INTRODUCTION
Reduced reproductive output extends life span in many species (e.g., Stearns 1992, Partridge et al. 2005; Flatt 2011). The disposable soma hypothesis suggests that when reproduction is reduced more nutrients are allocated to the soma, augmenting somatic maintenance and promoting longevity (Kirkwood 1987; 2002). This hypothesis has been supported by numerous cases of increased life span and reduced fecundity upon dietary restriction (e.g., Mobbs et al. 2007; Fontana et al. 2010). Also supporting this nutrient allocation hypothesis are examples of increased storage of lipid or carbohydrate upon reduced reproduction (Djawdan et al. 1996; Simmons and Bradley 1997; Tatar et al. 2001; Flatt et al. 2005). These increases in storage have been presumed to be accompanied by an increase in allocation of ingested nutrients to these tissues, concomitant with decreased allocation to reproduction. But, rigorous testing of a possible role of nutrient allocation in extending life span upon reduced reproduction requires tracking recently ingested nutrients into somatic storage.
Tracking of ingested nutrients in a long-lived model has only been done with Drosophila on dietary restriction, a treatment that indirectly reduces reproduction (Min et al. 2006; O’Brien et al. 2008; Tatar 2011). Absolute investment in the soma by flies on dietary restriction was not greater than absolute investment in the soma by control flies. However, investment in reproduction was greatly reduced by dietary restriction, so the investment in the soma was increased relative to the investment in reproduction. These results are inconsistent with strictly interpreted predictions from the disposable soma hypothesis, but show that the physiological mechanisms underlying nutrient allocation can be changed by manipulations that extend life span (O’Brien et al. 2008).
Some experiments manipulating diet, but not tracking ingested nutrients, have provided evidence inconsistent with the disposable soma hypothesis. For example, Grandison et al. (2009) reared Drosophila on a diet low in essential amino acids but with normal levels of methionine. These flies had extended life span and normal reproductive output. In this way, the authors demonstrate that diet-induced longevity does not necessarily result in reduced fecundity. If life span can be extended via diet manipulation without reducing fecundity, then allocation of a nutrient cannot underlie the extension of life span (Grandison et al. 2009). Whether directly reduced reproduction extends life span through nutrient allocation is untested.
Tracking of stable isotopes has become the method of choice for testing allocation of ingested nutrients to specific body tissues (as in Min et al. 2006; O’Brien et al. 2008; see Gannes et al. 1997; Karasov and Martinez del Rio 2007). Hence, we tracked ingested carbon and nitrogen allocation to multiple somatic pools using diets with distinct stable isotope signatures. We have previously shown that these diets produce similar reproductive tactics yet are clearly different in 13C and 15N concentrations (Judd et al. 2010).
Here, we test predictions of the disposable soma hypothesis by tracking the allocation of ingested nutrients into somatic storage in long-lived, ovariectomized grasshoppers (Romalea microptera). We have previously shown that ovariectomized grasshoppers have ~20% greater median life span than sham-operated controls (Hatle et al. 2008; Drewry et al. 2011). In contrast to other models in which the ovary is missing, ovariectomized grasshoppers continue to make the egg yolk-precursor protein vitellogenin, which accumulates in the hemolymph (Hatle et al. 2003). Therefore, ovariectomized grasshoppers have the reproductive organ removed, have reproductive output blocked, and have life span extended, but they retain the ability to invest nutrients in reproduction.
Previous experiments identified the time after first oviposition in sham-operated, fully reproductive females as the time at which they diverge physiologically from ovariectomized females (Hatle et al. 2008; Drewry et al. 2011). During clutch 1, investment in reproductive proteins is similar between ovariectomized and sham females (Hatle et al. 2008). Similarly, nutrient allocation during clutch 1 is affected little by ovariectomy, with only a 37% increase in 15N allocation to the fat body, and no changes in 15N allocation to femur muscle or in 13C allocation to either fat body or femur muscle (Judd et al. 2010). Hence, in this paper we focus on the period from first to second oviposition. We define the period from adult molt to median age of first oviposition by sham-operated females as ‘clutch 1’, for both sham and ovariectomized females. Similarly, we define the period from first oviposition to median age at second oviposition by sham-operated females as ‘clutch 2’ for both sham and ovariectomized females.
We track incorporation of ingested nutrients in three somatic tissues, namely hemolymph storage proteins, femur muscle, and the fat body. Lubber grasshoppers have three storage proteins, which all belong to the hexamerin family (Hathaway et al. 2009). The storage proteins make up 80% of the non-vitellogenin protein in the hemolymph, and their levels are positively associated with reproductive output (Hatle et al. 2001). Levels of storage proteins in ovariectomized females increase steadily throughout adulthood, implying increased storage, and consistent with the disposable soma hypothesis (Hatle et al. 2008). Femur muscle is a wholly somatic, amitotic tissue. It is one of the largest skeletal muscles in this flightless grasshopper. Fat body is the main metabolic and storage organ of insects (Chapman 1998), combining many of the functions of the liver and adipose tissues in vertebrates. It produces storage proteins, vitellogenin, and glycogen, and is the major depot of lipids. We separated the aqueous and lipid fractions of the fat body before stable isotope analysis. This allowed us to largely distinguish between the machinery for intermediary metabolism (i.e., the aqueous portion) and storage (i.e., the lipid portion).
The disposable soma hypothesis predicts that long-lived, ovariectomized grasshoppers will have increased storage (e.g., storage protein levels, fat body masses) relative to sham-operated controls. Further, the hypothesis predicts that incorporation of ingested nitrogen and carbon into these somatic tissues should be proportionally increased upon ovariectomy. That is, if a physiological shift in the allocation of nutrients from reproduction to somatic maintenance is responsible for life span extension in ovariectomized individuals, we expect that ovariectomized females will allocate proportionally more carbon and nitrogen from the clutch 2 period into somatic tissues than do sham-operated females.
RESULTS
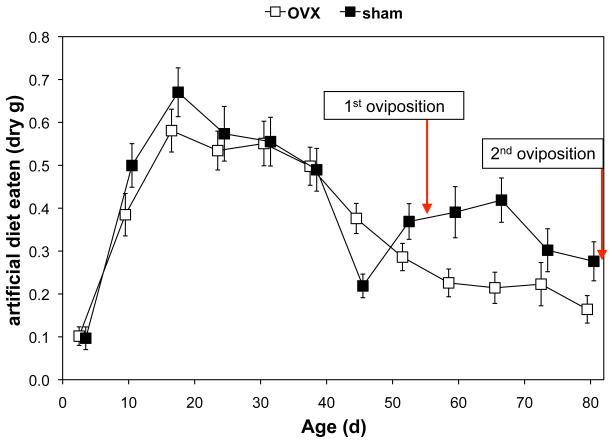
Ovariectomy resulted in ~40% lower ingestion during clutch 2 (Fig. 1). MANOVA revealed a significant effect of age (Pillai’s Trace F9,20 = 25.42; P < 0.001) and a marginal interaction of age and surgery (F9,20 = 2.21; P = 0.0675) on ingestion of artificial diet. Ovariectomy affected ingestion significantly at 45 and 66 d, marginally at 52 d, but not significantly on other days. Feeding rate is typically low about a week before oviposition, and sham females oviposited at 54.3 ± 0.9 d (mean ± SE), providing a likely explanation for the dips in feeding rate in sham females at 45 and 73 d.
Figure 1.
Feeding rates in sham-operated and ovariectomized (=OVX) lubber grasshoppers. Initial samples sizes were n = 23 for OVX females and n = 25 for sham females. During the period from adult molt until first oviposition in sham-operated females, feeding rates were similar between the two groups. In contrast, during the period from first oviposition to second oviposition in sham-operated females (i.e., clutch 2), ovariectomy decreased feeding on artificial diet by ~40%.
Hemolymph storage proteins
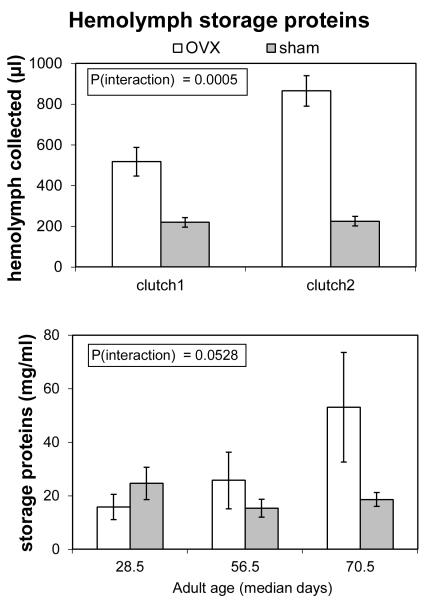
Ovariectomized females had greater quantities of hemolymph storage proteins (Fig. 2). The interaction of age and surgery significantly affected the volume of hemolymph collected (two-way MANOVA; F1,89 = 12.99; P = 0.0005). For hemolymph storage protein levels, the interaction of age and surgery was marginal (F2,91 = 3.04; P = 0.0528). Together, significantly greater collected hemolymph volumes and marginally greater storage protein levels in ovariectomized females yields a greater quantity of storage proteins in ovariectomized than in sham females.
Figure 2.
Volumes of hemolymph collected and levels of hemolymph storage proteins in sham-operated and ovariectomized (=OVX) lubber grasshoppers. Samples sizes for hemolymph collected were n = 37 for clutch 1 and n = 56 for clutch 2. Sample sizes for levels of hemolymph storage proteins were n = 38 at 28 d, n = 31 at 56 d, and n = 28 at 70 d. For hemolymph collected, clutch 1 is the period from adult molt to first oviposition in sham-operated females, and clutch 2 is the period from first oviposition to second oviposition in sham-operated females. For comparing these times to the bottom panel, 56 d is about the time of first oviposition. During clutch 2, quantities of hemolymph storage protein increased in ovariectomized females. This increase was due in part to increases in hemolymph volume and in part to increases in the concentration of storage proteins in the hemolymph.
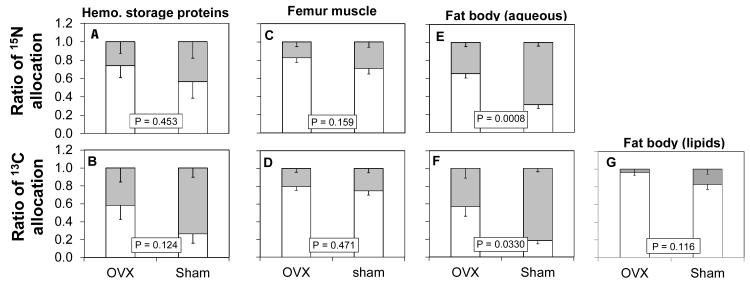
In contrast, ovariectomy did not affect the proportion of allocation of 15N or 13C to storage proteins during clutch 2 (Fig. 3A,B). Across surgeries, hemolymph storage proteins collected after clutch 2 were built from similar proportions of 15N ingested during clutch 1 and 15N ingested during clutch 2. Likewise, these same storage proteins were built of similar proportions of 13C ingested during clutch 1 and clutch 2 (P = 0.3853).
Figure 3.
Allocation of nitrogen and carbon to various tissues in sham-operated and ovariectomized lubber grasshoppers. All samples were collected about the time of second oviposition. Gray bars represent the proportion of the tissue built from food ingested during clutch 2, while white bars represent the proportion of the tissue built from food ingested during clutch 1. Across the entire experiment, the Bonferroni corrected α = 0.0036. A,B) hemolymph storage proteins, n = 6 sham-operated and n = 7 ovariectomized. C,D) femur muscle, n = 9 sham-operated and n = 9 ovariectomized. E,F) fat body aqueous extract, n = 8 sham-operated and n = 4 ovariectomized. G) fat body lipid extract, n = 8 sham-operated and n = 4 ovariectomized. None of the tissues tested showed a shift in nutrient allocation in the direction predicted by the disposable soma hypothesis.
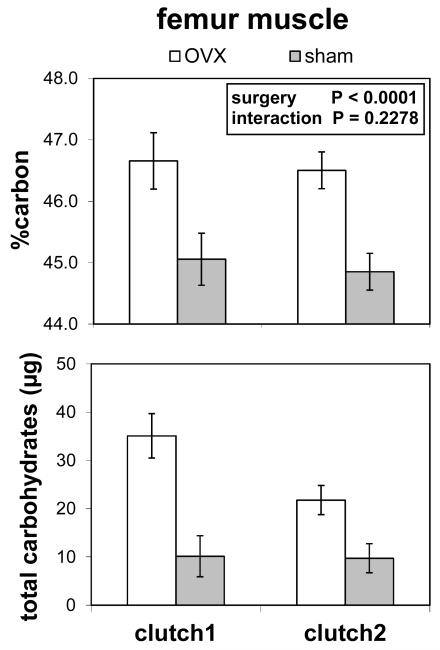
Femur muscle
Ovariectomy significantly increased storage in the femur muscle (two-way MANOVA; Pillai’s Trace F2,36 = 30.57; P < 0.001; Fig. 4). Both the percent carbon by weight (F1,37 = 18.56; P = 0.001) and the total carbohydrates in the muscle (F1,37 = 23.82; P < 0.0001) were increased by ovariectomy. Neither age (F2,36 = 0.1251) nor the interaction of age and surgery (F2,36 = 1.54; P = 0.2278) affected storage of carbon and carbohydrates in the femur muscle. The percent nitrogen by weight was not affected by surgery (F1,44 = 0.16; P = 0.693), age (P = 0.453), or interaction (P = 0.925; data not shown).
Figure 4.
Femur muscle carbon (n = 16 for clutch 1; n = 47 for clutch 2) and total carbohydrates (n = 13 for clutch 1; n = 28 for clutch 2) in sham-operated and ovariectomized (=OVX) lubber grasshoppers. Clutch 1 is the period from adult molt to first oviposition in sham-operated females, and clutch 2 is the period from first oviposition to second oviposition in sham-operated females. Ovariectomized females had higher levels of both carbon and carbohydrate than sham-operated females.
Ovariectomy did not affect the proportional allocation of either 15N or 13C to femur muscle during clutch 2 (Fig. 3C,D). Femur muscle was built primarily of nitrogen and carbon ingested during clutch 1. It incorporated little of the nutrients ingested during clutch 2 (t-tests; both P < 0.0001).
Fat body
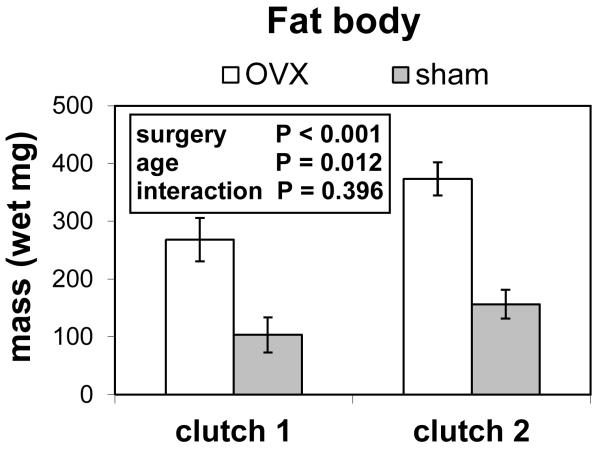
The visceral fat body of ovariectomized females was at least twice as large as that from sham females (Fig. 5). Ovariectomy significantly increased the total wet mass of fat body (two-way ANOVA; Pillai’s Trace F1,94 = 38.52; P < 0.001). Older females had significantly more wet fat body (F1,94 = 6.65; P = 0.0115). The interaction of age and surgery did not affect fat body mass (F1,94 = 0.73; P = 0.3955). Dry fat body masses were measured, and there were no obvious differences in the patterns for wet or dry masses. These tissue masses quantify investment to both lipid storage and metabolic machinery.
Figure 5.
Wet masses of total fat bodies (n = 38 for clutch 1; n = 60 for clutch 2) in sham-operated and ovariectomized (=OVX) lubber grasshoppers. Clutch 1 is the period from adult molt to first oviposition in sham-operated females, and clutch 2 is the period from first oviposition to second oviposition in sham-operated females. Ovariectomized females had larger fat bodies than sham-operated females, and females dissected after clutch 2 had larger fat bodies than females dissected after clutch 1.
For the aqueous fraction of the fat body, ovariectomy significantly decreased the allocation of 15N (Fig. 3E,F), in the opposite direction of that predicted by the disposable soma hypothesis. Ovariectomy did not significantly affect the allocation of 13C to the aqueous fraction of the fat body, although the trend was similar to that for nitrogen. Aqueous fraction of fat body collected after clutch 2 was built of more 13C ingested during clutch 2 than 13C ingested during clutch 1 (t-test; P = 0.0009). However, the same samples were built of similar proportions of 15N ingested during clutch 1 and clutch 2 (P = 0.0695; Bonferroni corrected α = 0.0036).
For the lipid fraction of the fat body, ovariectomy did not affect the proportion of allocation of 13C collected after clutch 2 (Fig. 3G.). The lipid in the fat body was built primarily of carbon ingested during clutch 1 (t-test; P < 0.0001). Lipid does not contain sufficient nitrogen for stable isotope analysis.
DISCUSSION
Our present data suggest that, in long-lived ovariectomized grasshoppers, standard measures of somatic storage remain high or increase during clutch 2. Hence, ovariectomized grasshoppers show the type of elevated storage that has been taken as support of the nutrient allocation prediction of the disposable soma hypothesis. In stark contrast, the 15N and 13C ingested during clutch 2 are allocated to these tissues in similar proportions in ovariectomized and sham-operated females. Therefore, direct tracking of nutrients from ingestion to these somatic tissues fails to support a role for allocation of incoming nutrients as the physiological mechanism underlying longevity. To our knowledge, this is the first combination of measuring somatic storage and tracking ingested nutrients to these depots in a long-lived animal. We observe an experimentally induced trade-off among reproductive output, storage, and longevity, yet our data suggest that a bias in proportional allocation of recently ingested nutrients toward somatic storage is not the mechanism underlying this trade-off.
Nutrient allocation in flies on dietary restriction was examined by O’Brien et al. (2008). They found an increase in somatic allocation relative to reproductive allocation in flies on dietary restriction. Our study on effects of reduced reproduction has similar, but not identical, conclusions. Both studies find that absolute allocation to somatic tissues is not increased. In our study, we were unable to compare allocation to reproduction directly, because sham-operated females had insufficient vitellogenin to isolate for stable isotope analysis.
Our study is consistent with the results of Grandison et al. (2009). These authors fed fruit flies on a diet with normal levels of methionine but restricted in the other essential amino acids. This produced flies that were long lived yet with normal reproductive output. By showing that the trade-off between reduced reproduction and longevity in response to diet is not absolute, they demonstrate that longevity in response to dietary restriction does not necessarily rely on a shift in allocation of ingested nutrients. Here, we provide evidence that longevity in response to reduced reproduction does not necessarily occur via a change in allocation of ingested nutrients. In comparison to Grandison et al. (2009), our approach rigorously tracks ingested nutrients, but does not identify which nutrients are critical to longevity.
Physiological changes from clutch 1 to clutch 2
Storage protein quantities and feeding rates best demonstrate the physiological shift from clutch 1 to clutch 2. By the middle of clutch 2, storage protein quantities had risen to high levels in ovariectomized females, while in sham females quantities had changed little. These results are in agreement with previous work using hemolymph protein levels as evidence of a shift toward storage during clutch 2 (Hatle et al. 2008). Similarly, ovariectomy decreased feeding rates during clutch 2, after not affecting ingestion rates during clutch 1. Together, these justify our focus on this period for measuring nutrient allocation.
Storage is increased greatly in ovariectomized females
Multiple physiological metrics suggest ovariectomized females invest more in storage than do sham females during clutch 2. Storage as hemolymph hexamerins, femur muscle carbohydrates, and total fat body mass were all at least 2-fold higher in ovariectomized females. In concert with the extension of life span by ovariectomy (Hatle et al. 2008), these data are consistent with organismal somatic storage being associated with the trade-off between reproductive output and longevity.
Notably, storage of all three major nutrients was increased. Fat bodies of adult male lubber grasshoppers contain lipid, protein, and glycogen (Spring and Gäde 1987). Visceral fat body is the main depot of lipid storage in insects (Arrese and Soulages 2010). Fat body is also the primary site of carbohydrate storage, while skeletal muscle and hemolymph are secondary carbohydrate stores (Chapman 1998). Lubber grasshoppers are flightless, and the femur muscle is one of the largest muscles. Because skeletal muscle has little other carbohydrate, the increase in total carbohydrates in the femur muscle of ovariectomized females likely represents increased glycogen. Last, hemolymph storage proteins are a critical depot of amino acid storage in many insects, and reproduction in grasshoppers requires dietary protein (Chapman 1998; Hatle et al. 2006a; Judd et al. 2010). Our data showing increased amounts of hemolymph hexamerins and fat body mass indicate that organismal protein storage is increased.
During clutch 2, ingestion of artificial diets (the greatest amount of protein available for the grasshoppers) was reduced by ~40% in ovariectomized grasshoppers. Yet at the same time, some aspects of somatic storage doubled. Together, these results raise interesting questions regarding the source of the nutrients responsible for this increased storage. In another experiment, we found that ovariectomy does not affect total body weight of females fed ad libitum (Drewry et al. 2011). Ovariectomized females ingest less, but they also do not deposit eggs.
Our results show that growth of somatic organs (e.g., fat body, hemolymph) occurs upon ovariectomy. The regulation of this organ growth may be associated with life extension. Indeed, it may be that organ-level changes in signaling for growth factors is more important to the body changes upon ovariectomy than is nutrient allocation (see Hatle et al. 2006a for a similar conclusion). We hypothesize that these growing somatic organs increase expression of receptors for growth factors, such as insulin-like molecules. This would allow increased growth in some tissues (e.g., storage tissues), but not in all tissues (e.g., reproductive tissues).
Considering each of the three focal tissues, our estimates of storage come with a few caveats. First, storage protein may not be an irreversible investment in the soma, because amino acids from storage proteins end up in eggs in other insects. However, storage protein itself is clearly not a direct reproductive investment, because these amino acids can also be allocated to somatic roles (Pan and Telfer 1998 for moths). Also, collected hemolymph is best taken as a qualitative (not quantitative) measure of hemolymph volume. Our estimates could be biased by different handling of the ovariectomized and sham females. That said, one of our initial goals of this project was to collect sufficient hemolymph to measure stable isotope levels in vitellogenin, and we anticipated vitellogenin levels would be lower in sham females. If anything, we feel we squeezed sham-operated females harder to collect as much hemolymph as possible. Next, total carbohydrate in femur muscle may be our weakest measure of storage, but because these grasshoppers had a carbohydrate-rich diet, muscle carbohydrates are probably not a very important store for grasshoppers. Third, similar to storage proteins, fat body is not wholly somatic; it synthesizes vitellogenin, storage proteins, lipophorin, and other secreted proteins (Arrese and Soulages 2010). However, only ~10% of the total protein produced by fat body at the middle of clutch 1 is vitellogenin, with most of the protein produced being storage proteins (KM Jaskowiak, JD Hatle, and DW Borst, unpublished data; Hatle et al. 2006a). Last, newly synthesized lipids do not fully incorporate the 13C signature of the diet (Wessels and Hahn, 2010). This may contribute to the low levels of clutch 2 diet components appearing in fat body lipids. Tracking of lipid allocation by heavy water might provide better information on allocation of ingested nutrients to lipids (e.g., Bruss et al. 2010). Despite these minor caveats, the general conclusion remains that ovariectomy greatly enhances storage at the organismal level.
The proportion of nutrient allocation to somatic tissues is not affected by ovariectomy
In contrast to the increase in storage upon ovariectomy, the physiological process of allocation of ingested nutrients to somatic tissues was not increased by ovariectomy. This means there is no bias for ingested nutrients to be routed more to storage sites in ovariectomized females. Hence, we find no evidence that allocation of ingested nutrients toward somatic storage is a possible mechanism for the trade-off between reproductive output and longevity. This is inconsistent with the disposable soma hypothesis.
Our estimates of stable isotope incorporation represent mass-specific proportional allocation. These were observed in concert with increased storage protein amounts and fat body masses. Despite these mass differences, estimates of the total amounts of nutrients being incorporated in these tissues also appear to be similar in ovariectomized and sham females (serial t-tests; all P > 0.25; supplemental Fig. 1). To makes these estimates of ‘total’ allocation, for each tissue we multiplied the percent allocation of each isotope by the size of the tissue for that individual. For example, ‘total’ allocation of 15N to storage proteins in an ovariectomized female was estimated by multiplying its ratio of 15N allocation (see Fig. 3) by the collected hemolymph volume for that individual (see Fig. 2). This approach has limitations but nonetheless provides a first test of whether the magnitudes of the significant differences in tissue sizes were great enough to overwhelm the lack of significant differences observed in allocation rates. They were not; even total allocation to hemolymph proteins and fat body aqueous extracts do not appear to be affected by ovariectomy.
Despite the general lack of significant differences in proportional allocation due to ovariectomy, our experiment had sufficient sensitivity to detect differences where they existed. For example, femur muscle nitrogen and carbon were deposited primarily during clutch 1, and regardless of surgery the muscle grew little during clutch 2. Hence, the experimental power was strong enough to identify significant differences.
The duration of the experiment is also appropriate, as shown by tissue turnover rates. The stable isotope data suggests that 23% of the nitrogen and 22% of the carbon in femur muscle came from food ingested during clutch 2. This was the slowest turnover rate of all the tissues, yet even for femur muscle, the 95% confidence intervals do not overlap zero. This suggests that all the tissues were clearly incorporating the label from the diet during the period studied, so the trial was sufficiently long. Further, continuing the experiment much longer could have resulted in obscuring treatment effects that might have existed. Fully 81% of the carbon in the fat body aqueous extracts was from food ingested during clutch 2. Had the experiment been extended for one week more, this could have reached a plateau of 100% incorporation. After 100% incorporation was reached, any possible surgery effects on allocation could be buried.
The sole significant effect of ovariectomy on nutrient allocation was the decrease in allocation of nitrogen to fat body aqueous extracts, which is the opposite direction of that predicted by the disposable soma hypothesis. These extracts represent the cellular machinery of intermediary metabolism and likely reflect protein synthesis and turnover. These data are coincident with our observation that feeding on artificial diet was decreased by ~40% upon ovariectomy. Together, these observations raise the possibility that ovariectomy, perhaps via a reduced feeding rate, results in overall decreased intermediary metabolism.
There are multiple physiological steps from ingestion of a nutrient to incorporating that nutrient into a tissue, including: chemical digestion, absorption, transport to the tissue, and uptake by the tissue. Our approach fed labeled diets to individuals and then sampled the tissue to measure its composition, so it encompassed all these steps. In general, there were no effects of ovariectomy on the overall outcome of this complex process. Despite this, it may be that particular steps in this long chain are altered upon ovariectomy, but that the net effect is no difference. These individual steps could be measured in short-term in vivo experiments using radiolabelled tracers. For example, Zera and Zhao have done elegant work showing the allocation of nutrients from the hemolymph into flight muscle and the ovary in wing dimorphic crickets (Zera and Zhao 2006; Zhao and Zera 2006). An additional possibility is that ovariectomized females may be taking resources allocated to reproduction during clutch 1 and shifting them to somatic storage during clutch 2. Our previous work on ovariectomized grasshoppers identified high levels of vitellogenin during clutch 1 and a decrease in vitellogenin during clutch 2 (Hatle et al. 2008). The degradation of this protein may contribute to the increased storage. This question could be addressed with compound-specific isotope techniques, such as labeling vitellogenin during clutch 1 and identifying turnover of the constituent amino acids in clutch 2.
The disposable soma hypothesis predicts that increasing allocation of nutrients to the soma permits greater maintenance of the soma. Hence, fully testing the disposable soma hypothesis requires testing levels of cellular maintenance (e.g., anti-oxidant activities, chaperones) in concert with nutrient allocation. The present data suggests that any enhanced cell maintenance should not be due to increased allocation of nutrients to somatic tissues. In future studies, we plan to test simultaneous nutrient allocation and levels of cellular maintenance upon dietary restriction (Hatle et al. 2006b).
EXPERIMENTAL PROCEDURES
Experimental animals
Lubber grasshoppers (Romalea microptera) were collected as juveniles in Miami, FL, USA and shipped to Jacksonville, FL. Juveniles were reared en masse on a 14L:10D photoperiod, ad libitum Romaine lettuce, oats, and occasional chopped green onions. Juveniles were offered mostly Romaine lettuce and ate little else.
Assignment to diet and surgery regimes
Upon molt to adult, females were serially assigned to a surgery group, either ovariectomy (=OVX) or sham-operated (=sham). Surgeries were performed according to Hatle et al. (2003b) within two days of molt to adulthood. Within each surgery group, individuals were then serially assigned to one of six artificial diet regimes, all starting at adult molt: a high 13C diet switched to a low 13C diet at first oviposition (HL), a continuous high 13C diet (HH), a low 13C diet switched to a high 13C diet at first oviposition (LH), or a continuous low 13C diet (LL). For estimation of growth during clutch 2, some females were fed on either a low 13C diet (LX) or a high 13C diet (HX) starting at adult molt and then dissected after clutch 1.
To determine the date of diet switching or dissection for ovariectomized animals, individuals were grouped based on their molt date. When half of the sham individuals in a group laid their first clutch, all remaining individuals on a ‘switch’ diet regime (i.e., HL or LH), including sham females who had not yet oviposited, were switched to their new diet. Similarly, when half of the sham individuals in a group oviposited for a second time (~27 days later), all individuals in that group were dissected. For the baseline controls to which the switched groups were compared (e.g., HH), n = 3.3±0.3 (mean±SE) for hemolymph storage proteins, n = 3.5±0.2 for femur muscles, n = 3.5±0.2 for fat body aqueous fractions, and n = 3.0±0.4 for fat body lipid fractions.
Feeding
The artificial diets have previously been described and shown to result in similar reproductive tactics (Judd et al. 2010). They contained either a high 13C protein source (δ13C = −13.98%thou) or a low 13C protein source (δ15C = −22.56%thou). In addition to differing in 13C, the protein sources also differed in 15N (δ15N = 2.15%thou and 7.73%thou respectively). For simplicity, we refer to the complete diets (including both lettuce and artificial diet) as ‘low 13C’ or ‘high 13C’. The high 13C artificial diet was 11.9% non-fibrous carbohydrate, while the low 13C artificial diet was 10.6% non-fibrous carbohydrate. The protein:carbohydrate ratio for both artificial diets was ~1:2. Romaine lettuce, the preferred lab diet for grasshoppers, has a protein:carbohydrate ratio of ~1:3.5.
Each day, individuals were fed their artificial diet (H or L) ad libitum. Uneaten artificial diet was collected daily, dried completely, and the mass was measured to determine the amount consumed. In addition, each individual was fed 0.5 g Romaine lettuce daily because females cannot reproduce on the artificial diets alone. The lettuce portion of the meal was almost always eaten in entirety. This amount of lettuce by itself is insufficient for egg production (e.g., Hatle et al. 2001).
During clutch 1, incorporation of 13C was complete (Judd et al. 2010). That is, the 13C levels in the diet nearly matched the 13C levels in the tissues, after adjusting for discrimination (see Martinez del Rio and Wolf 2005). For 15N, incorporation was ~60% for femur muscle and aqueous extract of fat body (Judd et al., 2010). Nonetheless, the qualitative conclusions for 13C and 15N during clutch 2 (i.e., this work) differed only for incorporation into the aqueous extract of fat body, and even for this tissue the trends were very similar (Fig. 3E,F).
Hemolymph storage proteins
The concentration of the three combined hexameric storage proteins in the hemolymph (Hathaway et al. 2009) were estimated as total hemolymph protein (Bradford 1976) from 5 μl samples that were collected about every two weeks (e.g., Hatle et al. 2001; 2006a). Vitellogenin levels were measured from the same samples by ELISA (Hatle et al. 2001). Hemolymph storage proteins were estimated as total hemolymph protein minus vitellogenin.
After clutch 2, hemolymph volume was estimated by exsanguinating the animal and estimating the volume of recovered hemolymph. Our estimation provides insight into relative hemolymph volumes between treatments. Samples were immediately frozen at −20°C.
Total storage proteins were isolated for stable isotope analysis. Samples were thawed and raw hemolymph was centrifuged at 12,000 rpm for 5 min. Supernatant (95 μl) was then added to 5 ml loading solution (50 mM tris, pH 8.2). This mixture was then centrifuged at 3000 rpm for 10 min to remove any precipitate. The supernatant was transferred to a 2 ml centrifugal filter device (Centricon, Bedford, MA, USA) with a 30,000 MWCO and again centrifuged at 5000 g for 1 hr at 4°C to remove any particles that could interfere with chromatography. The filtrate was discarded and the filter was washed with 7 ml loading solution to re-suspend proteins.
The proteins were then fractionated by anion-exchange chromatography (after Hathaway et al., 2009) using a 5 ml HiTrap Q FF ion exchange column (GE Healthcare, Uppsala, Sweden) at 5 ml/min with 50 mM tris, pH 8.2, with a NaCl step gradient of 0.0 M, 0.10 M, 0.23 M, 0.28 M, 0.40 M, 0.50 M, 0.60 M, and 1.00 M. The three hexameric storage proteins all eluted in the 0.23 M NaCl fraction (Hathaway et al., 2009). The tris was removed by dialysis. The samples were then acetone precipitated, pelleted, and the acetone was evaporated. Part of each pelleted precipitate (1300 - 1500 μg each) was used for stable isotope analysis (see below). This procedure also isolated vitellogenin in another distinct fraction. We were unable to isolate sufficient vitellogenin from individuals for stable isotope analysis because levels are low at the time of oviposition (e.g., Hatle et al. 2001).
Femur muscle
Femur muscle was removed by making an incision down the length of the femur and scraping out the muscle tissue with forceps. The muscle was then lyophilized, bead homogenized, and then 600 – 800 μg of each sample was used for stable isotope and percent carbon and nitrogen analysis (see below). This weighing procedure typically resulted in some spillage, which was not quantified but appeared to be small compared to the total sample. The remainder of the sample was used for quantification of total carbohydrates, measured as total anthrone positive material (e.g., Hatle and Spring 1999). These total carbohydrate measures do not include a correction for the size of the muscle or the size of the individual.
Fat body
Total visceral fat body samples were collected and weighed wet. Samples were stored at −20°C, lyophilized for at least 2 d, and then stored frozen.
Lipids were extracted from thawed fat body using a protocol modified from Folch et al. (1957). Lyophilized fat body was homogenized in a glass-glass tissue homogenizer in 10 ml of 2:1 chloroform: methanol solution. The homogenate was transferred to a glass 15 ml centrifuge tube and centrifuged at 2500 rpm for 5 min. The supernatant was removed and stored in a 10 ml Teflon capped glass vial. The pellet was re-suspended in 10 ml of 2:1 chloroform: methanol solution, and the extraction procedure was repeated twice more for each sample. The pellet was taken as the aqueous fraction of the fat body, and a portion was weighed (600 - 800 μg) for stable isotope analysis.
The combined chloroform: methanol supernatants were transferred to a 25 ml glass separatory flask and phase partitioned by adding ~5 ml of 0.1% NaCl and shaking for 30 s. The phases were allowed to partition and the organic layer was removed. This lipid layer was weighed for stable isotope analysis.
Stable isotope analyses
Each sample was weighed into a Costech 5×9 mm pressed tin capsule (Valencia, CA, USA) for stable isotope analysis. Analysis of δ13C and δ15N for each sample was determined by mass spectroscopy at the University of Florida Stable Isotope Geochemistry Lab, as previously (Judd et al. 2010).
Mixing model analysis
All tissues (i.e., hemolymph storage proteins, femur muscle, or fat body) for stable isotope analysis were collected after clutch 2. Hence, our mixing models always estimate the percentage of a tissue that is built from food ingested during clutch 2.
The contributions of particular dietary sources to tissues were estimated using linear mixing models (Martinez del Rio and Wolf 2005). When using stable isotopes, the discrimination of the isotope as it is metabolized by the animal must be taken into account. Discrimination results in differences in the composition of the diet and the composition of the consumer’s tissues. For example, animals tend to preferentially excrete 14N and therefore the soma accumulates 15N with age (Martinez del Rio and Wolf 2005). Perhaps the best way to account for such discrimination (as was done in this experiment) is to rear individuals on a constant diet over a long period and measure the difference between the dietary 13C concentration and the 13C composition of the tissue of interest (and the dietary 15N concentration and the 15N composition of the tissue of interest).
We raised control groups of grasshoppers on our high 13C diet during both clutch 1 and clutch 2 (HH), and other grasshoppers on our low 13C diet (LL). We also reared two groups of grasshoppers on switching diet regimens where they were fed the low 13C diet during clutch 1 and then switched to the high 13C diet during clutch 2 (LH), or visa versa (HL). With these data we were able to quantify the incorporation of 13C and 15N ingested during clutch 2 into a given somatic tissue using a two-source linear mixing model (Equation 1):
| Equation 1 |
The denominator of Equation 1 simply describes the entire range of possible stable isotope concentrations of the tissue of interest. This is divided into the degree of movement toward the composition that would exist if the animal had been continuously fed the final diet (i.e., the L diet for HL individuals tested during clutch 2). This estimates the portion of the tissue that is built from food ingested before the diet switch. Equation 2 is simply one minus this portion, to estimate the portion of the tissue that is built from food ingested after the diet switch.
| Equation 2 |
All mixing models were calculated using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA).
Statistics
Because the samples for nutrient allocation for the various tissues came from different individuals, MANOVA could not be used. Instead, for analyzing nutrient allocation by stable isotopes we used multiple Student’s t-tests with Bonferroni’s correction (α = 0.05/14 = 0.0036). The 14 comparisons include all possible comparisons between OVX and sham females, and all possible comparisons between clutch 1 and clutch 2 within a single tissue.
Amount of food eaten was analyzed via MANOVA, using time as a dependent variable.
Analyses of storage were made with two-way (surgery * clutch) ANOVAs for each of these response variables: hemolymph volume collected, storage protein concentration, and fat body mass. Storage in the femur muscle was analyzed with a two-way (surgery * clutch) MANOVA with percent carbon and total carbohydrates as response variables. Similarly, percent nitrogen in the femur muscle was analyzed with a two-way ANOVA, and fat body aqueous extracts were analyzed with a two-way MANOVA.
Supplementary Material
Supplemental Figure 1. Estimates of total allocation of nitrogen and carbon to storage protein and fat body aqueous extract from sham-operated (n = 6 for storage proteins, n = 7 for fat body aqueous extract) and ovariectomized (=OVX, n = 7 for storage proteins, n = 4 for fat body aqueous extract) lubber grasshoppers, during the period from first oviposition to second oviposition in intact females (i.e., clutch 2). These estimates incorporate tissue sizes into the allocation estimates. Both amount of storage protein and wet mass of fat body were increased by ovariectomy. In contrast, allocation of neither nitrogen nor carbon to storage protein and fat body were increased by ovariectomy. Combining these results provides an estimate of total allocation to storage proteins and aqueous extracts of the fat body, and neither of these was significant. Fat body aqueous extracts are best interpreted as the machinery for intermediary metabolism.
ACKNOWLEDGEMENTS
We thank Jason Curtis for analyzing the stable isotope samples. This study was funded by NIA awards 1R15 AG028512-01 and 2R15AG028512-02A1 to JDH and by NSF award IOS-641505 to DAH.
Footnotes
AUTHOR CONTRIBUTIONS
EJ, KW, FW, DH, and JH designed the experiments. EJ and MD reared the animals. EJ, MD, and MG analyzed the samples. JH, EJ, DH, and FW analyzed the data and wrote the paper.
REFERENCES
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Ann. Rev. Entomol. 2010;55:207–255. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmer ST. Insect herbivore nutrient regulation. Annual Review of Entomology. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 2010;298:E108–E116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RF. The Insects: Structure and Function. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Djawdan M, Sugiyama TT, Schlaeger LK, Bradley TJ, Rose MR. Metabolic aspects of the trade-off between fecundity and longevity in Drosophila melanogaster. Physiol. Biochem. Zool. 1996;69:1176–1195. [Google Scholar]
- Drewry MD, Williams JM, Hatle JD. Life-extending dietary restriction and ovariectomy result in similar feeding rates but different physiologic responses in grasshoppers. Experimental Gerontology. 2011 doi: 10.1016/j.exger.2011.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T. Survival costs or reproduction in Drosophila. Experimental Gerontology. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu M-P, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannes LZ, O’Brien DM, Martinez del Rio C. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology. 1997;78:1271–1276. [Google Scholar]
- Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway M, Hatle JD, Li S, Ding X, Barry T, Hong F, Wood D, Borst DW. Characterization of hexamerin protein and their mRNAs in the adult lubber grasshopper: the effects of nutrition and juvenile hormone on their levels. Comp. Biochem. Physiol. A. 2009;154:323–332. doi: 10.1016/j.cbpa.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Spring JH. Tests of potential adipokinetic hormone precursor related peptide (APRP) functions: lack of responses. Arch. Insect Biochem. Physiol. 1999;42:163–166. doi: 10.1002/(SICI)1520-6327(199910)42:2<163::AID-ARCH6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Eskew ME, Juliano SA. Maximum titers of vitellogenin and total hemolymph protein occur during the canalized phase of grasshopper egg production. Physiol. Biochem. Zool. 2001;74:885–893. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Juliano SA, Borst DW. Hemolymph ecdysteroids do not affect vitellogenesis in the lubber grasshopper. Arch. Insect Biochem. Physiol. 2003;52:45–57. doi: 10.1002/arch.10067. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Paterson CS, Jawaid I, Lentz C, Wells SM, Fronstin RB. Protein accumulation underlying lifespan extension via ovariectomy in grasshoppers is consistent with the disposable soma hypothesis but is not due to dietary restriction. Exp. Gerontol. 2008;43:900–908. doi: 10.1016/j.exger.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Wells SM, Fuller LE, Allen IC, Gordy LJ, Melnyk S, Quattrochi J. Calorie restriction and late-onset calorie restriction extend lifespan but do not alter protein storage in female grasshoppers. Mech. Age. Dev. 2006b;27:883–891. doi: 10.1016/j.mad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Waskey T, Jr, Juliano SA. Plasticity of grasshopper vitellogenin production in response to diet is primarily a result of changes in fat body mass. J. Comp. Physiol. B. 2006a;176:27–34. doi: 10.1007/s00360-005-0028-9. [DOI] [PubMed] [Google Scholar]
- Judd ET, Hatle JD, Drewry MD, Wessels FJ, Hahn DA. Allocation of nutrients to somatic tissues in young ovariectomized grasshoppers. Integ. Comp. Biol. 2010;50:818–828. doi: 10.1093/icb/icq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, Martínez del Rio C. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. Princeton University Press; Princeton: 2007. [Google Scholar]
- Kirkwood TBL. Immortality of the germ-line versus the disposability of the soma. In: Finch CE, Schneider EL, editors. Handbook of the Biology of Aging. 2nd ed. Van Nostrand Reinhold; New York: 1987. [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Mech. Age. Dev. 2002;123:737–74. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Martínez del Rio C, Wolf BO. Mass-balance models for animal isotopic ecology. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield: Science Publishers, Inc.; 2005. [Google Scholar]
- Min K-J, Hogan MF, Tatar M, O’Brien DM. Resource allocation to reproduction and soma in Drosophila: a stable isotope analysis of carbon from dietary sugar. J. Insect Physiol. 2006;52:763–770. doi: 10.1016/j.jinsphys.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Yen K, Hof PR, editors. Interdisciplinary Topics in Gerontology. Vol. 35. Karger; Basel: 2007. Mechanisms of Dietary Restriction in Aging and Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehrlin GS, Juliano SA. Plasticity of insect reproduction: testing models of flexible and fixed development in response to different growth rates. Oecologia. 1998;115:492–500. doi: 10.1007/s004420050546. [DOI] [PubMed] [Google Scholar]
- O’Brien DM, Min K-J, Larsen TL, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr. Biol. 2008;18:R155–R156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Pan ML, Telfer WH. Methionine-rich hexamerin and arylphorin as precursor reservoirs for reproduction and metamorphosis in female luna moths. Arch. Insect Biochem. Physiol. 1998;33:149–162. doi: 10.1002/(SICI)1520-6327(1996)33:2<149::AID-ARCH5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Simmons FH, Bradley TJ. An analysis of resource allocation in response to dietary yeast in Drosophila melanogaster. J. Insect Physiol. 1997;43:779–788. doi: 10.1016/s0022-1910(97)00037-1. [DOI] [PubMed] [Google Scholar]
- Spring JH, Gäde G. Factors regulating carbohydrate and lipid metabolism isolated from the corpus cardiacum of the Eastern Lubber Grasshopper, Romalea microptera. J. Exp. Zool. 1987;241:41–50. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- Tatar M. The plate half-full: status of research on the mechanisms of dietary restriction in Drosophila melanogaster. Experimental Gerontology. 2011;46:363–368. doi: 10.1016/j.exger.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Tu M-P, Yin C-M, Garofalo RS. A mutant Drosophila insulin receptor extends lifespan and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Wessels FJ, Hahn DA. Carbon 13 discrimination during lipid biosynthesis varies with dietary concentration of stable isotopes: implications for stable isotope analyses. Funct. Ecol. 2010;24:1017–1022. [Google Scholar]
- Yang Y, Joern A. Compensatory feeding in response to variable food quality by Melanoplus differentialis. Physiol. Entomol. 1994;19:75–82. [Google Scholar]
- Zera AJ, Zhao Z. Intermediary metabolism and life-history trade-offs: differential metabolism of amino acids underlies the dispersal-reproduction trade-off in a wing-polymorphic cricket. Am. Nat. 2006;167:889–900. doi: 10.1086/503578. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zera AJ. Biochemical basis of specialization for dispersal vs. reproduction in a wing-polymorphic cricket: morph-specific metabolism of amino acids. J. Insect Physiol. 2006;52:646–58. doi: 10.1016/j.jinsphys.2006.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Estimates of total allocation of nitrogen and carbon to storage protein and fat body aqueous extract from sham-operated (n = 6 for storage proteins, n = 7 for fat body aqueous extract) and ovariectomized (=OVX, n = 7 for storage proteins, n = 4 for fat body aqueous extract) lubber grasshoppers, during the period from first oviposition to second oviposition in intact females (i.e., clutch 2). These estimates incorporate tissue sizes into the allocation estimates. Both amount of storage protein and wet mass of fat body were increased by ovariectomy. In contrast, allocation of neither nitrogen nor carbon to storage protein and fat body were increased by ovariectomy. Combining these results provides an estimate of total allocation to storage proteins and aqueous extracts of the fat body, and neither of these was significant. Fat body aqueous extracts are best interpreted as the machinery for intermediary metabolism.