Abstract
During Leishmania major infection in mice, gamma interferon (IFN-γ) plays an essential role in controlling parasite growth and disease progression. In studies designed to ascertain the role of IFN-γ in Leishmania amazonensis infection, we were surprised to find that IFN-γ could promote L. amazonensis amastigote replication in macrophages (MΦs), although it activated MΦs to kill promastigotes. The replication-promoting effect of IFN-γ on amastigotes was independent of the source and genetic background of MΦs, was apparently not affected by surface opsonization of amastigotes, was not mediated by interleukin-10 or transforming growth factor β, and was observed at different temperatures. Consistent with the different fates of promastigotes and amastigotes in IFN-γ-stimulated MΦs, L. amazonensis-specific Th1 transfer helped recipient mice control L. amazonensis infection established by promastigotes but not L. amazonensis infection established by amastigotes. On the other hand, IFN-γ could stimulate MΦs to limit amastigote replication when it was coupled with lipopolysaccharides but not when it was coupled with tumor necrosis factor alpha. Thus, IFN-γ may play a bidirectional role at the level of parasite-MΦ interactions; when it is optimally coupled with other factors, it has a protective effect against infection, and in the absence of such synergy it promotes amastigote growth. These results reveal a quite unexpected aspect of the L. amazonensis parasite and have important implications for understanding the pathogenesis of the disease and for developing vaccines and immunotherapies.
Leishmania parasites are dimorphic protozoans. They are transmitted to humans or other mammals by sandfly vectors in the form of flagellated promastigotes, but they propagate inside tissue macrophages (MΦs) in the form of aflagellate amastigotes (2, 38). Leishmania infection exhibits a spectrum of clinical manifestations, from relatively benign cutaneous pathology to life-threatening visceral diseases, depending on the infective parasite species and host immune responses (47).
Studies of experimental Leishmania infection in mice have been important to our understanding of the pathogenesis of the disease. In the murine model of Leishmania major infection, susceptibility and resistance are due to the development of interleukin-4 (IL-4)-dominated Th2 responses and gamma interferon (IFN-γ)-dominated Th1 responses in the infected host, respectively (35, 36). At the cellular level, IFN-γ activates microbicidal mechanisms of MΦs that kill intracellular L. major parasites (13, 14, 21), while cytokines, such as IL-4, IL-10, and transforming growth factor β (TGF-β), not only inhibit IFN-γ-mediated parasite killing (21, 48, 49) but also directly promote parasite growth inside MΦs (18, 19). Although this Th1-Th2 dichotomy is well established in the L. major infection model, it may not adequately explain the pathogenesis of murine infection by other Leishmania species. For example, infection by the New World species Leishmania amazonensis has many unique aspects (8). While most inbred mouse strains are susceptible to L. amazonensis infection, this susceptibility is not associated with polarized Th2 responses (1, 41). C3H/HeJ mice have been found to be relatively resistant to L. amazonensis infection, yet their cytokine profile during infection is not highly Th1 polarized (34). Furthermore, propagation of L. amazonensis parasites in vivo is significantly reduced when either CD4+ T-cell function or the B-cell-mediated antibody response is eliminated (22, 41). In contrast, mice deficient in CD4+ T cells succumb to L. major infection (7, 11, 16, 29). These immunological data indicate that there are important differences between the L. major and L. amazonensis parasites in terms of the biology of their interactions with the host. This point is strengthened by the recent finding that lipophosphoglycan is an essential virulence factor for L. major but not for Leishmania mexicana (17, 44). Thus, conclusions drawn from studies of one Leishmania species may not always be extended to other species. Therefore, it is necessary, in the context of L. amazonensis infection, to revisit some fundamental aspects of Leishmania-host interactions that have been determined based mainly on data for L. major infection. Given the fact that MΦs are the primary host cells for all Leishmania parasites, in this study we sought to ascertain the role of the Th1 cytokine IFN-γ in the dynamic interactions between L. amazonensis parasites and host MΦs. Our efforts led to the surprising observation that IFN-γ may promote the replication of L. amazonensis amastigotes.
MATERIALS AND METHODS
Mice.
Wild-type and IFN-γ-deficient BALB/c and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). They were maintained under specific-pathogen-free conditions and used when they were 6 to 10 weeks old. All protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, Tex.).
Reagents.
Recombinant IL-10, tumor necrosis factor alpha (TNF-α), and neutralizing monoclonal antibody (MAb) against IL-10 (clone JES5-16E3) were purchased from BD PharMingen (San Diego, Calif.). Neutralizing MAb against mouse TGF-β (clone 1D11) was purchased from R&D Systems (Minneapolis, Minn.). Recombinant murine IFN-γ was purchased either from R&D Systems or Leinco Technologies, Inc. (St. Louis, Mo.). Lipopolysaccharide (LPS) from Salmonella enterica serovar Typhimurium and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (Fab specific) were purchased from Sigma (St. Louis, Mo.). Horseradish peroxidase-conjugated goat anti-mouse IgG(H+L) was purchased from Bio-Rad Laboratories (Hercules, Calif.). The L. amazonensis-specific antisera for staining intracellular parasites were harvested from BALB/c mice that had been infected for 4 months.
Parasites
L. amazonensis (MHOM/BR/77/LTB0016) parasites were maintained by regular passage in BALB/c mice. To culture these parasites, Schneider's Drosophila media (Life Technologies, Rockville, Md.) supplemented with 20% fetal bovine serum were used; the pH used for promastigotes was 7, and the pH used for amastigotes was 5. Promastigotes were cultured at 23°C. Metacyclic promastigotes of L. amazonensis were purified by negative selection with the 3A1 MAb (a gift from David Sacks, National Institute of Allergy and Infectious Diseases) as previously described (10). Tissue-derived amastigotes were harvested from foot tissues of infected BALB/c mice and were cultured at 33°C for 24 to 48 h before they were used, as previously reported (15). To prepare MΦ-derived amastigotes, MΦs that were infected with tissue-derived amastigotes for 96 h were lysed to release intracellular parasites (see below). The released amastigotes were rested at 33°C overnight in complete Schneider's Drosophila media (pH 5) prior to use. Unlike amastigotes that were immediately harvested from lesion tissues, MΦ-derived amastigotes had no fluorescence-activated cell sorting-detectable surface opsonization of antibodies (unpublished data).
L. amazonensis infection of mice and evaluation of the disease course.
Mice (five to eight animals per group) were subcutaneously inoculated in the right hind foot with 2 × 106 stationary-phase promastigotes or 105 tissue-derived amastigotes. The lesion size was measured with a digital caliper (Control Company, Friendswood, Tex.). At different times, mice were sacrificed to determine the parasite burden by a limiting dilution assay as previously described (34). In certain experiments, as indicated below, mice were intravenously injected through the tail vein with 107 S1A Th1 cells in 150 μl of phosphate-buffered saline (PBS) 1 day before infection. Mice that received PBS were used as the control. The methods used for generation and characterization of the Th1 line S1A have been described previously (20).
MΦ culture.
Cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen GIBCO, Carlsbad, Calif.) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 50 μg of gentamicin per ml, and 100 U of penicillin per ml. Bone marrow-derived MΦs (BM-MΦs) were generated as described previously (43). Briefly, marrow cells were seeded in a petri dish at a concentration of 2 × 106 cells per 10 ml of medium supplemented with 10% L929 culture supernatant. After 5 days, nonadherent cells were discarded, and adherent cells were maintained for an additional 2 to 4 days before they were detached from the petri dish with cold PBS containing 2 mM EDTA. These BM-MΦs were washed twice with warm medium and then cultured on four-well Lab-Tek chamber slides (Nalge Nunc International, Naperville, Ill.) at a concentration of 1.5 × 105 cells/well for enumeration of intracellular parasites by fluorescence microscopy. For other assays, cells were cultured in 24-well tissue culture plates at a concentration of 3 × 105 cells/well. Peritoneal MΦs were obtained from peritoneal lavages of mice that had been intraperitoneally injected with 1 ml of 3% thioglycolate broth 7 days previously.
MΦ stimulation and parasite infection.
BM-MΦs or peritoneal MΦs that had been rested for at least 12 h in tissue culture plates or on chamber slides were washed once with warm media. They were then given different cytokines, LPS, or various combinations of these compounds 4 h before infection with Leishmania parasites. Alternatively, MΦs were first infected at a defined parasite-to-cell ratio and then stimulated with cytokines 4 h later. Unless indicated otherwise, MΦs exposed to parasites were kept at 33°C, a temperature consistent with the temperature of Leishmania-induced cutaneous lesions in mice (39). In some experiments, as noted below, promastigotes were incubated with 2% antisera or freshly prepared mouse sera for 20 min at 33°C before they were used for infection. To synchronize amastigote binding to MΦs, each culture plate was spun at 100 ×g for 5 min immediately after parasites were added. MΦ cultures were processed to evaluate intracellular parasite burdens at various times for up to 96 h postinfection; after this time the spontaneous lysis of MΦs with huge parasitophorous vacuoles made it difficult to accurately measure parasite loads (unpublished observations). Unless otherwise indicated, tissue-derived amastigotes were used for cell infection.
Enumeration of parasites in MΦs by fluorescent microscopy.
Fluorescent labeling of intracellular parasites was performed by using a previously described method (21). Briefly, an infected MΦ monolayer was fixed on a chamber slide with methanol at 4°C for 20 min and then washed twice with PBS. It was subsequently stained with antisera (obtained from BALB/c mice infected for 3 to 6 months, 1:200 dilution in PBS) for 20 min at 4°C, washed three times with PBS, and then stained with FITC-conjugated goat anti-mouse IgG(H+L) (1:200 dilution in PBS) at 4°C for 20 min. Cells were counterstained with 4′,6′-2-diamidino-2-phenylindole (DAPI) and then examined under a coverslip with an AxioPlan II fluorescence microscope (Zeiss, Thornwood, N.Y.). To enumerate intracellular parasites, each well of the chamber slide was divided into three approximately equal areas along the long axis, and an image of a random field from each of the three areas was obtained in both FITC and DAPI channels with a Plan-Neofluar 40×/0.75 lens. The software-merged image was then evaluated, and data representing the three random fields were pooled. Typically, 500 to 700 cells were counted for each chamber well.
Evaluation of the parasite burden in MΦs after cell lysis by SDS.
Exposure to a low concentration of sodium dodecyl sulfate (SDS) (0.01%, wt/vol) has been used previously to lyse infected MΦs in order to release intracellular parasites (33). Accordingly, at different times following infection, MΦs in 24-well tissue culture plates (three wells per condition) were washed with PBS and then exposed to 200 μl of 0.01% SDS in PBS at 37°C. The process of cell lysis was monitored with an inverted microscope. The lysis was typically completed within 10 to 15 min, and virtually all intracellular parasites were released. Each parasite suspension was immediately supplemented with 0.8 ml of complete culture media and was thoroughly resuspended by repeated pipetting. The number of parasites per well was determined with a hemocytometer.
Data analysis.
To evaluate the statistical significance of the difference between experimental groups in individual experiments, two-tailed t tests were used. To evaluate the effect of IFN-γ treatment across 21 experiments, control and treated groups from each experiment were considered a pair, and the paired t test was used. All graphs were generated with SigmaPlot software (SPSS Inc., Chicago, Ill.).
RESULTS
Polarized Th1 cells fail to control L. amazonensis amastigote infection.
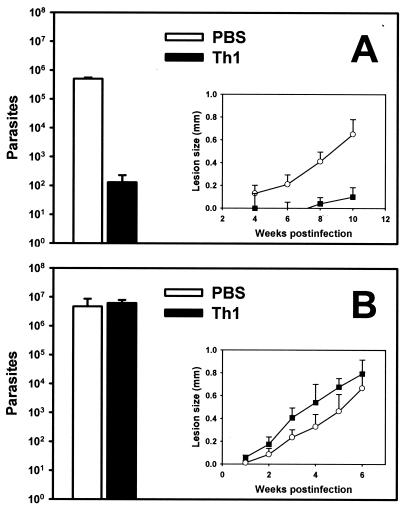
Murine infection by L. amazonensis parasites does not exhibit a Th1-Th2 dichotomy in association with resistance and susceptibility. Nonetheless, a highly polarized Th1 response is thought to be sufficient for controlling L. amazonensis infection in susceptible hosts. This is not only because Th1 responses play a protective role against many protozoan infections, including those caused by L. major (36), but also because vaccine protection against L. amazonensis infection is associated with greater enhanced Th1 responses (42). We recently generated an L. amazonensis-specific Th1 cell line, S1A, from splenocytes of infected C57BL/6 mice through repeated in vitro stimulation with amastigote lysates in the presence of IFN-γ and a neutralizing anti-IL-4 MAb. Intracellular staining and fluorescence-activated cell sorting analysis have indicated that more than 90% of S1A cells produce IFN-γ and TNF-α upon antigenic or polyclonal stimulation with no detectable IL-4 or IL-10 production (20). Upon intravenous transfer into naïve C57BL/6 mice that were challenged with L. amazonensis promastigotes 1 day later, S1A cells helped control lesion development and reduced the parasite burden by more than 3 orders of magnitude (Fig. 1A). This protection was clearly associated with enhanced Th1 responses (20). Curiously, however, when amastigotes were used for infection, the transfer of S1A cells failed to reduce the parasite burden (Fig. 1B). Importantly, mice into which S1A cells were transferred that were later infected with amastigotes did exhibit a strong Th1 cytokine pattern; when 106 lymph node cells were stimulated with amastigote lysates in 200 μl of complete medium for 72 h, the IFN-γ level was 220 ± 45 ng/ml and the IL-4 level was 0.12 ± 0.04 ng/ml (n = 5). Thus, S1A cells failed to control the amastigote infection despite the fact that they orchestrated a highly polarized Th1 response. Thus, although a polarized Th1 response is able to confer protection against promastigote infection, it may not be enough to limit amastigote propagation in tissues. One explanation for this dramatic distinction in the effectiveness of Th1 transfer against infections established with promastigotes and amastigotes is that the two forms of parasites may have quite different fates in immune-activated MΦs. Since MΦs are able to kill L. amazonensis promastigotes following IFN-γ activation in vitro (41), we sought to test the fate of amastigotes in IFN-γ-activated MΦs.
FIG. 1.
L. amazonensis infection in mice following adoptive transfer of Th1 cells. Groups of five to eight C57BL/6 mice were intravenously injected with 107 S1A Th1 cells or with PBS alone 1 day prior to infection with 2 × 106 stationary-phase promastigotes (A) or 105 tissue-derived amastigotes (B). Parasite burdens were assayed at 10 weeks after promastigote infection (A) or at 6 weeks after amastigote infection (B). (Insets) Lesion sizes at different times. The data represent the data from three independent experiments.
Enhanced amastigote replication in IFN-γ-treated murine MΦs.
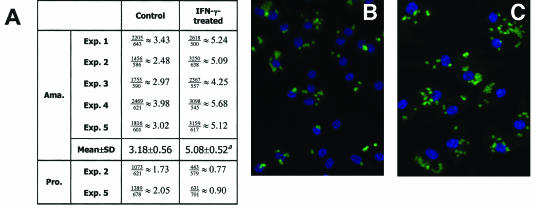
In agreement with a previous report (41), MΦs treated with 20 ng of IFN-γ per ml harbored fewer parasites than their untreated counterparts 48 h after promastigote infection (Fig. 2A). Strikingly, when MΦs were infected with L. amazonensis amastigotes, the same IFN-γ treatment significantly increased the number of parasites per cell at 48 h postinfection, from 3.18 parasites per control MΦ to 5.08 parasites per IFN-γ-treated MΦ (P < 0.01) (Fig. 2A). This increase in the average number of parasites per MΦ can be appreciated from the micrographs in Fig. 2B and C. Importantly, when MΦ monolayers were examined 1 or 5 h postinfection, no significant difference was observed between the IFN-γ-treated and control groups (data not shown). Therefore, the increased parasite/MΦ ratio in the IFN-γ-treated group at 48 h was probably a result of enhanced amastigote replication.
FIG. 2.
Microscopic evaluation of L. amazonensis infection in MΦs. BM-MΦs from BALB/c mice (Exp. 1 and 2) or C57BL/6 mice (Exp. 3, 4, and 5) were seeded at a concentration of 1.5 × 105 cells/chamber on chamber slides. Cells were not treated or were treated with 20 ng of IFN-γ per ml for 4 h prior to infection with 3 × 105 amastigotes (Ama.) or 7.5 × 105 stationary-phase promastigotes (Pro.). After 48 h of incubation, cells were processed for immunostaining of the parasites. (A) Summary of the average numbers of parasites per cell, as calculated by dividing the total number of parasites by the total number of MΦs examined. (B and C) Representative images of amastigote-infected control MΦs (B) and IFN-γ-treated MΦs (C). A superscript a indicates that the P value is <0.01.
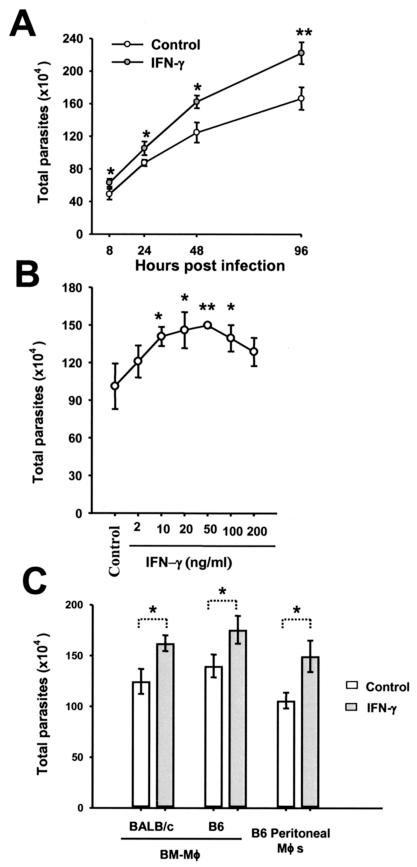
To further address this possibility, we directly determined the total parasite burden in an infected MΦ culture by counting the number of parasites released after MΦs were lysed with 0.01% SDS in PBS (33). The kinetics of amastigote replication were examined by using MΦs that were not treated or were treated with 20 ng of IFN-γ per ml for 4 h prior to infection. As shown in Fig. 3A, significantly more amastigotes were recovered from IFN-γ-treated MΦs than from control cells at 8, 24, 48, and 96 h postinfection. Next, MΦs were treated with different doses of IFN-γ, and the parasite load was determined 48 h after infection. As shown in Fig. 3B, the amastigote burden was significantly increased in MΦs treated with 10 to 100 ng of IFN-γ per ml. MΦs treated with 2 or 200 ng of IFN-γ per ml harbored more amastigotes, although the increase was not always statistically significant. To exclude the possibility that the increase in intracellular amastigote burdens after IFN-γ treatment is peculiar to a certain type of MΦs, different MΦ preparations were examined. As shown in Fig. 3C, the IFN-γ treatment increased the total number of amastigotes regardless of the genetic background (C57BL/6 or BALB/c) or the source of MΦs (BM-MΦs or peritoneal MΦs). Taken together, these data clearly demonstrate that IFN-γ treatment increases the amastigote load in murine MΦs.
FIG. 3.
Increased burdens of amastigotes in IFN-γ-treated MΦs. (A) C57BL/6 BM-MΦs (3 × 105 cells/well) were not treated or were treated with 20 ng of IFN-γ per ml for 4 h prior to infection with 4.5 × 105 amastigotes. The number of parasites in each well was determined at different times. The data are data from one of three experiments in which similar results were obtained. (B) Different concentrations of IFN-γ were tested by a procedure similar to that described above for panel A. The number of parasites in each well of a MΦ culture was determined at 48 h postinfection. The data represent the data from three independent experiments. (C) MΦs from different strains of mice and sources were treated with 20 ng of IFN-γ per ml for 4 h and then infected with amastigotes. The number of parasites was determined at 48 h postinfection. The data are data from one of two experiments in which similar results were obtained.
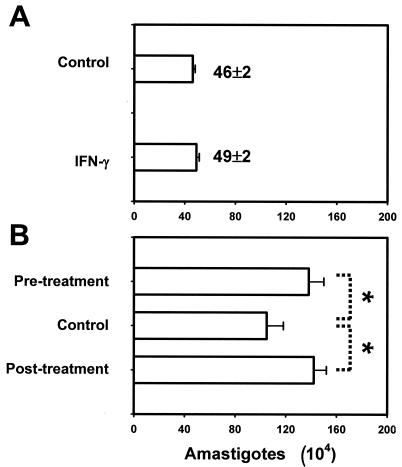
This unexpected phenomenon may be because MΦs are actually more permissive to intracellular amastigote replication after IFN-γ treatment. Alternatively, it could result from an increase in amastigote uptake by IFN-γ-treated MΦs. The latter possibility is particularly relevant since a fraction of amastigotes harvested from lesion tissues remain opsonized by host IgG even after 24 to 48 h in culture (15; unpublished data) and since IFN-γ could enhance Fc receptor-mediated phagocytosis by MΦs (51). In addition, IFN-γ might have direct effects on L. amazonensis amastigotes by shortening their doubling time. For example, IL-2 and IFN-γ were reported to promote the in vitro growth of L. amazonensis promastigotes and Trypanosoma brucei, respectively (3, 28). To exclude these possibilities, we treated MΦs with IFN-γ after they had internalized amastigotes. Phagocytosis of L. amazonensis amastigotes by MΦs is known to be a rapid and efficient process, taking approximately 30 min to complete from the time of parasite binding (25). In our hands, when the binding of amastigotes to the MΦ monolayer was synchronized by gentle centrifugation, virtually no amastigotes were observed outside MΦs by 1 h. At this time, the number of parasites recovered from lysed MΦs was essentially equal to the number in the initial inoculum (Fig. 4A), indicating that there was complete and synchronized uptake of amastigotes. Under these conditions of synchronized parasite uptake, significantly more amastigotes were recovered from MΦs that were stimulated with IFN-γ for either 4 h before or 4 h after the onset of amastigote infection (Fig. 4B). Therefore, the increased parasite burden was not due to enhanced parasite uptake following IFN-γ treatment or the direct effect of IFN-γ on the parasite. In a total of 21 experiments that involved MΦs from different sources or strains of mice, the amastigote load at 48 h postinfection was, on the average, increased by 34% ± 13% in MΦs treated with 20 ng of IFN-γ per ml, regardless of whether the IFN-γ treatment was 4 h prior to or after the onset of infection (P < 0.001, as determined by a paired t test). Together, these data strongly suggest that IFN-γ stimulation of MΦs promotes the replication of intracellular amastigotes.
FIG. 4.
Enhanced amastigote replication in IFN-γ-treated MΦs. C57BL/6 BM-MΦs (3 × 105 cells/well) were not treated or were treated with 20 ng of IFN-γ per ml for 4 h before or after infection with 4.5 × 105 amastigotes. The parasite binding to the MΦ monolayer was synchronized as described in Materials and Methods. The number of intracellular amastigotes was determined at 1 h (A) and 48 h (B) postinfection. Data are the mean +/− SD for a given condition in triplicate. The results for three independent experiments are shown.
Enhanced replication in IFN-γ-treated MΦs is a unique property of amastigotes that is not shared by metacyclic promastigotes.
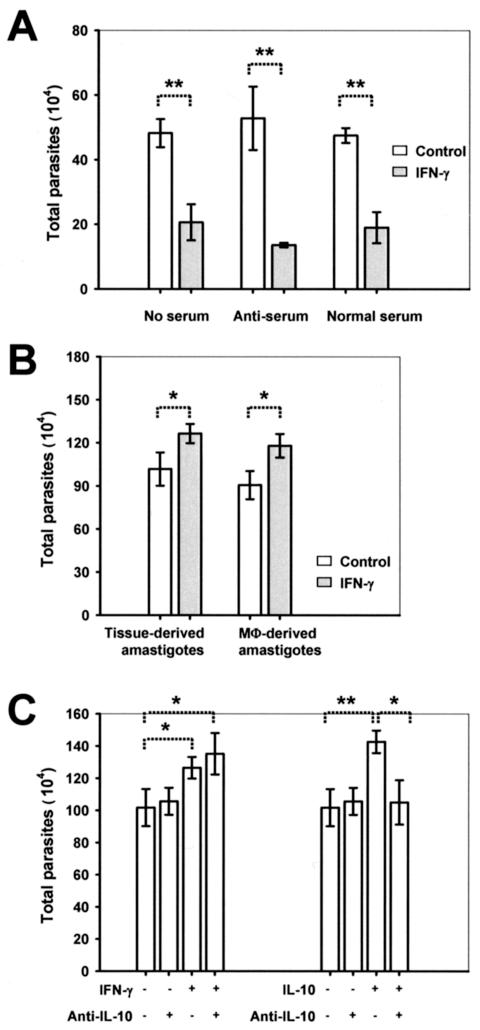
Data in Fig. 2, as well as in a previous report (41), clearly indicated that promastigotes are killed in IFN-γ-activated MΦs. However, these experiments involved the use of stationary-phase promastigote preparations, which typically contain only 10% highly infective metacyclic promastigotes. Metacyclic promastigotes directly give rise to amastigotes in a natural infection and are more resistant to innate killing by hosts (37, 38). This prompted us to further examine whether enhanced growth in IFN-γ-activated MΦs is a property shared by amastigotes and metacyclic promastigotes. As shown in Fig. 5A, significantly fewer parasites were recovered from metacyclic promastigote-infected, IFN-γ-treated MΦs. This was also true when metacyclic promastigotes were preincubated with freshly harvested normal sera or antisera, despite the fact that serum opsonization may assist certain Leishmania species in attaching to and surviving in MΦs (4, 30). Clearly, the ability to grow better in IFN-γ-activated MΦs is a unique feature of L. amazonensis amastigotes.
FIG. 5.
Enhanced replication in IFN-γ-treated MΦs is a unique property of amastigotes and is not mediated by IL-10. MΦs (3 × 105 cells/well) were infected with 1.5 × 106 metacyclic promastigotes or 4.5 × 105 amastigotes in 24-well plates. All parasite burdens were determined at 48 h postinfection. (A) C57BL/6 BM-MΦs either were not treated or were treated with 20 ng of IFN-γ per ml and then infected with metacyclic promastigotes that had been preincubated without serum, with 5% L. amazonensis-specific antiserum, or with normal mouse serum. (B) C57BL/6 BM-MΦs were not treated or were treated with 20 ng of IFN-γ per ml and then were infected with tissue-derived or MΦ-derived amastigotes. (C) Before infection with amastigotes, C57BL/6 BM-MΦs were pretreated with 20 ng of IFN-γ per ml in the presence or absence of 10 μg of anti-IL-10 MAb per ml. As controls, cells were treated with 20 ng of IL-10 per ml together with or without 10 μg of anti-IL-10 MAb per ml. All data are the means ± standard deviations for three wells. One asterisk indicates that the P value is <0.05, and two asterisks indicate that the P value is <0.01.
Enhanced replication in IFN-γ-treated MΦs is not mediated by IL-10.
To our knowledge, no biological activities that were ascribed previously to IFN-γ can directly account for the observed replication-promoting effect on amastigotes inside MΦs. However, IFN-γ might promote amastigote growth in MΦs through induction of another cytokine(s) that favors parasite replication. In this context, IL-10 is particularly relevant, because it can deactivate nitric oxide (NO)-mediated leishmanicidal effects of MΦs (21, 48, 49). It can also directly promote the growth of L. major parasites in MΦs by inducing arginase (18, 19), a key enzyme in the synthetic pathway of polyamines that are required for intracellular growth of Leishmania parasites (53). Interestingly, opsonized amastigotes can induce Fc receptor-dependent IL-10 production by MΦs, provided that the MΦs also receive concomitant inflammatory stimuli, such as bacterial LPS and hyaluronic acid (21). Thus, if IFN-γ is a costimulus that induces IL-10 production by infected MΦs, this may explain why IFN-γ can enhance the replication of L. amazonensis amastigotes. However, this does not appear to be the case. As shown in Fig. 5B, the growth-enhancing effect of IFN-γ was observed with both tissue-derived amastigotes and MΦ-derived amastigotes, which were bound by and free of host IgG, respectively (see Materials and Methods for details). In addition, no IL-10 was detectable by an enzyme-linked immunosorbent assay in amastigote-infected MΦs regardless of whether they were treated with IFN-γ (data not shown). More importantly, while exogenous IFN-γ and IL-10 promoted amastigote replication to similar extents, addition of a neutralizing anti-IL-10 MAb totally negated the effect of IL-10 but not the effect of IFN-γ (Fig. 5C). Similarly, a neutralizing MAb against TGF-β did not affect the growth-promoting effect of IFN-γ (data not shown). Since arginase I upregulation is responsible for the enhanced growth of L. major parasites in MΦs treated with IL-4, IL-10, or TGF-β (18, 19), we also examined the level of arginase I expression in IFN-γ-treated and L. amazonensis amastigote-infected MΦs by Western blotting. We found that there were no major changes in arginase expression due to IFN-γ treatment, amastigote infection, or a combination of these treatments (data not shown). Taken together, these results indicate that IFN-γ-enhanced amastigote replication is not likely to be mediated by induction of IL-10 or other arginase I-enhancing cytokines.
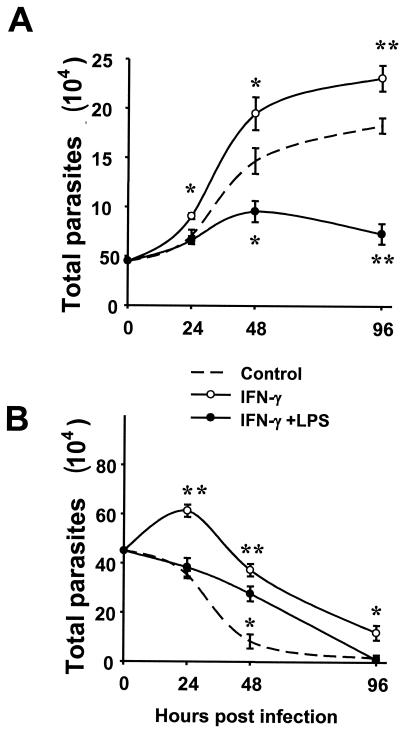
Control of L. amazonensis amastigote replication by IFN-γ plus LPS and its temperature dependence.
The combination of IFN-γ and LPS has been well established as one of the strongest inducing conditions for iNOS-mediated NO production (27), which is believed to be an esential leishmanicidal mechanism operating in vitro and in vivo (13, 14, 24, 31, 52). Given the finding that IFN-γ by itself may promote intracellular replication of L. amazonensis amastigotes, it was important to determine the effect of IFN-γ coupled with LPS. As shown in Fig. 6A, following treatment with IFN-γ and LPS, MΦ became significantly more resistant to intracellular proliferation of amastigotes. Importantly, MΦs stimulated with LPS alone harbored amounts of amastigotes similar that those of the untreated control (data not shown). These data demonstrate that when combined with LPS, IFN-γ is able to stimulate MΦs to limit amastigote replication. This result also correlates with the finding that IFN-γ together with LPS but not by itself can induce a significant amount of iNOS protein expression (unpublished observation).
FIG. 6.
Temperature dependence of amastigote replication in MΦs. C57BL/6 BM-MΦs (3 × 105 cells/well) were not treated or were treated with 20 ng of IFN-γ per ml alone or with 20 ng of IFN-γ per ml plus 10 ng of LPS per ml for 4 h and then were infected with 4.5 × 105 amastigotes. Infected MΦ cultures were kept at either 33°C (A) or 37°C (B) throughout the experiments. At different times, the amastigote burden in the group treated with IFN-γ or IFN-γ plus LPS was compared to the amastigote burden in the untreated control. One asterisk indicates that the P value is <0.01, and two asterisks indicate that the P value is <0.001. The data are the means ± standard deviations for three wells. The results of one of two independent experiments are shown.
A potential caveat of the above-described experiments is that amastigote infection and subsequent MΦ incubation were carried out at 33°C, a reduced temperature. This temperature is consistent with that of Leishmania-induced lesions in mice (39). However, it can be argued that microbicidal functions of MΦs are impaired at this reduced temperature. Thus, it is possible that 33°C is the temperature required for both IFN-γ and LPS to induce a leishmanicidal state in MΦs, while IFN-γ alone might be sufficient at 37°C. Therefore, we tested whether at 37°C IFN-γ alone or in combination with LPS can promote killing of L. amazonensis amastigotes in MΦs. A sharp decrease in the number of intracellular amastigotes was observed at 37°C in infected MΦs without any treatment (Fig. 6B). This was particularly evident after 24 h. Such parasite loss was most likely due to the spontaneous death of amastigotes rather than to active killing by untreated MΦs, because lesion-derived amastigotes quickly stopped replicating and spontaneously died when they were cultured at 37°C (15; unpublished data). This spontaneous loss of parasites at 37°C was in sharp contrast to the pronounced parasite propagation at 33°C (compare Fig. 6A and B). At 33°C, amastigote replication was clearly reduced in MΦs treated with IFN-γ plus LPS compared to amastigote replication in MΦs that were not treated (Fig. 6A). Interestingly, cotreatment with IFN-γ and LPS did not incresae the spontaneous loss of amastigotes at 37°C (Fig. 6B). Importantly, treatment of MΦs with IFN-γ alone also failed to accelerate the spontaneous loss of intracellular amastigotes at 37°C (Fig. 6B). Rather, it significantly induced amastigote replication during the first 24 h after the onset of infection. After this, the parasite load in IFN-γ-treated MΦs was not lower than that in the control at any time (Fig. 6B). Taken together, these data demonstrate that IFN-γ-enhanced amastigote replication in MΦs is not peculiar to a lower temperature. Rather, IFN-γ promotes amastigote growth even at a temperature that does not favor parasite survival.
DISCUSSION
Scott et al. showed two decades ago that despite being able to kill L. major amastigotes and Toxoplasma gondii parasites, lymphokine-activated MΦs failed to control intracellular replication by L. amazonensis amastigotes. However, no significant enhancement of amastigote replication was observed (40). The difference between the previous findings and the present results is probably due to the fact that Scott et al. used complete culture supernatants produced by splenocytes from L. amazonensis-sensitized mice as the lymphokine, while we used recombinant IFN-γ. Our results indicate that L. amazonensis amastigote replication is enhanced in IFN-γ-treated MΦs, which revealed a unique ability of L. amazonensis parasites to resist and to take advantage of host defense mechanisms. On the other hand, our data also show that L. amazonensis amastigotes can be controlled by MΦs if they are stimulated properly (i.e., by a combination of IFN-γ and LPS but by neither of these compounds alone) (Fig. 6). Because iNOS-deficient MΦs failed to control amastigote replication even when they were stimulated with IFN-γ and LPS (unpublished data), this killing is probably NO mediated, similar to what has been observed for L. major parasites (13, 14, 21). Apparently, depending on the presence of other factors, IFN-γ may either facilitate L. amazonensis amastigote replication or promote L. amazonensis amastigote killing.
While the present study did not pinpoint a mechanism by which stimulation of MΦs with IFN-γ alone could facilitate the intracellular replication of L. amazonensis amastigotes, several possibilities can be contemplated. First, amastigotes might be able to sense the activation of a particular signaling pathway downstream of the IFN-γ receptor and then accelerate their own replication. This accelerated amastigote replication may be sufficiently fast to compensate for the loss of parasites due to NO-mediated killing, especially when a microbicidal level of NO production requires de novo iNOS transcription, translation, and posttranslational modification (12). Second, L. amazonensis amastigotes may influence the balance of arginine metabolism in MΦs to their own favor. As mentioned above, cytokines such as IL-4, IL-10, and TGF-β enhance the activity of arginase I and promote the growth of L. major parasites in MΦs (18, 19). This is because arginase-mediated arginine metabolism, which by definition competes with iNOS for the same substrate, leads to the synthesis of polyamines that are essential for the replication of eukaryotic cells, including protozoans such as Leishmania (32, 53). Thus, increased polyamine production in the host cell favors parasite growth. While IFN-γ activates the iNOS but not the arginase pathway, it may enhance arginine transport into the MΦs (5, 6, 26). Therefore, if L. amazonensis amastigotes in IFN-γ-activated MΦs could somehow skew the balance between arginase and iNOS activities toward the former, increased polyamine synthesis and enhanced parasite replication may occur. Given that intracellular replication of Leishmania parasites occurs in acidified phagolysosomes, another possibility is that IFN-γ may facilitate maturation of amastigote-containing phagolysosomes, leading to an accelerated onset of amastigote replication. This possibility would explain our observation that IFN-γ-treated MΦs exhibit a discernible increase in total parasite burdens by 8 h postinfection (Fig. 3A). Currently, we are addressing these possibilities.
Our results have revealed that there is a striking contrast between the fates of L. amazonensis promastigotes and amastigotes in IFN-γ-activated MΦs. The finding that promastigotes are killed while amastigotes may continue to grow intracellularly correlates well with the observation that transferred Th1 cells controlled infection with promastigotes but not infection with amastigotes (Fig. 1). During a natural infection, however, all surviving promastigotes in the mammalian host must eventually transform into amastigotes. Thus, there seems to be a significant period of time between the entry of promastigotes into MΦs and the completion of amastigote transformation, during which the parasite is highly vulnerable to Th1-induced microbicidal attacks by host MΦs. Indeed, this period of transformation was estimated to be as long as 5 days (9). Conceivably, this 5-day period would be a window of opportunity for vaccine-generated memory Th1 cells to be recalled into action, activating MΦs to eliminate the parasite (as seen in successfully immunized mice). Beyond that, a polarized Th1 response may not be sufficient to control the infection or even have an exacerbating impact. Based on these analyses, it appears that the immune memory induced by an effective anti-L. amazonensis vaccine would have to be fast reacting and able to mount a Th1 response before the complete promastigote-amastigote transformation. Furthermore, Th1 enhancement should not be the sole basis for immunotherapies aimed at resolving L. amazonensis infections at a later stage. When amastigotes have established tissue residence in the host, for example, local administration of recombinant IFN-γ to L. amazonensis-infected lesions may be harmful rather than beneficial to the host.
Previous studies have provided conclusive evidence that IFN-γ plays a clear-cut protective role in controlling L. major infection in mice (45, 46, 50). Accordingly, the guiding principle for vaccine development and immunotherapeutics against Leishmania infection is to enhance Th1 responses. Results in this report point to a more complicated aspect of L. amazonensis infection regarding the role of IFN-γ and suggest that this infection requires a modified strategy for the development of vaccines and immunotherapies.
Acknowledgments
We are grateful to Judy Aronson for providing L929 cells, Timothy Denning for sharing expertise on cell transfer studies, Thomas Albrecht and Eugene Knutson at the Infectious Diseases & Toxicology Imaging Core-UTMB for technical assistance, and Mardelle Susman for comments on the manuscript.
This study was supported in part by NIAID grant AI43003 to L. Soong. H. Qi, J. Ji, and N. Wanasen were supported by the James W. McLaughlin Fellowship Fund.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J., and D. G. Russell. 1992. The interaction of Leishmania species with macrophages. Adv. Parasitol. 31:175-254. [DOI] [PubMed] [Google Scholar]
- 3.Bakhiet, M., T. Olsson, J. Mhlanga, P. Buscher, N. Lycke, P. H. van der Meide, and K. Kristensson. 1996. Human and rodent interferon-γ as a growth factor for Trypanosoma brucei. Eur. J. Immunol. 26:1359-1364. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, J. M., R. A. Ezekowitz, M. B. Roberts, J. Y. Channon, R. B. Sim, and S. Gordon. 1985. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 162:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogle, R. G., A. R. Baydoun, J. D. Pearson, S. Moncada, and G. E. Mann. 1992. l-Arginine transport is increased in macrophages generating nitric oxide. Biochem. J. 284:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caivano, M. 1998. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 429:249-253. [DOI] [PubMed] [Google Scholar]
- 7.Chakkalath, H. R., and R. G. Titus. 1994. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J. Immunol. 153:4378-4387. [PubMed] [Google Scholar]
- 8.Colmenares, M., S. Kar, K. Goldsmith-Pestana, and D. McMahon-Pratt. 2002. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S3-S7. [DOI] [PubMed] [Google Scholar]
- 9.Courret, N., C. Frehel, E. Prina, T. Lang, and J. C. Antoine. 2001. Kinetics of the intracellular differentiation of Leishmania amazonensis and internalization of host MHC molecules by the intermediate parasite stages. Parasitology 122:263-279. [DOI] [PubMed] [Google Scholar]
- 10.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 11.Erb, K., C. Blank, U. Ritter, H. Bluethmann, and H. Moll. 1996. Leishmania major infection in major histocompatibility complex class II-deficient mice: CD8+ T cells do not mediate a protective immune response. Immunobiology 195:243-260. [DOI] [PubMed] [Google Scholar]
- 12.Geller, D. A., and T. R. Billiar. 1998. Molecular biology of nitric oxide synthases. Cancer Metast. Rev. 17:7-23. [DOI] [PubMed] [Google Scholar]
- 13.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 14.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 15.Hodgkinson, V. H., and L. Soong. 1997. In vitro maintenance of Leishmania amastigotes directly from lesions: advantages and limitations. J. Parasitol. 83:953-956. [PubMed] [Google Scholar]
- 16.Holaday, B. J., M. D. Sadick, Z. E. Wang, S. L. Reiner, F. P. Heinzel, T. G. Parslow, and R. M. Locksley. 1991. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J. Immunol. 147:1653-1658. [PubMed] [Google Scholar]
- 17.Ilg, T. 2000. Lipophosphoglycan is not required for infection of macrophages or mice by Leishmania mexicana. EMBO J. 19:1953-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iniesta, V., L. C. Gomez-Nieto, and I. Corraliza. 2001. The inhibition of arginase by Nω-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 193:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iniesta, V., L. C. Gomez-Nieto, I. Molano, A. Mohedano, J. Carcelen, C. Miron, C. Alonso, and I. Corraliza. 2002. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 24:113-118. [DOI] [PubMed] [Google Scholar]
- 20.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 22.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 24.Liew, F. Y., S. Millott, C. Parkinson, R. M. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J. Immunol. 144:4794-4797. [PubMed] [Google Scholar]
- 25.Love, D. C., M. Mentink Kane, and D. M. Mosser. 1998. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp. Parasitol. 88:161-171. [DOI] [PubMed] [Google Scholar]
- 26.MacLeod, C. L. 1996. Regulation of cationic amino acid transporter (CAT) gene expression. Biochem. Soc. Trans. 24:846-852. [DOI] [PubMed] [Google Scholar]
- 27.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 28.Mazingue, C., F. Cottrez-Detoeuf, J. Louis, M. Kweider, C. Auriault, and A. Capron. 1989. In vitro and in vivo effects of interleukin 2 on the protozoan parasite Leishmania. Eur. J. Immunol. 19:487-491. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell, G. F. 1983. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J. Immunogenet. 10:395-412. [DOI] [PubMed] [Google Scholar]
- 30.Mosser, D. M., and P. J. Edelson. 1987. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature 327:329-331. [DOI] [PubMed] [Google Scholar]
- 31.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pegg, A. E. 1988. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 48:759-774. [PubMed] [Google Scholar]
- 33.Pham, T. V., and J. Mauel. 1987. Studies on intracellular killing of Leishmania major and lysis of host macrophages by immune lymphoid cells in vitro. Parasite Immunol. 9:721-736. [PubMed] [Google Scholar]
- 34.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 35.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 36.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 37.Sacks, D. L., and P. V. Perkins. 1985. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am. J. Trop. Med. Hyg. 34:456-459. [DOI] [PubMed] [Google Scholar]
- 38.Sacks, D. L., and P. V. Perkins. 1984. Identification of an infective stage of Leishmania promastigotes. Science 223:1417-1419. [DOI] [PubMed] [Google Scholar]
- 39.Scott, P. 1985. Impaired macrophage leishmanicidal activity at cutaneous temperature. Parasite Immunol. 7:277-288. [DOI] [PubMed] [Google Scholar]
- 40.Scott, P., D. Sacks, and A. Sher. 1983. Resistance to macrophage-mediated killing as a factor influencing the pathogenesis of chronic cutaneous leishmaniasis. J. Immunol. 131:966-971. [PubMed] [Google Scholar]
- 41.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 42.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 44.Spath, G. F., L. Epstein, B. Leader, S. M. Singer, H. A. Avila, S. J. Turco, and S. M. Beverley. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. 97:9258-9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swihart, K., U. Fruth, N. Messmer, K. Hug, R. Behin, S. Huang, G. Del Giudice, M. Aguet, and J. A. Louis. 1995. Mice from a genetically resistant background lacking the interferon-γ receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med. 181:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo, S. J., B. M. Sullivan, C. Stemmann, A. R. Satoskar, B. P. Sleckman, and L. H. Glimcher. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 295:338-342. [DOI] [PubMed] [Google Scholar]
- 47.Tapia, F. J., M. Caceres-dittmar, and M. A. Sanchez. 1996. Molecular and immune mechanisms in the pathogenesis of cutaneous leishmaniasis. R. G. Landes Company, Austin, Tex.
- 48.Vieth, M., A. Will, K. Schroppel, M. Rollinghoff, and A. Gessner. 1994. Interleukin-10 inhibits antimicrobial activity against Leishmania major in murine macrophages. Scand. J. Immunol. 40:403-409. [DOI] [PubMed] [Google Scholar]
- 49.Vouldoukis, I., P. A. Becherel, V. Riveros-Moreno, M. Arock, O. da Silva, P. Debre, D. Mazier, and M. D. Mossalayi. 1997. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur. J. Immunol. 27:860-865. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z. E., S. L. Reiner, S. Zheng, D. K. Dalton, and R. M. Locksley. 1994. CD4+ effector cells default to the Th2 pathway in interferon-γ-deficient mice infected with Leishmania major. J. Exp. Med. 179:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren, M. K., and S. N. Vogel. 1985. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 134:982-989. [PubMed] [Google Scholar]
- 52.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 53.Yarlett, N., and C. J. Bacchi. 1994. Parasite polyamine metabolism: targets for chemotherapy. Biochem. Soc. Trans. 22:875-879. [DOI] [PubMed] [Google Scholar]