona is partially funded by Grants 03/0019, 05/0302 and 06/0265 from Fondo de Investigaciones Sanitarias, Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the AGAUR 2009 SGR 1337 of the Catalan Government, Spain; by the European Commission under the 6th Framework Programme, Contract nº: LSHC-CT-2006-018702 (GenoMEL) and by the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript
ABBREVIATIONS
- DFU
digital follow-up
- MM
malignant melanoma
- TBP
total-body photography
- AMS
Atypical mole syndrome
- SD
standard deviation
Footnotes
Financial Disclosure: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gabriel Salerni, Email: gabrielsalerni@hotmail.com.
Cristina Carrera, Email: criscarrer@yahoo.es.
Louise Lovatto, Email: loulovatto@hotmail.com.
Joan Anton Puig-Butille, Email: jantonpuig@gmail.com.
Celia Badenas, Email: cbadenas@clinic.ub.es.
Estel Plana, Email: estel.plana@gmail.com.
Susana Puig, Email: susipuig@gmail.com, spuig@clinic.ub.es.
Josep Malvehy, Email: jmalvehy@clinic.ub.es.
References
- 1.Puig S, Argenziano G, Zalaudek I, et al. Melanomas that failed dermoscopic detection: a combined clinicodermoscopic approach for not missing melanoma. Dermatol Surg. 2007 Oct;33(10):1262–73. doi: 10.1111/j.1524-4725.2007.33264.x. [DOI] [PubMed] [Google Scholar]
- 2.Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006 Sep;142(9):1113–9. doi: 10.1001/archderm.142.9.1113. [DOI] [PubMed] [Google Scholar]
- 3.Halpern AC. Total body skin imaging as an aid to melanoma detection. Semin Cutan Med Surg. 2003 Mar;22(1):2–8. doi: 10.1053/sder.2003.50000. [DOI] [PubMed] [Google Scholar]
- 4.Fuller SR, Bowen GM, Tanner B, Florell SR, Grossman D. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007 Oct;33(10):1198–206. doi: 10.1111/j.1524-4725.2007.33254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malvehy J, Puig S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: a two-step method. Clin Dermatol. 2002 May-Jun;20(3):297–304. doi: 10.1016/s0738-081x(02)00220-1. [DOI] [PubMed] [Google Scholar]
- 6.Haenssle HA, Korpas B, Hansen-Hagge C, et al. Seven-point checklist for dermatoscopy: performance during 10 years of prospective surveillance of patients at increased melanoma risk. J Am Acad Dermatol. 2010 May;62(5):785–93. doi: 10.1016/j.jaad.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 7.Haenssle HA, Korpas B, Hansen-Hagge C, et al. Selection of patients for long-term surveillance with digital dermoscopy by assessment of melanoma risk factors. Arch Dermatol. 2010 Mar;146(3):257–64. doi: 10.1001/archdermatol.2009.370. [DOI] [PubMed] [Google Scholar]
- 8.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987 Oct;17(4):571–83. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 9.Stoltz W, Braun-Falco O, Bilek P, Landthaler M, Cognetta A. A color atlas of dermoscopy. Germany: Blackwell Science; 1994. [Google Scholar]
- 10.Argenziano G, Fabbrocini G, Carli P. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563–70. doi: 10.1001/archderm.134.12.1563. [DOI] [PubMed] [Google Scholar]
- 11.Puig S, Malvehy J, Badenas C, et al. Role of the CDKN2A locus in patients with multiple primary melanomas. J Clin Oncol. 2005 May 1;23(13):3043–5. doi: 10.1200/JCO.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- 13.Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. Evaluation of factors associated with skin self-examination. Cancer Epidemiol Biomarkers Prev. 1999 Nov;8(11):971–8. [PubMed] [Google Scholar]
- 14.Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986 May;14(5 Pt 1):857–60. doi: 10.1016/s0190-9622(86)70100-x. [DOI] [PubMed] [Google Scholar]
- 15.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005 Aug;141(8):998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 16.Wang SQ, Kopf AW, Koenig K, Polsky D, Nudel K, Bart RS. Detection of melanomas in patients followed up with total cutaneous examinations, total cutaneous photography, and dermoscopy. J Am Acad Dermatol. 2004 Jan;50(1):15–20. doi: 10.1016/s0190-9622(03)02794-4. [DOI] [PubMed] [Google Scholar]
- 17.Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: non-uniform dermoscopic features and growth. J Am Acad Dermatol. 2003 May;48(5):663–71. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]
- 18.Goodson AG, Florell SR, Hyde M, Bowen GM, Grossman D. Comparative analysis of total body and dermatoscopic photographic monitoring of nevi in similar patient populations at risk for cutaneous melanoma. Dermatol Surg. 2010 Jul;36(7):1087–98. doi: 10.1111/j.1524-4725.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risser J, Pressley Z, Veledar E, Washington C, Chen SC. The impact of total body photography on biopsy rate in patients from a pigmented lesion clinic. J Am Acad Dermatol. 2007 Sep;57(3):428–34. doi: 10.1016/j.jaad.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008 Sep;159(3):669–76. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 22.Pizzichetta MA, Talamini R, Piccolo D, et al. The ABCD rule of dermatoscopy does not apply to small melanocytic skin lesions. Arch Dermatol. 2001 Oct;137(10):1376–8. [PubMed] [Google Scholar]
- 23.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 24.Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. Long-term dermoscopic follow-up of melanocytic naevi: clinical outcome and patient compliance. Br J Dermatol. 2003;149:79–86. doi: 10.1046/j.1365-2133.2003.05409.x. [DOI] [PubMed] [Google Scholar]
- 25.Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980–5. doi: 10.1038/sj.jid.5700119. [DOI] [PubMed] [Google Scholar]
- 26.Bauer J, Blum A, Strohhacker U, Garbe C. Surveillance of patients at high risk for cutaneous malignant melanoma using digital dermoscopy. Br J Dermatol. 2005;152:87–92. doi: 10.1111/j.1365-2133.2005.06370.x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson JK, Nickoloff BJ. Digital epiluminescence microscopy monitoring of high-risk patients. Arch Dermatol. 2004;140:49– 56. doi: 10.1001/archderm.140.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43:467–76. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 29.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br J Dermatol. 2008 Aug;159(2):331–6. doi: 10.1111/j.1365-2133.2008.08649.x. Epub 2008 May 28. [DOI] [PubMed] [Google Scholar]
- 30.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol. 2001 Dec;137(12):1583–9. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 31.Weatherhead SC, Haniffa M, Lawrence CM. Melanomas arising from naevi and de novo melanomas--does origin matter? Br J Dermatol. 2007 Jan;156(1):72–6. doi: 10.1111/j.1365-2133.2006.07570.x. [DOI] [PubMed] [Google Scholar]
- 32.Argenziano G, Kittler H, Ferrara G, et al. Slow-growing melanoma: a dermoscopy follow-up study. Br J Dermatol. 2010 Feb 1;162(2):267–73. doi: 10.1111/j.1365-2133.2009.09416.x. [DOI] [PubMed] [Google Scholar]
- 33.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br J Dermatol. 2008 Aug;159(2):331–6. doi: 10.1111/j.1365-2133.2008.08649.x. Epub 2008 May 28. [DOI] [PubMed] [Google Scholar]