Abstract
Activating mutations in different domains of the ABCC8 gene-coded sulfonylurea receptor 1 (SUR1) cause neonatal diabetes. Here we show that a diabetogenic mutation in an unexplored helix preceding the ABC core of SUR1 dramatically increases open probability of (SUR1/Kir6.2)4 channel (KATP) by reciprocally changing rates of its transitions to and from the long-lived, inhibitory ligand-stabilized closed state. This kinetic mechanism attenuates ATP and sulfonylurea inhibition, but not Mg-nucleotide stimulation, of SUR1/Kir6.2. The results suggest a key role for L0 helix in KATP gating and together with previous findings from mutant KATP clarify why many patients with neonatal diabetes require high doses of sulfonylureas.
Keywords: Potassium channel, ABC protein, Gating mutation, Diabetes, Sulfonylurea therapy
1. Introduction
ABCC8/KCNJ11-coded KATP [1], stimulated by MgATP/ADP at the ABC ATPase and inhibited by ATP at the K+ inward rectifier [2,3], control the metabolism-dependent excitability of pancreatic β-cells and certain neurons [4,5]. Activating mutations in either KATP gene cause neonatal diabetes (ND), including severe ND with neurological abnormalities (see [6-8] and many additional reports reviewed in [9,10]). How ND mutations in different domains of SUR1 affect KATP open probability (PO) and inhibition by sulfonylureas (SU) needs to be better understood.
The first functional analysis of ND mutations in the canonical TMD1-NBD1-TMD2-NBD2 core of SUR1 defined its Mg-nucleotide-dependent hyperstimulation as the first, or A type, mechanism of pathogenic KATP overactivity [7]. Additional tests [11] uncovered that hyperstimulated KATP can show reduced responsiveness to sulfonylureas (SU), thus partly explaining why many ND patients need SU dozes exceeding those recommended by the FDA for treatment of adult-onset (type 2) diabetes (discussed in [9,10]).
A strikingly high percentage of ND mutations map to the L0 linker of the TMD0-L0 gatekeeper module (Fig. 1) that couples the SUR1 core with the KATP pore and controls its nucleotide-independent POmax [12,13]. The first ND mutation found in the L0 linker, L213R, is in the middle of the hotspot region in the putative interface helix [14] and causes severe ND with neurological abnormalities [7]. We hypothesized that the mutation in the domain not required for nucleotide binding to SUR1 [12] hyperactivates KATP through a mechanism that is different from A type mechanism. Consistent with our hypothesis, the diabetogenic F132L in TMD0 of SUR1 increased KATP activity in the absence of nucleotides [15].
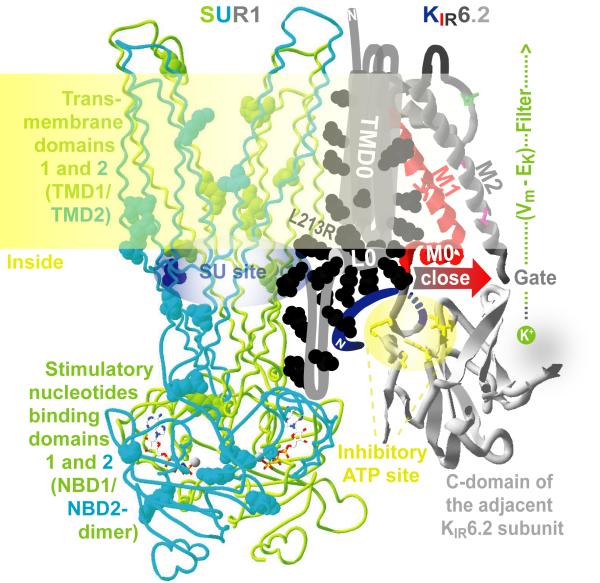
Fig. 1.
A working model of a KATP gating unit. One quarter of KATP complex is shown for clarity. ND ABCC8 mutations are mapped as balls. The SUR1 core is shown in a backbone wire presentation. Nucleotides are rendered in licorice style. KIR6.2 pore-forming domains are presented as ribbons. No high resolution structural template is available for the N-terminal domain of SUR1 or the KIR N-tail. L213R and other elements of the model discussed in the text are identified using color-coded labels. The model of SUR1/KIR6.2 coupling predicts the KIR-closing movement of L0 and M0 helices colocalized at the membrane-cytosol interface.
The present study validates the hypothesis and establishes the kinetic mechanism of KATP hyperactivity and reduced SU responsiveness.
2. Materials and methods
Genetic and clinical testing showing that the mutation caused ND by compromising insulin release was described elsewhere [7].
Homology models of the Sav1866-like human SUR1 core and the KirBac/KirChimera-like human KIR6.2 pore were built as described previously [11,13]. The mean helical hydrophobic moment was computed as described earlier [16].
Mutagenesis, sequencing, cell culture and transfections were done as described earlier [7,11]. Leu-213 is conserved in all SURs. L213R mutation was introduced into hamster SUR1 cDNA. SUR1L213R or WT receptor was expressed with human KIR6.2 and enhanced green fluorescent protein (a transfection marker) in COSm6 cells lacking any endogenous SUR or KIR.
Expression and SU binding/labeling of mature (complex-glycosylated, ~170 kDa) and immature (core-glycosylated, ~140 kDa) receptors were compared in SUR1L213R/KIR6.2- vs SUR1/KIR6.2-expressing cells’ membranes isolated and photolabeled with 125I-azidoglibenclamide as described previously [17]. The incorporation of 125I-azidoglibenclamide into different bands was estimated by densitometry of the autoradiographs and normalized to the membrane protein concentrations as described earlier [18].
Patch-clamp recording and single-channel kinetics analysis were done as described previously [7,11,19-21]. The pipette solution contained (in mM): 145 KCl; 1 MgCl2; 1 CaCl2; 10 HEPES; pH 7.4 (KOH). The multivalent cation-free internal solution contained (in mM): 140 KCl; 5 EDTA; 5 HEPES; 10 KOH; pH 7.2 (KOH). The bath intracellular solution contained (in mM): 140 KCl; 1 MgCl2; 5 EGTA; 5 HEPES; 10 KOH; pH 7.2 (KOH). The [Mg2+]i in nucleotide-containing solutions was kept at ~0.7 mM. The holding potential was −40 mV. COSm6 cells have negligible background currents, permitting measurements of virtually any low mean KATP currents, I, in the native-like environment of mammalian cell membranes. Analysis of currents allowed to verify the unitary current amplitude (i) in the cell-attached mode from all-points current amplitude histograms and determine the on-cell activity of N identical channels with the mean PO, N × PO = I × i−1. The Colquhoun-Hawkes test was used to evaluate the channel singularity. POmax determined from single-channel and multi-channel currents were similar. N from macrocurrent noise analysis reflected the density of functional channels in the plasma membrane. All-points dwell time destributions were used to determine mean life times of all kinetic states and rates of burst-interburst gating transitions.
Ligand responses of KATP currents were obtained using an automated multi-channel rapid solution changer as described previously [7,11,19-22]. To correct the ATP dose responses for partial rundown and/or refreshment of KATP currents the I value in the presence of each ATP concentration was normalized to the arithmetic mean of the I values before application of each [ATP] and after washout. Similar corrections were applied when estimating the steady-state activity in the presence of other ligands.
Statistical data analysis and curve fitting were done using OriginPro 8 (OriginLab Corporation, Northampton, MA) as described previously [11,22]. Averaged data were expressed as mean ± S.E. for n ≥ 5 with error bars equal to S.E. unless otherwise noted. Significance was evaluated using the unpaired t test. Differences with values of p < 0.05 were considered to be significant.
3. Results and discussion
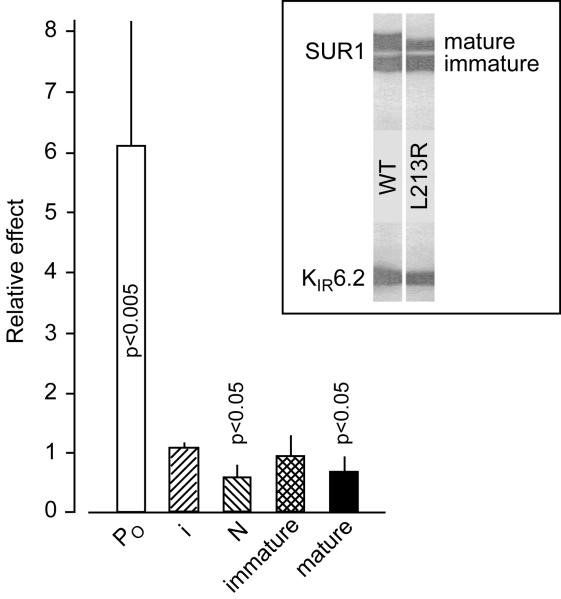
Fig. 2 shows that L213R dramatically increases the Po in intact cells while not affecting i and slightly decreasing N. The latter effect is consistent with the small negative effect of L213R on the amount of mature receptor, which is in line with observations that a comparable amphipathic L0 helix of ABCC1 attaches to the membrane [23] and L225P in a less conserved portion of the cytoplasmic linker of SUR1 does not affect N [24] or surface expression of SUR1 in the same cell line [25]. The results show that L213R can induce pathogenic currents in intact cells by hyperactivating KATP and support the notion that possible negative effects of some ND mutations on N (see also [25]) are overridden by their much stronger effect on PO.
Fig. 2.
L213R markedly elevates on-cell PO, does not affect the unitary conductance, and slightly decreases functional expression of (SUR1/KIR6.2)4 complexes without altering their labeling with 1 nM 125I-azidoglibenclamide. Inset shows similar affinity photolabeling of immature, core-glycosylated SUR1L213R vs SUR1 with the second generation SU, and modestly reduced amount of mature, complex-glycosylated mutants trafficking with colabeled KIR6.2. As was reported earlier, nanomolar glibenclamide or micromolar tolbutamide abolish labelling by displacing the photoreactive analogue of glibenclamide which does not label KIR6.2 expressed without SUR1 [17,18,26], the L0 loop apparently coordinates the photoreactive group-containing non-sulfonylurea moiety of the drug [22,27], and deleting the N-terminus of KIR6.2 eliminates its colabeling without disrupting KATP assembly [18] required for trafficking of either SUR1 or glycosylation site-free KIR6.2 to the plasma membrane [26,28]. Therefore, normal affinity labeling of SUR1L213R and KIR6.2, while slightly reduced amount of mature SUR1L213R, suggest that the mutation does not alter the SU binding site or the direct proximity of L0 to the KIR N-terminal helix, while slightly reducing KATP trafficking to the plasma membrane; n ≥ 5 for each bar.
To establish the principal mechanism of pathogenic increase in on-cell PO it is essential to determine if and how the mutation alters intrinsic gating kinetics (spontaneous bursting) and nucleotide inhibition vs Mg-nucleotide stimulation of the same population of channels before their significant rundown [11]. We obtained a significant number of single mutant and WT KATP records showing no significant rundown over minutes in the multivalent cation-free internal solution. Analysis of these records (Fig. 3) demonstrated that L213R nearly saturates the channel intrinsic activity, POmax → τO × (τO + τCs)−1 [12], by increasing TB and decreasing TIB, thereby limiting the availability of the long-lived, inhibitory nucleotide-/SU-stabilized [4,19,29,30] nonconductive C2 state. The analysis of long, continuous, >3 kHz low-pass filtered single-channel records was essential for determining highly significant mean life times of all kinetic states in each KATP and demonstrating that reciprocal changes in rates of transitions to and from the long-lived closed state is the mechanism of POmax saturation in the hyperactive KATP.
Fig. 3.
L213R saturates intrinsic activity of KATP by altering its slow gating kinetics. The top panel shows short segments of records of currents trough single WT and L213R KATP in inside-out patches in the nucleotide-free internal solution. Here and in the following figures, downward deflection of the current trace corresponds to inward current and the horizontal dashed line shows zero KATP current level. The next panels show the results of analysis of 10 L213R vs 10 WT KATP records like those shown in the top panel. A burst criterion was 2 ms. Each TIB reflected the slow tau determined from all-points closed time distribution fit, verifying no third, rundown-indicating component ([19,21], not shown here). The mutation-affected characteristics and transitions are boxed.
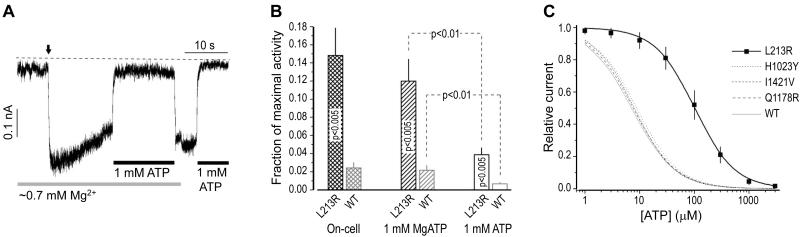
The mutation destabilizing the closed state with the Mg-independent micromoilar KD(ATP) [19,21,29] could attenuate ATP inhibition of macroscopic KATP currents without affecting their Mg-dependent nucleotide stimulation. To test this prediction we first compared the activities of the same macro-population of L213R channels in intact cells, in 1 mM MgATP, and in 1 mM ATP without Mg (Fig. 4A illustrates the protocol) vs similarly recorded activities of WT channels. We found (Fig. 4B) similarly elevated activities of mutant KATP on cells and in the quasi-physiologic submembrane [MgATP] and similarly significant similar-fold decreases in the activities of both channels upon Mg removal. The results suggested that L213R does not alter the Mg-nucleotide stimulatory action but compromises the Mg-independent nucleotide inhibition of KATP. Indeed, the steady-state inhibitory ATP dose-response (Fig. 4C) demonstrated that unlike A type mutations tested earlier under identical experimental conditions [7,11], L213R markedly (~20 times) increases IC50(ATP). Thus the mutation limiting the availability of C2 state opens KATP in the presence of high intracellular [ATP], even without MgADP. This biophysical mechanism of KATP hyperactivity, called B type mechanism for short, explains why pancreatic β-cells in the L213R patient did not release insulin (remained hyperpolarized) despite his very high blood glucose [7].
Fig. 4.
L213R compromises inhibitory nucleotide action. (A) L213R KATP currents under conditions indicated. The vertical arrow marks transition from the cell-attached (on-cell) to inside-out patch configuration in the intracellular solution. (B) The results from tests like in A (10 different cells for each KATP type). (C) The inhibitory ATP dose-response curve for L213R (IC50(ATP) = 101.7±4.1 μM; Hill coefficient = 1.19±0.04) vs those for WT and A type mutant KATP tested earlier under similar conditions (see [7,11] for details); n = 10 and R2 > 0.995 for each curve fit.
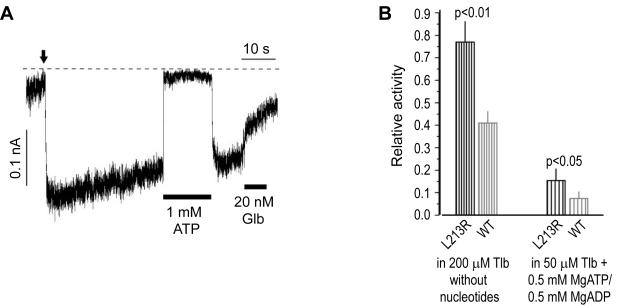
A dose of glibenclamide above the FDA recommended dose allowed to transfer the L213R patient from insulin injections to SU therapy [7]. We asked if the mutation attenuates KATP inhibition by SU despite essentially normal SU binding to SUR1L213R/KIR6.2 complexes (Fig. 2 inset legend). Our first test (Fig. 5A) indicated reduced inhibition of L213R channels by glibenclamide, but the slow washout of the second generation SU made it difficult to estimate its steady-state effect corrected for rundown (see Materials and methods and similar test for WT KATP in [22]). Therefore we used the rapidly unbinding SU tolbutamide to quantify the effect of L213R on KATP inhibition by [SU] saturating its specific, but not non-specific, sites [22,31]. We compared the SU inhibition of L213R vs WT KATP activity under non-stimulatory conditions, as well as in the presence of the lowest possible submembrane [MgATP] and the highest possible [MgADP] in resting β-cells [32,33], as we did earlier for A type mutant KATP [7,11]. We found that unlike A type mutations, L213R compromises SU inhibition under non-stimulatory conditions (Fig. 5B, left bars). While saturation of specific SU binding sites with 200 μM tolbutamide reduces spontaneous activity of WT KATP by about 60% [22,31], the same SU treatment of L213R channels produces significantly smaller inhibition. Thus, L213R alters the nucleotide-independent component of SU action by uncoupling its high-affinity binding from the channel closure. Fortunately for ND patients, even lower [SU] significantly decreased the activity of B type channels in Mg-nucleotides (Fig. 5B, right bars). This is consistent with findings that specific SU binding releases stimulatory nucleotides, thus abolishing KATP stimulation [22,34]. This effect unmasks the inhibitory action of nucleotides less effectively in B type mutant channels because of their increased IC50(ATP). Thus, the mechanism of reduced SU responsiveness is fundamentally linked to the mechanism of KATP hyperactivity.
Fig. 5.
L213R attenuates inhibition of KATP by sulfonylureas. (A) L213R KATP currents indicating their reduced response to the normally (tightly) binding, slowly dissociating, insulin secretagogue glibenclamide (Glb). Electrophysiological conditions are like in Fig. 4. The vertical arrow marks patch excision in the intracellular solution. (B) The steady-state inhibition of L213R vs WT KATP by tolbutamide (Tlb) under conditions indicated; n = 10 for each bar.
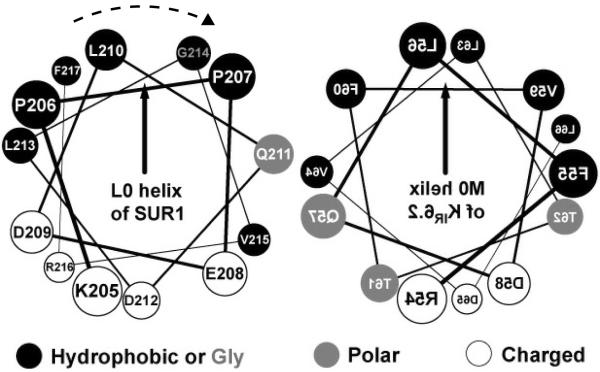
The established kinetic mechanism of ND-SUR1/Kir6.2 hyperactivity and SU tolerance is consistent with early findings that SUR1/ΔNKir6.2 channels with increased spontaneous activity show decreased sensitivity to ATP and SU [21,22,35-37] and inhibit insulin secretion in mice [38]. These and additional studies using chimeric SUR [20], N-terminal Kir6.2 peptides [18], and TMD0-L0 fragments [12] led us to the conclusion that TMD0-L0/M0-M1 interactions play key roles in KATP assembly and gating [13]. Consistent with the proposal, F132L in TMD0 and activating mutations in M0 alter intrinsic gating [15,39,40]. Strong effects of L213R in L0 helix on TB/TIB and KATP inhibition provide a new evidence in support of our working mechanistic model (Fig. 1). Only subtle and insignificant functional effects of E208K [25] are not surprising. Unlike L213R, E208K can be carried asymptomatically [41,42] and exchanges similarly hydrophilic side chains on the hydrophilic side of the submembrane amphipathic helix (Fig. 6). Normal POmax and ATP inhibition of SUR1L225P/Kir6.2 channels [24,25] are consistent with the prediction that a relatively short [14] interface L0 helix is a key element of the SUR1/Kir6.2 inhibitory machinery. So, it is tempting to speculate that L0 helix interacts with the equidistant [43] M0 “slide” [44] helix (Fig. 6) to lock the ligand-sensitive gate (Fig. 1). The mechanistic interpretation is consistent with homologous structures [44-48] and single particle electron microscopy analysis of (SUR1-KIR6.2)4 [49] verifying that TMD0-L0 gatekeepers can fit between the KIR tetramer and four SUR1 cores and thus might transduce signals from the receptor core to the pore [12,13]. A similar L0 helix was shown to interact with the conserved core in ABCC1 [23], and we provided biochemical (labeling) evidence for the close proximity of ABCC8 L0 to the N-terminal domain of KIR6.2 in inhibited KATP [18]. L213R does not alter the direct proximity of L0 to Kir6.2 (Fig. 2 inset), but could disrupt optimized L0/M0 interactions by rotating the L0 helix along its axis (Fig. 6) as L213R changes the helical hydrophobic moment. The paradigm of evolutionary optimized L0/Kir6.2 interactions provides a basic explanation for the high concentration of spontaneous ND mutations in L0. Although our mechanistic model reconciles available data, it needs to be refined in additional studies.
Fig. 6.
Helical wheel plots of L0 and M0 helices. The two helices are in an antiparallel orientation and their matching hydrophobic moments (vectors) point toward the membrane. The expected L213R-induced rotation of L0 helix is indicated.
Further functional analyses of new ND mutations in different parts of the TMD0-L0 gatekeeper module should shed more light on functional coupling between SUR1 and Kir6.2 and clarify if most of these mutations, causing ~40% of ND-ABCC8 cases, overactivate KATP via B type mechanism. Previous studies showed that ND mutations in every major domain of the ABC core of SUR1 can increase KATP PO via A type mechanism (reviewed in [9,10]; see also [25] on V324M in TMD1). As we continue our search for the third, or C, type diabetogenic mutations altering the SUR1-induced decrease in the KD for inhibitory ATP [19], it becomes evident that A and B mechanisms underlie the majority of ND-ABCC8 cases. This can explain why most ND-ABCC8 patients require body weight-normalized SU doses exceeding those prescribed to type 2 diabetes patients. Pharmacological testing of new ND-ABCC8/KCNJ11 recombinants will help evaluate this argument and identify SU resistant ND cases requiring alternative personalized therapeutic approaches.
Diabetogenic L213R in SUR1 L0 hyperactivates KATP by destabilizing its closed state
This kinetic mechanism attenuates KATP inhibition by ATP and sulfonylureas
Our analysis reveals a key role for L0 helix in controlling KATP gating
Acknowledgments
This work was supported by National Institutes of Health Grant DK077827 (to A.P.B.). We thank Adrian Sculptoreanu and Guiling Zhau for technical assistance. We are grateful to Dr. Joseph Bryan (Pacific Northwest Research Institute, Seattle, WA) for his advice on labeling. We thank Dr. Lydia Aguilar-Bryan (PNRI) and Drs. Philippe Froguel, Michel Polak, Hélène Cave, Kanetee Bushiah, Paul Chernichow and Raphael Sharfmann (members of the French Network for the Study of Neonatal Diabetes Mellitus) for encouragement. We also thank the Department of Defense and the Pacific Northwest Research Foundation for support.
Abbreviations
- SUR1
sulfonylurea receptor 1
- KATP
(SUR1/Kir6.2)4 channel
- ND
neonatal diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- [2].Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, IV, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- [3].Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- [4].Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- [5].Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- [6].Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- [7].Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- [8].Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum. Mol. Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- [9].Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr. Rev. 2008;29:265–291. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev. Endocr. Metab. Disord. 2010;11:193–198. doi: 10.1007/s11154-010-9149-x. [DOI] [PubMed] [Google Scholar]
- [11].Babenko AP. A novel ABCC8 (SUR1)-dependent mechanism of metabolism-excitation uncoupling. J. Biol. Chem. 2008;283:8778–8782. doi: 10.1074/jbc.C700243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Babenko AP, Bryan J. SUR domains that associate with and gate KATP pores define a novel gatekeeper. J. Biol. Chem. 2003;278:41577–41580. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- [13].Babenko AP. KATP channels “vingt ans apres”: ATG to PDB to Mechanism. J. Mol. Cell. Cardiol. 2005;39:79–98. doi: 10.1016/j.yjmcc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [14].Granseth E, von Heijne G, Elofsson A. A study of the membrane-water interface region of membrane proteins. J. Mol. Biol. 2005;346:377–385. doi: 10.1016/j.jmb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- [15].Proks P, Shimomura K, Craig TJ, Girard CA, Ashcroft FM. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum. Mol. Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. [DOI] [PubMed] [Google Scholar]
- [16].Eisenberg D, Weiss RM, Terwilliger TC. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 1982;299:371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- [17].Sharma N, Crane A, Clement JP, IV, Gonzalez G, Babenko AP, Bryan J, Aguilar-Bryan L. The C terminus of SUR1 is required for trafficking of KATP channels. J. Biol. Chem. 1999;274:20628–20632. doi: 10.1074/jbc.274.29.20628. [DOI] [PubMed] [Google Scholar]
- [18].Babenko AP, Bryan J. SUR-dependent modulation of KATP channels by an N-terminal KIR6.2 peptide: Defining intersubunit gating interactions. J. Biol. Chem. 2002;277:43997–44004. doi: 10.1074/jbc.M208085200. [DOI] [PubMed] [Google Scholar]
- [19].Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Sulfonylurea receptors set the maximal open probability, ATP sensitivity and plasma membrane density of KATP channels. FEBS Lett. 1999;445:131–136. doi: 10.1016/s0014-5793(99)00102-7. [DOI] [PubMed] [Google Scholar]
- [20].Babenko AP, Gonzalez G, Bryan J. Two regions of sulfonylurea receptor specify the spontaneous bursting and ATP inhibition of KATP channel isoforms. J. Biol. Chem. 1999;274:11587–11592. doi: 10.1074/jbc.274.17.11587. [DOI] [PubMed] [Google Scholar]
- [21].Babenko AP, Gonzalez G, Bryan J. The N-terminus of KIR6.2 limits spontaneous bursting and modulates the ATP-inhibition of KATP channels. Biochem. Biophys. Res. Commun. 1999;255:231–238. doi: 10.1006/bbrc.1999.0172. [DOI] [PubMed] [Google Scholar]
- [22].Babenko AP, Gonzalez G, Bryan J. The tolbutamide site of SUR1 and a mechanism of its functional coupling to KATP channel closure. FEBS Lett. 1999;459:367–376. doi: 10.1016/s0014-5793(99)01215-6. [DOI] [PubMed] [Google Scholar]
- [23].Bakos E, Evers R, Calenda G, Tusnady GE, Szakacs G, Varadi A, Sarkadi B. Characterization of the amino-terminal regions in the human multidrug resistance protein (MRP1) J. Cell. Sci. 2000;113(Pt 24):4451–4461. doi: 10.1242/jcs.113.24.4451. [DOI] [PubMed] [Google Scholar]
- [24].Masia R, De Leon DD, MacMullen C, McKnight H, Stanley CA, Nichols CG. A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM) Diabetes. 2007;56:1357–1362. doi: 10.2337/db06-1746. [DOI] [PubMed] [Google Scholar]
- [25].Zhou Q, Garin I, Castano L, Argente J, Munoz-Calvo MT, de Nanclares G. Perez, Shyng SL. Neonatal diabetes caused by mutations in sulfonylurea receptor 1: interplay between expression and Mg-nucleotide gating defects of ATP-sensitive potassium channels. J. Clin. Endocrinol. Metab. 2010;95:E473–478. doi: 10.1210/jc.2010-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clement JP, IV, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- [27].Mikhailov MV, Mikhailova EA, Ashcroft SJ. Molecular structure of the glibenclamide binding site of the beta-cell KATP channel. FEBS Lett. 2001;499:154–160. doi: 10.1016/s0014-5793(01)02538-8. [DOI] [PubMed] [Google Scholar]
- [28].Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- [29].Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J. Physiol. (Lond.) 1987;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gillis KD, Gee WM, Hammoud A, McDaniel ML, Falke LC, Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am. J. Physiol. 1989;257:C1119–1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- [31].Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- [32].Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, Henquin JC. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J. Biol. Chem. 1998;273:33905–33908. doi: 10.1074/jbc.273.51.33905. [DOI] [PubMed] [Google Scholar]
- [33].Fridlyand LE, Ma L, Philipson LH. Adenine nucleotide regulation in pancreatic beta-cells: modeling of ATP/ADP-Ca2+ interactions. Am. J. Physiol. Endocrinol. Metab. 2005;289:E839–848. doi: 10.1152/ajpendo.00595.2004. [DOI] [PubMed] [Google Scholar]
- [34].Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koster JC, Sha Q, Shyng S, Nichols CG. ATP inhibition of KATP channels: control of nucleotide sensitivity by the N-terminal domain of the Kir6.2 subunit. J. Physiol. (Lond.) 1999;515:19–30. doi: 10.1111/j.1469-7793.1999.019ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koster JC, Sha Q, Nichols CG. Sulfonylurea and K+-channel opener sensitivity of KATP channels. Functional coupling of Kir6.2 and SUR1 subunits. J. Gen. Physiol. 1999;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reimann F, Tucker SJ, Proks P, Ashcroft FM. Involvement of the N-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J. Physiol. (Lond.) 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell KATP channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- [39].Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- [41].Vaxillaire M, Dechaume A, Busiah K, Cave H, Pereira S, Scharfmann R, de Nanclares GP, Castano L, Froguel P, Polak M. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56:1737–1741. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- [42].Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Edmonds DT. The alpha-helix dipole in membranes: a new gating mechanism for ion channels. Eur. Biophys. J. 1985;13:31–35. doi: 10.1007/BF00266307. [DOI] [PubMed] [Google Scholar]
- [44].Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- [45].Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- [46].Dawson RJ, Locher KP. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. [DOI] [PubMed] [Google Scholar]
- [47].Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified K(ATP) channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]