Dear Editor:
The Gardos channel inhibitor senicapoc is proven to diminish haemolytic anaemia in a Phase II study conducted in adults with sickle cell anaemia (Ataga, et al 2008), and later confirmed in a Phase III study in sickle cell disease subjects as reported in a recent article in the British Journal of Haematology (Ataga, et al 2011).
Brain natriuretic peptide is a hormone secreted by ventricular cardiomyocytes in response to stretch (Levin, et al 1998). The plasma level of its propeptide (NT-proBNP) provides a convenient biomarker of cardiac stress, correlating in sickle cell subjects with pulmonary hypertension proven by pulmonary artery catheterization, or estimated by noninvasive Doppler echocardiography (Machado, et al 2006). Increased NT-proBNP in sickle cell adults is associated with lower haemoglobin, high serum lactate dehydrogenase (LDH) and other laboratory variables (Machado, et al 2006, Mekontso Dessap, et al 2008, Mokhtar, et al 2010, van Beers, et al 2008, Voskaridou, et al 2007).
Because senicapoc is proven to increase haemoglobin and decrease LDH in sickle cell anaemia (Ataga, et al 2008), we hypothesized that NT-proBNP levels would fall in sickle cell anaemia subjects who respond to senicapoc treatment with increased haemoglobin level. We obtained local regulatory approval to study coded plasma samples from the 2008 Phase II senicapoc trial (Ataga, et al 2008). We procured archived plasma samples from that trial, and measured NT-proBNP by the same standard clinical laboratory assay we have previously reported in sickle cell anaemia (Machado, et al 2006). In the 53 treated subjects, NT-proBNP levels declined by a nonsignificant degree. However, when the analysis was restricted to the 35 subjects (66%) who responded to senicapoc with a rise in total Hb of at least 5 gm/L, significant changes were observed in NT-proBNP and other markers. Subjects who responded to 6mg daily or 10mg daily were analyzed together as a single group.
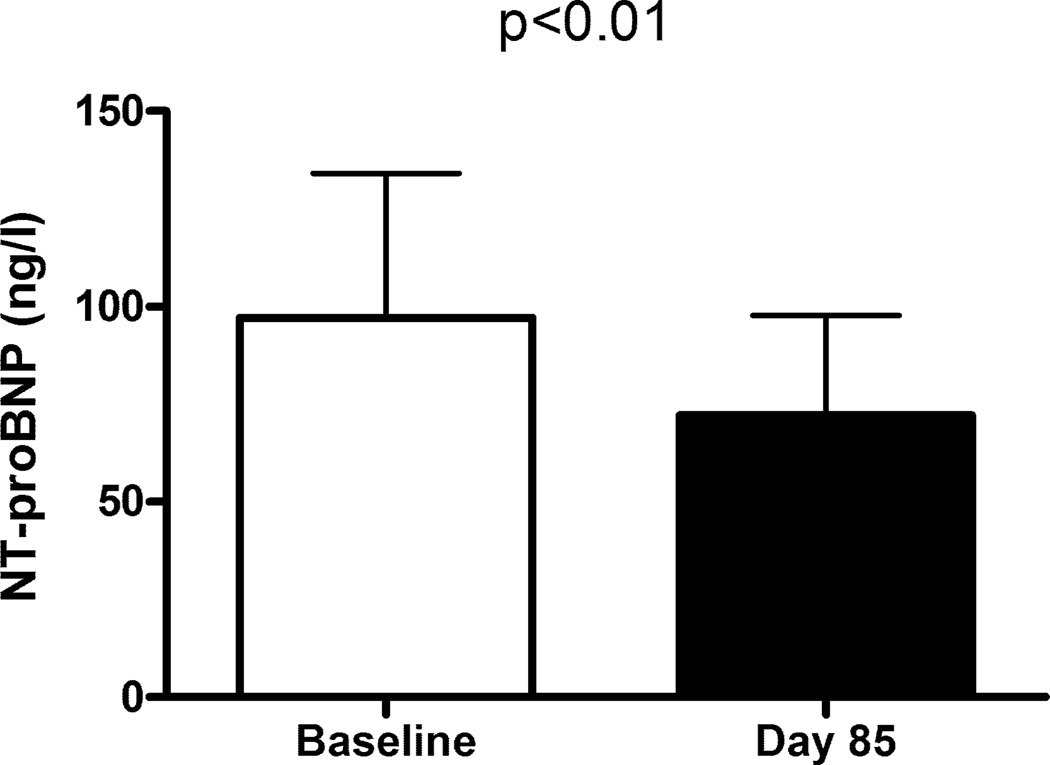
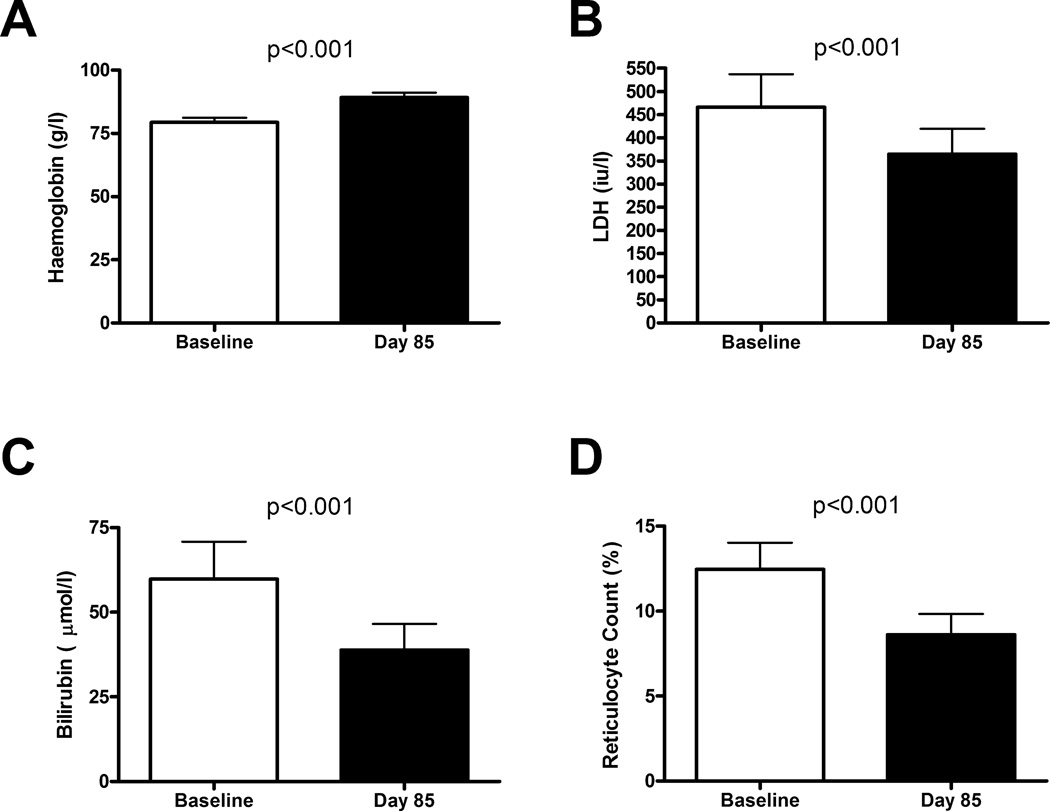
Among these 35 senicapoc responders, baseline NT-proBNP fell by 26% (geometric mean 97 vs. 72 ng/l, p<0.01, paired t-test, log transformed values; Fig. 1). Haemoglobin levels increased by 13% (mean 79 ± 2 vs. 89 ± 2 gm/L, mean ± SEM, p<0.001, paired t-test; Fig. 2A), as expected in this group defined by senicapoc-induced rise in haemoglobin. Serum LDH levels fell 22% (geometric mean 466 vs. 365 iu/l, p<0.001, paired t-test, log transformed values; Fig. 2B), serum bilirubin levels dropped 35% (67.2 vs. 44.5 µmol/l, p<0.001; Fig. 2C), and percentage reticulocytes declined (12.5 vs. 8.6%, p<0.001; Fig. 2D). These results suggest that senicapoc simultaneously diminished both haemolysis and NT-proBNP in a subgroup of subjects.
Figure 1.
NT-proBNP levels before and after senicapoc therapy. White bar indicates baseline plasma NT-proBNP level prior to initiating oral senicapoc therapy in 35 adults with sickle cell anaemia. The black bar indicates plasma NT-proBNP level on day 85 of senicapoc therapy in the same subjects. Bars indicate geometric mean and error bars indicate 95% confidence interval. P value was calculated by paired t-test on log-transformed values. Analysis included only subjects whose haemoglobin level rose by at least 5 g/l on senicapoc therapy.
Figure 2.
Indicators of haemolytic severity before and after senicapoc therapy. White bars indicate baseline values prior to initiating oral senicapoc therapy in 35 adults with sickle cell anaemia. The black bars indicate values on day 85 of senicapoc therapy in the same subjects. (A) Haemoglobin (bars indicate mean values, and error bars indicate standard error of the mean; p value calculated by paired t-test); (B) Serum lactate dehydrogenase (LDH), (C) Serum total bilirubin, (D) Reticulocyte count. Bars indicate geometric mean and error bars indicate 95% confidence interval; p value was calculated by paired t-test on log transformed values. Analysis included only subjects whose haemoglobin level rose by at least 5 g/l on senicapoc therapy.
A limitation in our study is that only two-thirds of these senicapoc subjects had a sufficient increase in haemoglobin to observe the linked decrease in NT-proBNP, and inclusion of the senicapoc nonresponders obscures this NT-proBNP change. This study was also not prospectively designed to determine the efficacy of senicapoc in reducing NT-proBNP, and the results should be interpreted with caution. However, the findings support important concepts linking haemolytic anaemia to elevated pulmonary arterial pressure. A similar decline in NT-proBNP also has been reported in patients with another chronic haemolytic anaemia, paroxysmal nocturnal hemoglobinuria, in whom hemolysis was reduced by use of the drug eculizumab (Hill, et al 2010). We also have reported downward trends in NT-proBNP and significant decline in pulmonary artery systolic pressure estimated by Doppler echocardiography in several sickle cell anaemia patients in whom hydroxycarbamide produced robust increase in foetal haemoglobin and significant reduction in serum LDH (Olnes, et al 2009). Severity of chronic haemolytic anaemia is linked to high levels of the functional cardiac marker NT-proBNP, and three mechanistically different pharmacological interventions that attenuate intravascular hemolysis also reduced NT-proBNP in these two pathologically distinct haemolytic anaemias.
Our result suggests that reducing hemolysis in sickle cell anaemia results in lower NT-proBNP, previously shown to serve in this population more as a biomarker of mean pulmonary artery pressure than of left ventricular dysfunction (Machado, et al 2006). Additional research directed at reducing hemolysis is warranted as a potential means to reduce NT-proBNP and improve pulmonary hypertension in sickle cell disease.
Acknowledgments
The authors are grateful to Mary K. Hall for regulatory support. The NIH authors are supported by research funds from the Division of Intramural Research of the National Heart, Lung and Blood Institute at the National Institutes of Health (1ZIAHL006014).
Footnotes
Conflict of Interest
Greg C. Rigdon is an employee of Icagen, Inc., which provided the blood specimens. Jonathan W. Stocker is a consultant to the pharmaceutical industry and was employed by Icagen during this senicapoc trial. The authors declare no other conflict of interest.
Authorship
CPM and GJK conceived the study, obtained local regulatory approvals and wrote the manuscript. GCR and JWS coordinated the senicapoc clinical trial, obtained regulatory approval for this study, provided coded plasma specimens and linked laboratory data from the Icagen archives. LM coordinated specimen transfer and assays. AR supervised NT-proBNP assays. JW and GJK performed statistical analysis. Each author had access to the data and played a role in editing the final manuscript.
References
- Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH, Stocker JW. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153:92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC, Stocker JW. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- Hill A, Rother RP, Wang X, Morris SM, Jr, Quinn-Senger K, Kelly R, Richards SJ, Bessler M, Bell L, Hillmen P, Gladwin MT. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2010;149:414–425. doi: 10.1111/j.1365-2141.2010.08096.x. [DOI] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, Hunter L, Barton B, Waclawiw M, Castro O, Gladwin MT. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, Godeau B, Galacteros F, Brun-Buisson C, Brochard L, Maitre B. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177:646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- Mokhtar GM, Adly AA, El Alfy MS, Tawfik LM, Khairy AT. N-terminal natriuretic peptide and ventilation-perfusion lung scan in sickle cell disease and thalassemia patients with pulmonary hypertension. Hemoglobin. 2010;34:78–94. doi: 10.3109/03630260903554621. [DOI] [PubMed] [Google Scholar]
- Olnes M, Chi A, Haney C, May R, Minniti C, Taylor Jt, Kato GJ. Improvement in hemolysis and pulmonary arterial systolic pressure in adult patients with sickle cell disease during treatment with hydroxyurea. Am J Hematol. 2009;84:530–532. doi: 10.1002/ajh.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers EJ, Nur E, Schaefer-Prokop CM, Mac Gillavry MR, van Esser JW, Brandjes DP, Kappers-Klunne MC, Duits AJ, Muskiet FA, Schnog JJ, Biemond BJ. Cardiopulmonary imaging, functional and laboratory studies in sickle cell disease associated pulmonary hypertension. Am J Hematol. 2008;83:850–854. doi: 10.1002/ajh.21272. [DOI] [PubMed] [Google Scholar]
- Voskaridou E, Tsetsos G, Tsoutsias A, Spyropoulou E, Christoulas D, Terpos E. Pulmonary hypertension in patients with sickle cell/beta thalassemia: incidence and correlation with serum N-terminal pro-brain natriuretic peptide concentrations. Haematologica. 2007;92:738–743. doi: 10.3324/haematol.11136. [DOI] [PubMed] [Google Scholar]