Abstract
The distribution and potential bioavailability of polycyclic aromatic hydrocarbons (PAHs) in soil from a former manufactured-gas plant (MGP) site were examined before and after long-term biostimulation under simulated in situ conditions. Treated soil was collected from the oxygenated zones of two continuous-flow columns, one subjected to biostimulation and the other serving as a control, and separated into low- and high-density fractions. In the original soil, over 50% of the total PAH mass was associated with lower-density particles, which comprised < 2% of the total soil mass. However, desorbable fractions of PAHs were much lower in the low-density material than in the high-density material. After over 500 d of biostimulation, significant removal of total PAHs occurred in both the high- and low-density materials (77% and 53%, respectively), with three- and four-ring PAHs accounting for the majority of the observed mass loss. Total PAHs that desorbed over a 28-d period were substantially lower in treated soil from the biostimulated column than in the original soil for both the high-density material (23 versus 63%) and low-density material (5 versus 20%). The fast-desorbing fractions quantified by a two-site desorption model ranged from 0.1 to 0.5 for most PAHs in the original soil but were essentially zero in the biostimulated soil. The fast-desorbing fractions in the original soil underestimated the extent of PAH biodegradation observed in the biostimulated column, and thus was not a good predictor of PAH bioavailability after long-term, simulated in situ biostimulation.
Keywords: Polycyclic aromatic hydrocarbons, Bioavailability, Desorption, Kinetics, Two-site model
Introduction
Polycyclic aromatic hydrocarbon (PAH)-contaminated soils at former manufactured gas plant (MGP) sites are a complex mixture of soil organic matter, humic substances, inorganic minerals, and various waste residues from past gas operations including pyrogenic residues, oils, tars, and other nonaqueous-phase liquids [1, 2]. Sorption of PAHs to these various compartments can greatly influence overall PAH transport, degradation, and bioavailability and, therefore, ultimately governs the success of bioremediation strategies [3, 4]. Polycyclic aromatic hydrocarbon bioavailability is commonly equated with the amount of a given PAH that can be desorbed relatively rapidly from a solid-phase or nonaqueous compartment to the aqueous phase, where the compound can be accessed by indigenous PAH-degrading bacteria [5]. However, the distribution and bioavailability of PAHs varies among the soil compartments, reflecting differences in compound hydrophobicity and sorption capacities of the soil domains. Soil organic matter and other natural and anthropogenic domains such as residual coal tar, pitch, coke, soot, coal, and lampblack have been identified as important reservoirs for sorption of PAHs in MGP soils [6–9] and tend to exhibit significantly lower PAH availability than mineral particles [10].
Desorption of PAHs in natural soils is generally biphasic, with an initial phase of rapid PAH release followed by a longer period of slow PAH desorption. During the initial phase, the bioavailability of PAHs is high and degradation rates may be limited by microbial processes; however, as available PAHs are consumed, mass transfer mechanisms such as desorption and diffusion gain importance, becoming critical factors in defining PAH bioavailability [11]. Polycyclic aromatic hydrocarbons that are associated with compartments characterized by slow release to the aqueous phase have been regarded as largely unavailable for microbial activity [12, 13], although evidence of repartitioning from slow- to fast-desorbing domains has been documented [14]. A number of physical [5, 15–17], chemical [18–23], and biological [13, 24] techniques have been proposed for estimating the bioavailability of PAHs and other hydrophobic organic chemicals in field-contaminated soils. Solid-phase extraction is one of the more common estimation methods, in which polymeric adsorbent resins (such as Tenax TA and Amberlite XAD) function as an infinite sink, maintaining a steep concentration gradient between the aqueous and solid phases for maximum desorption [25].
An empirical model is most commonly used to quantify the biphasic nature of PAH desorption [26], which assumes that desorption occurs from two compartments (or two sites) in the soil defined by fast and slow rates, each following first-order kinetics [5, 27, 28]. The rapidly desorbing fraction is often defined as the bioavailable fraction and has been used successfully to predict the extent of PAH degradation in field-contaminated sediments [15, 17, 24]; however, other studies have found that the rapidly desorbing fraction underestimates the extent of biodegradation [5, 29]. Although the two-site model output parameters are empirical and have no direct mechanistic relationship with physical compartments of the soil matrix [5], they have proven valuable in quantifying variations in PAH bioavailability between different soils and sediments [30, 31] and under different treatment conditions [5, 15, 31].
The effects of bioremediation on PAH distribution and bioavailability in field-contaminated soils and sediments has been well-documented in a variety of ex-situ treatment systems [5, 7, 15, 20, 32, 33]. However, only a few of these studies have examined variations in PAH bioavailability among different soil fractions after treatment [7, 33] and even fewer have investigated these effects in situ [20]. The present study examines the effects of long-term in situ biostimulation on PAH distribution and potential bioavailability in contaminated soil from a former MGP site. Two continuous-flow columns packed with MGP soil were operated for over 500 d; one column was subjected to continuous biostimulation and the other served as a control. Samples of the original and treated soils were density-separated into primarily carbonaceous (lower-density) and primarily mineral (higher-density) fractions to evaluate the impact of biostimulation on PAH distribution within these fractions and to estimate the contribution of each fraction to overall PAH desorption.
Materials and Methods
Chemicals
Acetone (>99.5%), acetonitrile (>99.9%), dichloromethane (>99.5%), methanol (>99%), cesium chloride (>99%), sodium azide, and sodium sulfate (>99%) were purchased from Fisher Scientific. Anthracene-d10 (98%) was purchased from Cambridge Isotope Laboratories.
Soil
Contaminated soil was collected from a former MGP site in Salisbury, NC, USA in the vicinity of the former tar well at a depth of 4 ft below ground surface and processed according to the method outlined in the Supplemental Data. The processed soil contained 83% sand, 14% silt, and 3% clay, with total organic matter of 8.3% and extractable organic matter of 0.64%. Total PAH concentration (sum of 13 of the 16 EPA-regulated PAHs) was 295 ± 65 mg/kg dry soil (n = 33), with phenanthrene comprising 44% of the total PAH mass (Figure 1). In subsequent discussion, the processed soil is referred to as column soil. The column soil was demonstrated to contain bacteria capable of growing on a range of PAHs from two to four rings [34].
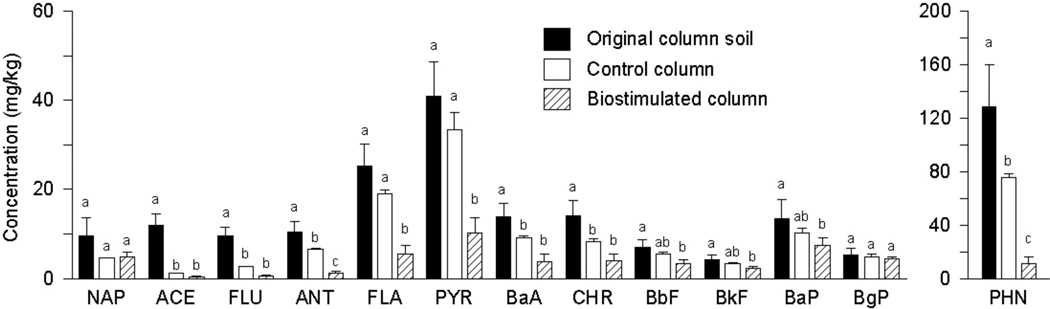
Figure 1.
Polycyclic aromatic hydrocarbons concentrations in the original (untreated) column soil and in samples collected from Port A of the control and biostimulated columns at day 534. Note that phenanthrene concentration is plotted separately. Error bars represent one standard deviation. The letters above the bars represent the results of significance analyses using the Tukey-Kramer Honestly Significant Difference (HSD) test. For each analyte, conditions sharing a common letter are not significantly different. Abbreviations: NAP – naphthalene, ACE – acenaphthene, FLU – fluorene, PHN – phenanthrene, ANT – anthracene, FLA – fluoranthene, PYR – pyrene, BaA – benz[a]anthracene, CHR – chrysene, BbF – benzo[b]fluoranthene, BkF – benzo[k]fluoranthene, BaP – benzo[a]pyrene, BgP – benzo[ghi]perylene.
Experimental Design
Details of the column design and operation are presented in the Supplemental Data. Briefly, two 110-cm long, 10.2-cm diameter (outer diameter) stainless-steel columns, each containing a 100-cm zone of column soil, were operated for approximately 780 d at 20°C, receiving a continuous supply of simulated groundwater in a downward flow direction. One of the columns was subjected to biostimulation by amending the simulated groundwater with pure oxygen, nitrogen (1.0 mg/L final concentration) and phosphorus (0.3 mg/L final concentration); the second column served as a control, receiving air-saturated, unamended groundwater. Both columns were run under control conditions for eight months before biostimulation was initiated in one of the columns (equilibration phase). Each column was equipped with three ports for soil sample collection, along with nine additional smaller ports for monitoring porewater dissolved oxygen concentrations.
As part of a larger study [35], soil samples were collected from the surface soil and Ports A, B, and C of the control and biostimulated columns immediately after the equilibration phase (t = 0) and 31, 93, 184, 380, and 534 d after biostimulation commenced. For the present study, samples from the control and biostimulated columns were collected from Port A, located 30 cm below the top of each column. Port A was selected because the dissolved oxygen concentration was saturated at this depth in both columns at the time of sampling.
Desorption experiments, soil extraction, and quantification of PAHs were conducted on the column soil (untreated) and treated soil from the control and biostimulated columns 534 d after biostimulation commenced (subsequently referred to as day 534). Additional soil was collected from both columns at day 593 for density separation into two soil fractions: the bulk mineral fraction (primarily sand, silt, and clays) and carbon-rich particles [36], defined as high-density and low-density material, respectively. Based on trends in PAH removal over the entire time course (data not shown), we do not believe that there would have been large differences in soil samples between day 534 and day 593.
Density Separation
A cesium chloride solution (specific gravity 1.8) was used to separate the low-density carbonaceous particles and wood fragments from the high-density, bulk mineral fraction of the column soil and treated soils removed from the columns on day 593 [8, 36]. Aliquots (10 g wet weight) of each soil sample were distributed into nine 30-ml centrifuge vials and combined with 20 ml of the cesium chloride solution. Contents of the vials were shaken vigorously for 24 to 48 h and centrifuged for 15 min at 2,800 g to divide the soil into the high- and low-density fractions. The floating, low-density material was transferred to a 0.2 µm pore-size nylon filter using a stainless-steel spatula, rinsed several times with reagent water to remove residual cesium chloride, and placed in a desiccator to dry; volatilization of PAHs during sample storage was minimal (< 0.03% of total PAH mass), as determined with a Tenax trap placed in the desiccator. The above steps were repeated three to five times or until low-density material was no longer visible at the top of the vial after centrifugation. All low-density material from a given soil sample that was collected on the filters was combined in an aluminum pan and weighed. Prior to desorption and PAH analyses, the low-density material for each sample was lightly ground using a mortar and pestle to ensure uniformity among replicates. The remaining high-density material was also rinsed with reagent water, vigorously mixed, and centrifuged several times to remove residual cesium chloride. Subsamples of the high-density material were used to calculate moisture content by loss of weight at 105°C for 24 h. Moisture content of the low-density material was not determined due to its low overall mass; however, we assumed that water content was minimal after extended desiccation. For the column soil and treated soils, PAH mass recovered in the whole samples removed from the columns on day 534 was compared to the sum of the respective PAH masses in the high- and low-density materials in the samples obtained on day 593.
Desorption
Tenax TA® polymeric adsorbent beads (Alltech) were used as an infinite sink to continuously uptake dissolved PAHs and establish a steep concentration gradient between the soil and aqueous phases. Prior to use, Tenax beads were cleaned in 50:50 acetone:hexane for 12 to 16 h (Soxhlet extraction), rinsed with methanol, and air-dried overnight. Five-gram (wet weight) aliquots of the column soil and treated samples obtained on day 534 were placed into triplicate 30-ml glass centrifuge vials for desorption experiments and another set of triplicate vials for initial PAH analyses (six vials per condition). To each desorption replicate was added 20 ml of simulated groundwater (without nitrogen or phosphorus amendment), 0.2 ml of 330 g/L sodium azide to inhibit biological activity, and 0.1 g of clean Tenax beads. The vials were sealed with screw-top caps (with Teflon-lined septa), covered in aluminum foil to eliminate light exposure, and placed on a wrist-action shaker at room temperature (19 to 23°C). After 1, 3, 6, 14, and 28 d of continuous shaking, the vials were centrifuged for 15 min at 2,800 g and allowed to stand for 1 to 2 h for separation of the beads and soil particles. The beads were removed by raising the water surface in each vial with sterile-filtered reagent water to access the floating beads. Once the beads were removed, the supernatant was carefully decanted and the vial replenished with simulated groundwater, sodium azide, and new beads and returned to the shaker. The 28-d desorption period is beyond the time frame of 7 to 12 d recommended [37] to capture the presumptive bioavailable fraction of PAHs in field-contaminated soils and sediments.
For each time point, the beads were retrieved using a stainless-steel spatula, transferred to a clean centrifuge tube containing reagent water, and vortexed briefly to detach any residual soil particles from the beads; any settled material collected during this bead washing step was returned to the original desorption vial. The beads were then transferred to 15 ml amber screw-top vials containing methanol and shaken for 24 h. Each methanol extract was filtered through a 0.2 µm pore-size nylon filter (Millipore) and transferred to a 25-ml volumetric flask for subsequent high-performance liquid chromatography analysis. In preliminary experiments, the mass recovery of beads for this method was 96 ± 2 %. Following the last time point (t = 28 d), the residual PAH concentration in the desorbed soil replicates was determined to evaluate total PAH recovery at the end of the desorption experiment. A mass balance for each analyte was assessed by comparing the initial PAH mass of the soil sample to the sum of the residual mass and cumulative mass desorbed.
The same desorption procedure described above was used for the high-density material of the column soil and treated soil samples obtained on day 593. Because of the limited mass of low-density material, only 0.2 g (dry weight) was added to duplicate desorption incubations, along with 1 g of Tenax beads to account for the higher organic content of this fraction [36]. Desorption studies on the low-density material of the column soil were extended to 58 d to determine the effect of longer desorption time periods on overall PAH release.
Chemical Analyses
Soil samples and density-separated materials were extracted with dichloromethane and acetone, and PAHs were quantified by high-performance liquid chromatography as outlined elsewhere [38], with the following exceptions: sodium sulfate was not added to the low-density extractions because solvent-water interferences were expected to be minimal following desiccation; and residual low-density material (after the desorption period) was dried at 60°C overnight to eliminate residual water prior to extraction. A third extraction step (with an additional 10 ml each of dichloromethane and acetone) was performed for the low-density material to account for the higher PAH and organic carbon content; however, the improvement in PAH mass recovery beyond the first two extractions was marginal (< 2% of total mass). An internal standard, 0.2 ml of 100 mg/L anthracene-d10 in acetonitrile, was included in all soil extractions to evaluate the recovery efficiency of the solvent extraction procedure. Recovery of anthracene-d10 was ≥90%. Where necessary, extracts of the treated soils were concentrated to improve detection of two- and three-ring PAHs. Of the 16 EPA-regulated PAHs, acenaphthylene was not detected using this quantification method; dibenz[ah]anthracene and indeno[123-cd]pyrene were detected at concentrations near their respective method detection limit and are excluded from subsequent analyses.
Data Analysis
An empirical two-compartment model (Eqn. 1) [5, 15, 27, 30, 31] was used to describe the biphasic (fast- and slow-desorbing) desorption data for each PAH in the whole, high-density, and low-density fractions of the column soil and treated soil samples:
| (1) |
where Ct is the concentration of PAH (mg/kg dry soil) desorbed after time t (h), C0 is the initial concentration of PAH in the soil, f is the fast-desorbing fraction, and k1 and k2 are the first-order rate constants for fast and slow desorption, respectively (h−1). Best-fit values of f, k1, and k2 were determined by non-linear regression of duplicate (low-density material) and triplicate (whole soil and high-density material) data points of PAH desorbed at each time point using ProStat® 4.02 (Poly Software International). The fraction of mass desorbed for a given PAH at each time point was normalized to the respective initial PAH concentration.
For samples where biphasic desorption behavior was not observed and unique values of f, k1, and k2 could not be obtained from the two-site model, a simplified form of Equation 1 (where f and k1 were set to 0) was used to fit the data:
| (2) |
To ensure the most appropriate model was selected, the simple first-order model (Eqn. 2) was also fit to all datasets and the resulting r2 values compared to the two-site model fit. Student t tests and the Tukey-Kramer Honestly Significant Difference test were performed using JMP® 7.0.1 (SAS Institute); differences between experimental conditions are noted as significant if p < 0.05.
Results
PAH Removal and Distribution
Polycyclic aromatic hydrocarbons concentrations in the column soil and treated soil samples from Port A (day 534) are presented in Figure 1. After continuous biostimulation, significant reductions in concentration were observed for all PAHs with the exception of benzo[ghi]perylene (BgP). In the control column, significant reductions were limited to three- and some four-ring PAHs. Total PAH mass removal at Port A of the biostimulated and control columns was 80 and 37%, respectively, and generally decreased with increasing molecular weight of the PAH. We also monitored soil PAH concentration with depth, aqueous PAH concentration in the column effluent, and the abundance of specific PAH-degrading bacteria, as part of a companion study [35]. That study confirmed that PAH reductions were indeed due to the presence of oxygen and associated microbial activity rather than colloid-facilitated transport through the columns.
Low-density material constituted < 2% of the total mass of the original column soil, but contained more than 50% of the total PAHs (Figure 2). Low-density material similarly accounted for a small fraction of the total soil mass in samples removed from the biostimulated and control columns (1.7% and 1.5% of the total soil mass, respectively); however, poor PAH mass balances (which we attribute to the small mass of material to work with and the high PAH concentrations) made it difficult to accurately quantify the fraction of total PAHs in the low-density material from both columns.
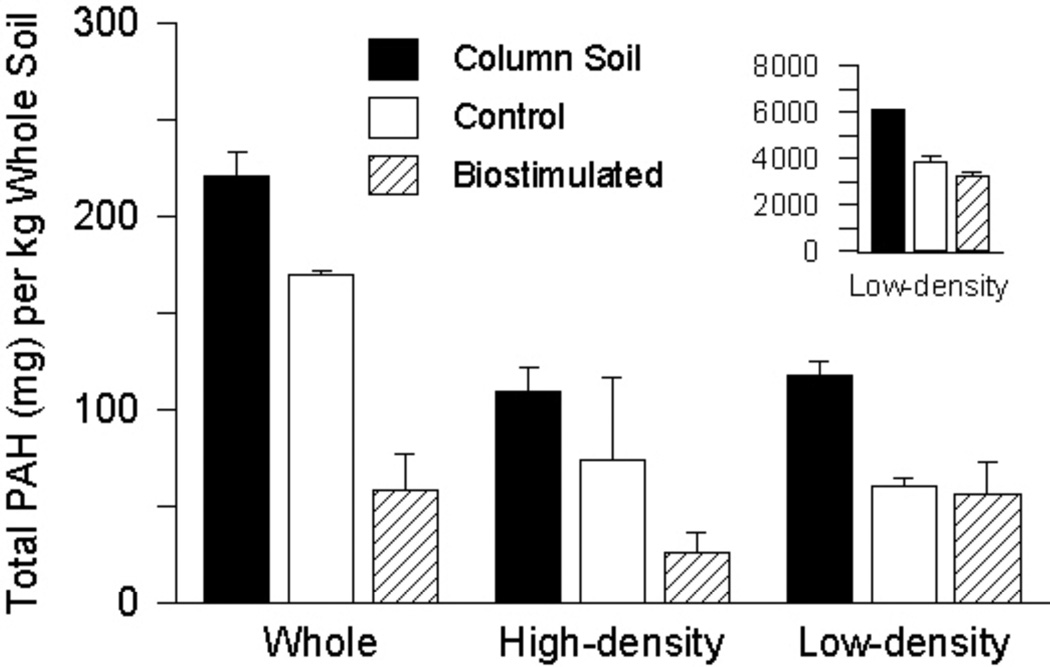
Figure 2.
Total polycyclic aromatic hydrocarbons mass per unit dry mass of the whole (unseparated) soil for the whole soil itself and the high-density and low-density materials in the original column soil and soil collected from the control and biostimulated columns. The inset shows the actual total polycyclic aromatic hydrocarbon (PAH) concentration in the low-density material (mg/kg dry material) of the original column soil and the treated soils.
Total PAH concentrations in the high- and low-density material of the treated soils (day 593) were lower than the respective fractions in the original column soil (Figure 2). For the high-density material, PAH removal was greater in the biostimulated soil than the control, with average total PAH concentrations of 29 mg/kg and 81 mg/kg, respectively. Significant reductions in three- and four-ring PAH concentrations accounted for the majority of PAH removal following biostimulation, while only acenaphthene, phenanthrene, and pyrene decreased significantly in the control soil (Supplemental Data, Figure S2a). Reductions in five- and six-ring PAH concentrations were observed in the high-density material of the biostimulated soil; however, concentrations were not significantly different than those in the original column soil (Figure S2a). For the low-density material, average total PAH concentration was reduced from 6,140 mg/kg in the original column soil to 3,890 mg/kg and 3,220 mg/kg in the control and biostimulated soils, respectively (Figure 2 inset). Concentrations of three- and four-ring PAHs in the low-density material were significantly reduced following biostimulation, while no significant removal of five- and six-ring PAHs was noted. Similar results for the low-density material from the control column were observed, except benz[a]anthracene and chrysene were also not removed (Supplemental Data, Figure S2b).
PAH Desorption from Whole (Unfractionated) Soil
Desorption data for all PAHs in the original column soil and treated soils are presented in the Supplemental Data (Figure S3), with selected PAHs shown in Figure 3. Recovery of the initial PAH mass was generally good, ranging from 79 to 142% for all PAHs except naphthalene, which is consistent with observations from other desorption studies [5, 14, 31]. Poor mass recoveries for naphthalene were attributed to volatilization losses, although volatilization was not measured.
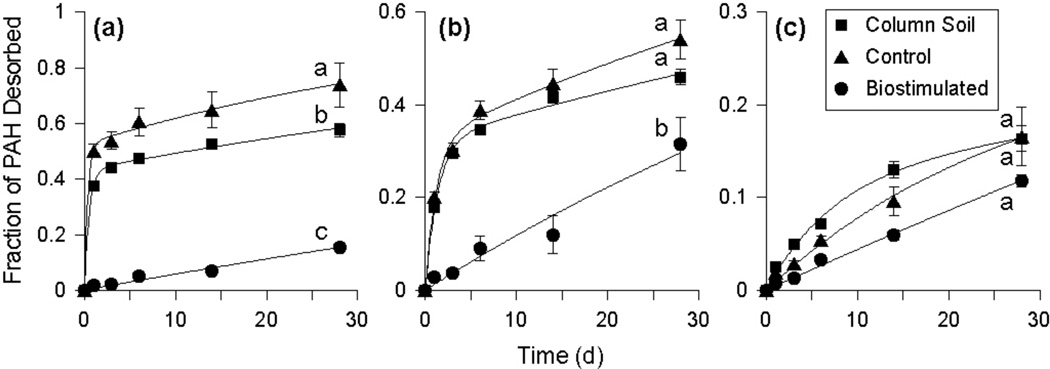
Figure 3.
Desorption curves for phenanthrene (a), pyrene (b), and benzo[a]pyrene (c) for the original column soil and for soil collected from the control and biostimulated columns at day 534. Symbols are the mean values of duplicate analyses from triplicate vessels. Error bars represent the standard deviation and are within the size of the symbol if not visible. The solid lines are the best-fit curves from the simple first-order (biostimulated) or two-site desorption models (untreated column soil and soil from the control column). The letters adjacent to the 28-d time points represent the results of significance analyses using the Tukey-Kramer Honestly Significant Difference (HSD) test. For each analyte, conditions sharing a common letter have total desorbable fractions after 28 d that are not significantly different (p > 0.05). Note the different scales on the y-axes.
After the 28-d desorption period, the amount of PAH desorbed generally decreased with increasing PAH molecular weight in the column soil, with average desorbable fractions of 59% for three-ring PAHs, 43% for four-ring PAHs, and 16% for five- and six-ring PAHs (data not shown). Similar results were observed for the soil from the control column (day 534), for which there was no significant difference in the fraction desorbed compared to the untreated column soil for most PAHs (Figures 3 and S3). For the biostimulated soil (day 534), the amount of PAH desorbed was lower than for the soil from the control column and the untreated column soil for most PAHs, with significant differences noted for all three- and four-ring PAHs (Figures 3 and S3). The trend of decreasing desorbable fraction with increasing PAH molecular weight was not evident in the biostimulated soil, with fractions of PAH desorbed remaining below 33% for all PAHs.
Best-fit parameters of the two-site desorption model (Eqn. 1) for the data in Figures 3 and S3 are summarized in Table S1 of the Supplemental Data. For the untreated column soil and the treated soil from the control column, unique values of the three model parameters were obtained for all PAHs except BgP, for which the simple first-order model (Eqn. 2) was used. The first-order model also provided better fits than the two-site model for the treated soil from the biostimulated column for all PAHs, indicating that the majority of the labile (fast-desorbing) fraction of the PAHs had already been removed by biodegradation (f, k1 = 0).
The fast-desorbing fraction (f) of PAHs in the untreated column soil and soil from the control column ranged from 0 (BgP) to 0.85 (acenapthene; ACE) and, like the total desorbable fractions, decreased with increasing PAH molecular weight (Supplemental Data, Table S1). Desorption of the fast-desorbing fraction occurred within 2 to 4 d for three- and four-ring PAHs and within 9 to 15 d for five- and six-ring PAHs, consistent with observations from other desorption studies [5, 31, 37]. Average f values for five- and six-ring PAHs in the soil from the control column were significantly lower than those in the untreated column soil, reinforcing that some PAH removal had occurred in the control column.
Rate constants for the fast-desorbing fraction (k1) were higher in the soil from the control column than the untreated column soil for all PAHs, although the differences were not statistically significant. Rate constants for the slow-desorbing fraction (k2) for all PAHs ranged from 3 × 10−6 (BgP) to 1.2 × 10−3 h−1 (ACE), which falls within the range of reported literature values [26]. For the untreated column soil and soil from the control column, k2 values generally decreased with increasing PAH molecular weight; however, this was not the case for k2 values for the biostimulated soil. Values of k2 were one to two orders of magnitude lower than k1 for all PAHs in the untreated column soil and soil from the control column, consistent with other studies in which the two-site desorption model has been employed [39].
PAH Desorption from Density-Separated Materials
Polycyclic aromatic hydrocarbon desorption data for the high- and low-density material of the untreated column soil and the treated soils are presented in Supplemental Data (Figures S4 and S5), with selected PAHs shown in Figure 4. Mass recoveries for all PAHs in the high-density materials were comparable to those for the whole soil (65 to 150%); however, mass recoveries were much lower for the low-density material, ranging from 35 to 89%, with the majority of PAH recoveries between 50 and 70%. Desorption experiments with the low-density material involved very small quantities of material (< 0.2 g) that were dried at 60 °C before PAH extraction at the end of the desorption period, with the corresponding potential for PAH loss (any such loss would not have affected the measurement of PAH mass sorbed by the Tenax beads, however).
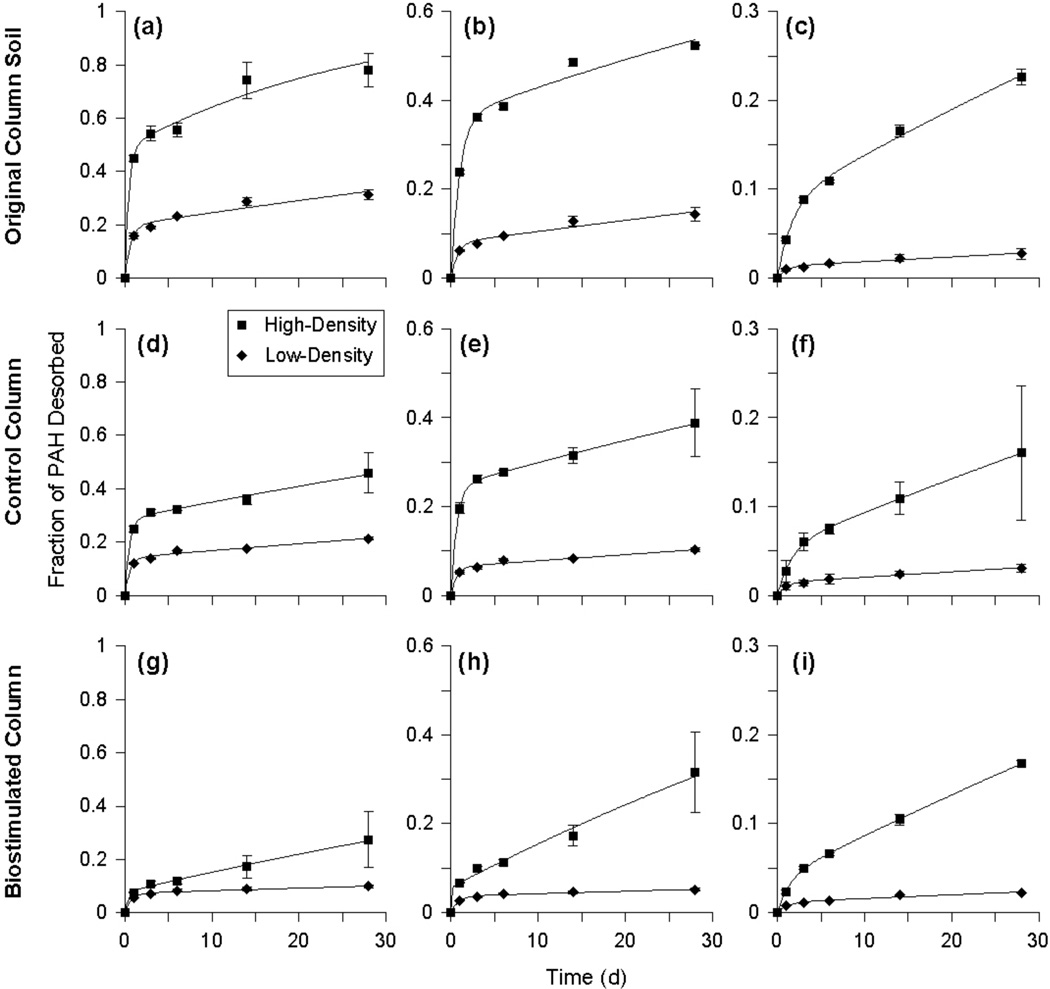
Figure 4.
Desorption curves for phenanthrene, pyrene, and benzo[a]pyrene for the high-density and low-density materials from the original column soil (a–c, respectively) and soil collected from the control column (d–f, respectively) and biostimulated column (g–i, respectively) at day 593. Symbols are the mean values of duplicate analyses from duplicate or triplicate vessels. Error bars represent the range or standard deviation and are within the size of the symbol if not visible. The solid lines are the best-fit curves from the two-site desorption model. Note the different scales on the y-axes.
The amount of PAH desorbed after 28 d from the untreated column soil and the treated soil samples was greater in the high-density material than the corresponding low-density material for all PAHs (Figure 4). For the untreated column soil, desorbable fractions in the high-density material were as high as 86% (ACE) while in the low-density material, desorbable fractions were less than 37% for all PAHs. For the soil from the control and biostimulated columns, up to 46% (phenanthrene; PHN) and 32% (fluoranthene; FLA), respectively, of PAHs were desorbed from the high-density material while up to 22% (fluorene; FLU) and 13% (FLU), respectively, were desorbed from the low-density material. For most PAHs, the total fraction desorbed was significantly lower in the treated soil samples than in the untreated column soil for the high-density material (Supplemental Data, Figure S4). In the low-density material, significant differences were limited to three- and four-ring PAHs (Supplemental Data, Figure S5). Desorption results for the separated soils differ from the whole soil data, for which the fractions desorbed were similar between the untreated column soil and soil from the control column for most PAHs. Soil heterogeneities, the amount of time elapsed between collection of the whole soil samples (day 534) and the high- and low-density materials (day 593), or the extra processing associated with density separation of the soil samples may have contributed to these observed differences.
The two-site desorption model provided the best fits for the high- and low-density desorption data for the column soil and the treated soils. Best-fit parameter values for the high- and low-density material in the column soil and treated soils are presented in the Supplemental Data (Tables S2 and S3). Fast-desorbing fractions (f) for the untreated column soil ranged from 0.02 (BgP) to 0.56 (FLU) in the high-density material and 0.01 (BgP) to 0.25 (FLU) in the low-density material, while f values for the soil from the control column were lower (< 0.28 and 0.14, respectively). For the biostimulated soil, f values for most PAHs in the high- and low-density material were significantly lower than those of the untreated column soil and the soil from the control column.
No significant differences in k1 values were noted between the original column soil and treated soil samples for both the high- and low-density materials (Tables S2 and S3). For most PAHs in all samples, k2 values were an order of magnitude higher in the high-density material than the low-density material. The k2 values for the high-density material were significantly lower for the treated soils than for the untreated column soil for all PAHs, while for the low-density material, significant differences were observed only for three- and four-ring PAHs.
Discussion
Over 50% of the PAH mass in the original (untreated) column soil was distributed in the low-density, carbonaceous fraction (Figure 2), consistent with observations in previous studies on density-based separation of PAH-contaminated sediments [33, 36]. Khalil et al. [8] further separated the low-density fraction of a variety of PAH-contaminated sediments by particle type (wood, charcoal, coal/coke, cenospheres, coal tar pitch), revealing that coal tar pitch, a suspected waste product of former MGP operations, was a major reservoir for PAHs (containing > 90% of PAH mass in the low-density material).
Long-term biostimulation under simulated in situ conditions resulted in significant PAH removal, particularly for three- and four-ring PAHs, in both the high- and low-density materials of the original column soil (Supplemental Data, Figure S2). These results are in contrast to an earlier study [33] in which aerobic bioslurry treatment of PAH-contaminated sediment was found to reduce PAH concentrations only in the high-density material. However, in a companion study by the same group [7], significant reductions were also reported for PAHs associated with low-density material recovered from different contaminated sediments. The authors attributed the observed differences between sediments to the differences in carbon composition in the low-density material, suggesting that PAHs associated with coal tar pitch (a softer, semi-solid domain) were more bioavailable than those bound to highly aromatic, coal-derived particles [7]. Their hypothesis was supported by differences in PAH desorption from the low-density material between sediments. Although the presence of coal tar pitch in the low-density material of the column soil was not confirmed in the present study, it is clear from our results that PAHs in the low-density material were amenable to desorption and biodegradation.
Long-term biostimulation removed the rapidly desorbing fractions of all PAHs in the column soil, including five-ring PAHs (Table S1). This finding is in contrast to a previous study [5] where significant reductions in the fast-desorbing fractions were limited to two-, three-, and four-ring PAHs after aerobic bioreactor treatment of field-contaminated sediment; the fast-desorbing fraction of five- and six-ring PAHs remained unchanged after treatment. Variations in carbon composition and associated PAH sorption capacity of the tested soils/sediments, or differences between the microbial communities, might explain the greater removal of five-ring PAHs in the present study.
Although the fast-desorbing fractions of all PAHs were depleted after biostimulation, up to 30% of the remaining PAHs in soil from the biostimulated column continued to be desorbable over the 28-d assay period (Figures 4g–i). Model fits for the whole soil (Figure 3) and the high-density material (Figure 4) did not reach an asymptote, so the extent to which desorption might have continued beyond 28 d is unknown. For the low-density material, slopes of the model fits generally approached an asymptote after 28 d, particularly in the biostimulated soil. This suggests that there is a very slow (or effectively irreversible) desorption domain present in the low-density material. After extending the desorption period to 58 d, additional mass released from the low-density material of the column soil accounted for less than 3% of the total PAH mass desorbed (data not shown).
Implications of the Two-Site Model
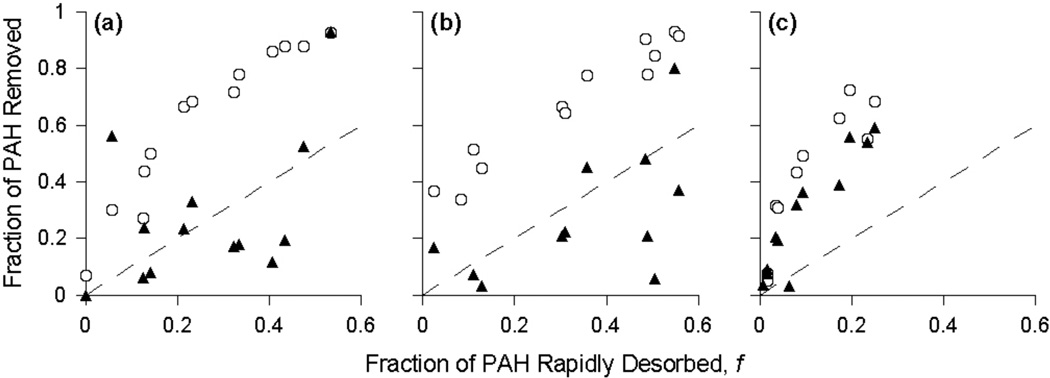
Results from several studies [15, 17, 24] have suggested that the fast-desorbing fraction can be used as a predictor of PAH bioavailability and, correspondingly, the achievable extent of PAH degradation in a bioremediation system. For the present study, we compared the fraction of each PAH removed after 534 d of biostimulation and control conditions versus the respective fast-desorbing fractions in the original column soil for the whole soil and the high- and low-density materials (Figure 5). For the soil from the control column, the fractions of each PAH removed after 534 d were mostly near or below the corresponding f values of the original column soil in both the whole soil and the high-density material (Figures 5a and b). In contrast, for all PAHs in the whole soil and the high-density material from the biostimulated column, the fraction removed was greater (up to 3.5 times) than the corresponding f values of the column soil. The fast-desorbing fraction was, therefore, not predictive of PAH biodegradation under long-term, simulated in situ biostimulation conditions. Other studies have also reported that f values from the two-site desorption model underestimated the extent of PAH degradation in field-contaminated soils and sediments [5, 29]. Birdwell et al [14] recently suggested that PAHs partitioned into apparently slow-desorbing compartments are capable of re-partitioning to a more bioaccessible domain. Such re-partitioning could explain the inability of a short-term desorption assay to predict long-term biodegradation in a biologically active system. It is also possible that the microbial community could influence the mass-transfer behavior of sorbed contaminants, although we have no experimental evidence for this in the present study.
Figure 5.
Fractions of polycyclic aromatic hydrocarbon (PAH) removed in the control (▲) and biostimulated (○) columns versus the fraction of PAH rapidly desorbed (f) in the original column soil for the whole soil (a), the high-density material (b), and the low-density material (c). Each point represents the mean value for an individual PAH. Standard deviations have been omitted for clarity. The dashed line represents a 1:1 correlation. Naphthalene, benzo[a]pyrene, and benzo[ghi]perylene are not included in plot (c) because no removal was observed.
In the low-density material (Figure 5c), the fractions of PAH removed under both the biostimulated and control conditions were up to six times greater than the respective f values of the untreated column soil. Although the f values for the low-density material were much lower than the corresponding values for the whole soil or the high-density material, these results reinforce that the fast-desorbing fraction of a PAH is not necessarily predictive of long-term biodegradation.
Using the best-fit model parameters in Tables S1–S3 of the Supplemental Data, extrapolation of Equation 1 to a desorption period of 534 d results in PAH concentrations well below those actually observed in either of the column systems. Such overestimation of PAH removal by extrapolation of the two-site model suggests that there may be a third effective domain characterized by very slow desorption that is not captured over the time frame (28 d) used in our desorption experiments; similar desorption periods have been used in a number of other studies [5, 7, 30, 31]. Ghosh et al. [36] found that even after a 100-d desorption period with PAH-contaminated sediment, the desorption curve for the high-density material had not reached an asymptote. It appears, therefore, that longer desorption periods may be necessary to accurately predict the long-term extent of PAH biodegradation in field-contaminated soils.
Supplementary Material
Acknowledgement
We thank Randall Goodman and Glenn Walters for their help in the design and construction of the columns. We also thank Wei Sun of the University of North Carolina Department of Biostatistics for assistance with statistical analyses. This work was supported by the National Institute of Environmental Health Sciences (grant number 5 P42 ES005948).
References
- 1.Luthy RG, Dzombak DA, Peters CA, Roy SB, Ramaswami A, Nakles DV, Nott BR. Remediating tar-contaminated soils at manufactured gas plant sites. Environ Sci Technol. 1994;28:266A–276A. doi: 10.1021/es00055a718. [DOI] [PubMed] [Google Scholar]
- 2.Jonker MTO, Sinke AJC, Brils JM, Koelmans AA. Sorption of polycyclic aromatic hydrocarbons to oil contaminated sediment: Unresolved complex? Environ Sci Technol. 2003;37:5197–5203. doi: 10.1021/es0300564. [DOI] [PubMed] [Google Scholar]
- 3.Aitken CM, van Duin ACT, Collins MJ. Understanding bioavailability of polycyclic aromatic hydrocarbons: Mechanisms and prediction. In: Head IM, Singleton I, Milner MG, editors. Bioremediation: A critical review. Norfolk, England: Horizon Scientific Press; 2003. pp. 185–204. [Google Scholar]
- 4.Ehlers LJ, Luthy RG. Contaminant bioavailability in soil and sediment. Environ Sci Technol. 2003;37:295A–302A. doi: 10.1021/es032524f. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen G, Rigterink H, Ferdinandy MMA, van Noort PCM. Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environ Sci Technol. 1998;32:966–970. [Google Scholar]
- 6.Hong L, Ghosh U, Mahajan T, Zare RN, Luthy RG. PAH sorption mechanism and partitioning behavior in lampblack-impacted soils from former oil-gas plant sites. Environ Sci Technol. 2003;37:3625–3634. doi: 10.1021/es0262683. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh U, Zimmerman JR, Luthy RG. PCB and PAH speciation among particle types in contaminated harbor sediments and effects on PAH bioavailability. Environ Sci Technol. 2003;37:2209–2217. doi: 10.1021/es020833k. [DOI] [PubMed] [Google Scholar]
- 8.Khalil MF, Ghosh U, Kreitinger JP. Role of weathered coal tar pitch in the partitioning of polycyclic aromatic hydrocarbons in manufactured gas plant site sediments. Environ Sci Technol. 2006;40:5681–5687. doi: 10.1021/es0607032. [DOI] [PubMed] [Google Scholar]
- 9.Ahn S, Werner D, Luthy RG. Physicochemical characterization of coke-plant soil for the assessment of polycyclic aromatic hydrocarbon availability and the feasibility of phytoremediation. Environ Toxicol Chem. 2005;24:2185–2195. doi: 10.1897/04-564r.1. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh U. The role of black carbon in influencing availability of PAHs in sediments. Hum Ecol Risk Assess. 2007;13:276–285. [Google Scholar]
- 11.Bosma TNP, Middeldorp PJM, Schraa G, Zehnder AJB. Mass transfer limitation of biotransformation: Quantifying bioavailability. Environ Sci Technol. 1997;31:248–252. [Google Scholar]
- 12.Kan AT, Fu G, Hunter M, Chen W, Ward CH, Tomson MB. Irreversible sorption of neutral hydrocarbons to sediments: Experimental observations and model predictions. Environ Sci Technol. 1998;32:892–902. [Google Scholar]
- 13.Stroo HF, Jensen R, Loehr RC, Nakles DV, Fairbrother A, Liban CB. Environmentally acceptable endpoints for PAHs at a manufactured gas plant site. Environ Sci Technol. 2000;34:3831–3836. [Google Scholar]
- 14.Birdwell JE, Thibodeaux LJ. PAH repartitioning in field-contaminated sediment following removal of the labile chemical fraction. Environ Sci Technol. 2009;43:8092–8097. doi: 10.1021/es9016798. [DOI] [PubMed] [Google Scholar]
- 15.Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC. PAH release during water desorption, supercritical carbon dioxide extraction, and field bioremediation. Environ Sci Technol. 2001;35:4577–4583. doi: 10.1021/es010771i. [DOI] [PubMed] [Google Scholar]
- 16.Cuypers C, Pancras T, Grotenhuis JTC, Rulkens WH. The estimation of PAH bioavailability in contaminated sediments using hydroxypropyl-[beta]-cyclodextrin and Triton X-100 extraction techniques. Chemosphere. 2002;46:1235–1245. doi: 10.1016/s0045-6535(01)00199-0. [DOI] [PubMed] [Google Scholar]
- 17.Lei L, Suidan MT, Khodadoust AP, Tabak HH. Assessing the bioavailability of PAHs in field-contaminated sediment using XAD-2 assisted desorption. Environ Sci Technol. 2004;38:1786–1793. doi: 10.1021/es030643p. [DOI] [PubMed] [Google Scholar]
- 18.Hatzinger PB, Alexander M. Effect of aging of chemicals in soil on their biodegradability and extractability. Environ Sci Technol. 1995;29:537–545. doi: 10.1021/es00002a033. [DOI] [PubMed] [Google Scholar]
- 19.Kelsey JW, Kottler BD, Alexander M. Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ Sci Technol. 1997;31:214–217. [Google Scholar]
- 20.Breedveld GD, Karlsen DA. Estimating the availability of polycyclic aromatic hydrocarbons for bioremediation of creosote contaminated soils. Appl Microbiol Biotechnol. 2000;54:255–261. doi: 10.1007/s002530000362. [DOI] [PubMed] [Google Scholar]
- 21.Reid BJ, Stokes JD, Jones KC, Semple KT. Nonexhaustive cyclodextrin-based extraction technique for the evaluation of PAH bioavailability. Environ Sci Technol. 2000;34:3174–3179. [Google Scholar]
- 22.Cuypers C, Grotenhuis JTC, Joziasse J, Rulkens WH. Rapid persulfate oxidation predicts PAH bioavailability in soils and sediments. Environ Sci Technol. 2000;34:2057–2063. [Google Scholar]
- 23.Liste HH, Alexander M. Butanol extraction to predict bioavailability of PAHs in soil. Chemosphere. 2002;46:1011–1017. doi: 10.1016/s0045-6535(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 24.Braida WJ, White JC, Pignatello JJ. Indices for bioavailability and biotransformation potential of contaminants in soils. Environ Toxicol Chem. 2004;23:1585–1591. doi: 10.1897/03-162. [DOI] [PubMed] [Google Scholar]
- 25.Pignatello JJ. Slowly reversible sorption of aliphatic halocarbons in soils. I. Formation of residual fractions. Environ Toxicol Chem. 1990;9:1107–1115. [Google Scholar]
- 26.Birdwell J, Cook RL, Thibodeaux LJ. Desorption kinetics of hydrophobic organic chemicals from sediment to water: A review of data and models. Environ Toxicol Chem. 2007;26:424–434. doi: 10.1897/06-104r.1. [DOI] [PubMed] [Google Scholar]
- 27.Connaughton DF, Stedinger JR, Lion LW, Shuler ML. Description of time-varying desorption kinetics: release of naphthalene from contaminated soils. Environ Sci Technol. 1993;27:2397–2403. [Google Scholar]
- 28.Shor LM, Rockne KJ, Taghon GL, Young LY, Kosson DS. Desorption kinetics for field-aged polycyclic aromatic hydrocarbons from sediments. Environ Sci Technol. 2003;37:1535–1544. doi: 10.1021/es025734l. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Pignatello JJ, Smets BF, Grasso D, Monserrate E. Bench-scale evaluation of in situ bioremediation strategies for soil at a former manufactured gas plant site. Environ Toxicol Chem. 2005;24:741–749. doi: 10.1897/04-247r.1. [DOI] [PubMed] [Google Scholar]
- 30.Jonker MTO, Hawthorne SB, Koelmans AA. Extremely slowly desorbing polycyclic aromatic hydrocarbons from soot and soot-like materials: Evidence by supercritical fluid extraction. Environ Sci Technol. 2005;39:7889–7895. doi: 10.1021/es0505191. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Roper JC, Pfaender F, Aitken MD. Effects of anaerobic incubation on the desorption of polycyclic aromatic hydrocarbons from contaminated soils. Environ Toxicol Chem. 2008;27:837–844. doi: 10.1897/07-166.1. [DOI] [PubMed] [Google Scholar]
- 32.Hawthorne SB, Grabanski CB. Correlating selective supercritical fluid extraction with bioremediation behavior of PAHs in a field treatment plot. Environ Sci Technol. 2000;34:4103–4110. [Google Scholar]
- 33.Talley JW, Ghosh U, Tucker SG, Furey JS, Luthy RG. Particle-scale understanding of the bioavailability of PAHs in sediment. Environ Sci Technol. 2002;36:477–483. doi: 10.1021/es010897f. [DOI] [PubMed] [Google Scholar]
- 34.Jones MD, Singleton SD, Sun W, Aitken MD. Multiple DNA extractions coupled with stable-isotope probing of anthracene-degrading bacteria in contaminated soil. Appl Environ Microbiol. 2011;77:2984–2991. doi: 10.1128/AEM.01942-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson SD. PhD thesis. Chapel Hill, NC, USA: University of North Carolina; 2010. Effects of in situ bioremediation strategies on the biodegradation and bioavailability of polycyclic aromatic hydrocarbons in weathered manufactured gas plant soil. [Google Scholar]
- 36.Ghosh U, Gillette JS, Luthy RG, Zare RN. Microscale location, characterization, and association of polycyclic aromatic hydrocarbons on harbor sediment particles. Environ Sci Technol. 2000;34:1729–1736. [Google Scholar]
- 37.Loehr RC, Lamar MR, Poppendieck DG. A protocol to estimate the release of anthropogenic hydrocarbons from contaminated soils. Environ Toxicol Chem. 2003;22:2202–2208. doi: 10.1897/02-463. [DOI] [PubMed] [Google Scholar]
- 38.Richardson SD, Lebron BL, Miller CT, Aitken MD. Recovery of phenanthrene-degrading bacteria after simulated in situ persulfate oxidation in contaminated soil. Environ Sci Technol. 2011;45:719–725. doi: 10.1021/es102420r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC. Comparing PAH availability from manufactured gas plant soils and sediments with chemical and biological tests. 1. PAH release during water desorption and supercritical carbon dioxide extraction. Environ Sci Technol. 2002;36:4795–4803. doi: 10.1021/es020626k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.