SUMMARY
Activation of Sir2-orthologs is proposed to increase lifespan downstream of dietary restriction (DR). Here we describe an examination of the effect of 32 different lifespan-extending mutations and four methods of dietary restriction on replicative lifespan (RLS) in the short-lived sir2Δ yeast strain. In every case, deletion of SIR2 prevented RLS extension; however, RLS extension was restored when both SIR2 and FOB1 were deleted in several cases, demonstrating that SIR2 is not directly required for RLS extension. These findings indicate that suppression of the sir2Δ lifespan defect is a rare phenotype among longevity interventions and suggest that sir2Δ cells senesce rapidly by a mechanism distinct from that of wild-type cells. They also demonstrate that failure to observe life span extension in a short-lived background, such as cells or animals lacking sirtuins, should be interpreted with caution.
Keywords: aging, replicative lifespan, longevity, yeast, epistasis
Combining two or more longevity-altering interventions and determining the resulting effect on lifespan is a common method for examining the relationship between such interventions. An important subset of this type of analysis occurs when one of the factors under study promotes longevity, such as daf-16 in Caenorhabditis elegans or SIR2 in Saccharomyces cerevisiae. For both of these genes, several studies have combined a lifespan shortening null allele with an intervention that extends lifespan. A resulting lifespan similar to that of the short-lived single mutant has generally been interpreted as suggesting that the factors act in the same pathway. In contrast, an intervention extending the lifespan of the short-lived mutant has been interpreted as suggesting that the factors act in genetically distinct pathways. Specific examples of this type of comparison are studies in which DR fails to extend lifespan in yeast (Lin et al. 2000), invertebrates (Rogina & Helfand 2004; Wang & Tissenbaum 2006), and mice (Li et al. 2008) when Sir2-orthologs are mutated. These data have been, and continue to be, interpreted by some to support a model in which DR promotes longevity and healthspan through activation of sirtuins (Baur et al. 2010).
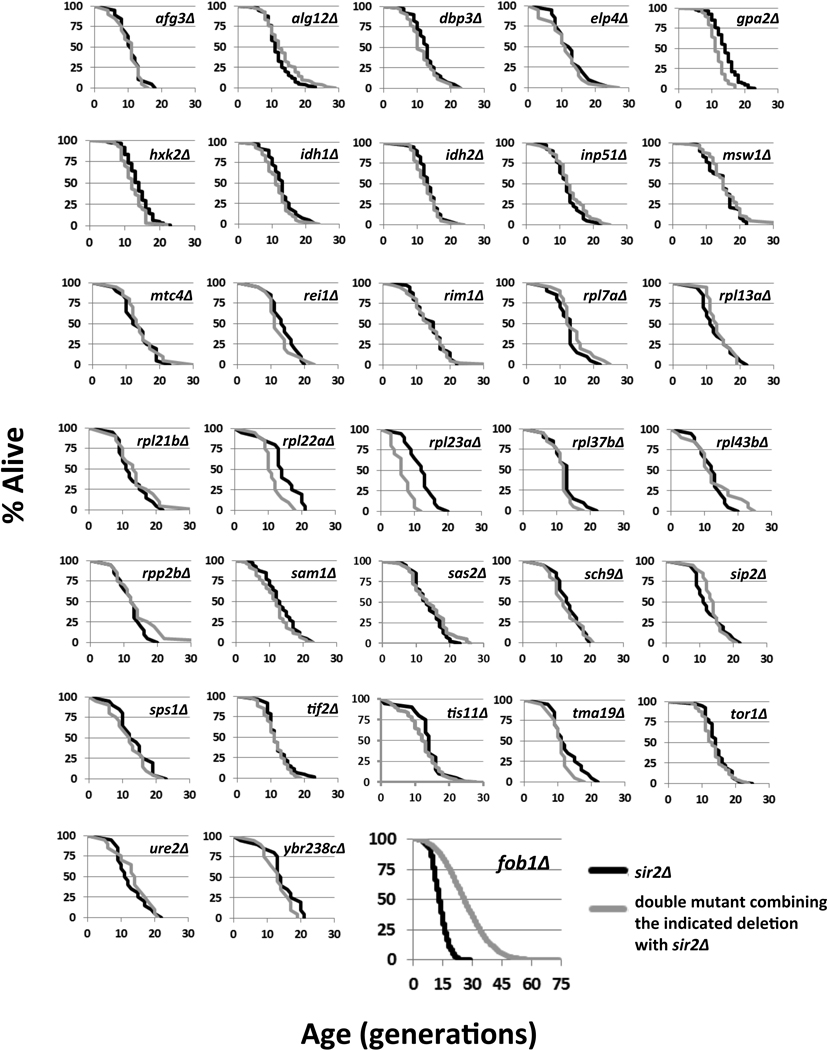
It has been previously reported that deletion of SIR2 blocks RLS extension from DR by reduction of glucose and in strains lacking GPA2 or HXK2, two genetic mimics of DR, but not in a strain lacking the rDNA replication fork block protein, FOB1 (Kaeberlein et al. 2004). In order to examine the influence of deleting SIR2 on RLS extension more generally, we generated 30 additional double mutant strains in which a RLS extending deletion was combined with deletion of SIR2. We also tested three additional methods of DR involving growth on alternative carbon sources (ethanol, glycerol, or raffinose). Strikingly, none of these interventions resulted in a significant RLS extension relative to sir2Δ cells (Figure 1; Figure S2; Table S1).
Figure 1. Single-gene deletions that extend RLS in wild-type cells do not extend RLS of sir2Δ cells.
Replicative survival curves are provided for 33 double mutant strains combining a known long-lived gene deletion with deletion of SIR2.
One possible interpretation of these data is that each of the RLS-extending interventions acts upstream of Sir2, perhaps by promoting Sir2 activity. Two observations are inconsistent with this model. First, at least eight single-gene deletions that increase wild type RLS, and all four forms of DR, significantly extend the RLS of sir2Δ fob1Δ cells (Figure S1A; Figure S2; Table S1), demonstrating that SIR2 is not absolutely required for RLS extension in these cases. Second, at least five long-lived deletion mutants show no indication of enhanced Sir2 activity in vivo, as measured by rDNA recombination or rDNA silencing (Figure S3). A similar lack of increased Sir2 activity has been previously reported in cells subjected to DR (Kaeberlein et al. 2005; Riesen & Morgan 2009; Smith et al. 2009). Interestingly, deletion of TOR1 caused a significant decrease in rDNA recombination, but this effect was independent of SIR2 (Figure S3A).
An alternative explanation for these data is that loss of SIR2 alters aging such that molecular processes that do not limit RLS in wild-type cells become limiting in sir2Δ cells. Sir2 has multiple functions, including repression of extrachromosomal rDNA circle formation (Kaeberlein et al. 1999), enhancing global rDNA stability and silencing (Gottlieb & Esposito 1989; Smith & Boeke 1997), promoting asymmetric inheritance of damaged proteins (Aguilaniu et al. 2003), and maintaining telomeric chromatin during aging (Dang et al. 2009). Our observation that only deletion of FOB1 is sufficient to suppress the short RLS of sir2Δ cells suggests that (1) the primary RLS-limiting defect in sir2Δ cells is likely related to rDNA instability and (2) none of the 32 deletions tested that slow aging in wild-type cells is able to overcome this defect. One prior study reported that overexpression of Hsp104 could also suppress the short RLS of sir2Δ cells (Erjavec et al. 2007), raising the possibility that accumulation of damaged proteins in sir2Δ mother cells may also contribute to the reduced longevity.
While it is likely that many of the genes examined in this study do not require Sir2 for their effect on RLS, we do not believe that all of the 32 long-lived single gene deletion mutants examined here necessarily act via Sir2-independent mechanisms. For example, deletion of SAS2, a histone acetyltransferase known to antagonize Sir2 effects on chromatin (Dang et al. 2009), extends wild-type RLS but fails to extend the RLS of sir2Δ fob1Δ cells (FigureS2b). Thus, both functional and genetic evidence suggest that Sas1 likely acts in the same longevity pathway as Sir2.
This study provides a clear demonstration of the challenges associated with interpreting longevity epistasis data. In particular, the failure of a longevity-intervention to extend lifespan in a short-lived background may not be informative regarding the mechanism of lifespan extension in the wild-type context. In the absence of strong evidence indicating that the lifespan shortening is caused by acceleration of the wild-type aging process, caution is warranted when interpreting these types of data.
Supplementary Material
Acknowledgements
This work was supported by NIH Grant R01AG025549. JRD, GLS, and SJ were supported by NIH Training Grant T32AG000057. JS was supported by NIH Training Grant T32ES007032. XL is supported by The National Natural Science Foundation of China (30672205, 30871440, 30900739, 30971620, 31101051), The Natural Science Foundation of Guangdong Province (7301506, 8452402301001450, 9252402301000002), and Key Foundation of Natural Science Research for Guangdong Universities (06Z015). MK is an Ellison Medical Foundation New Scholar in Aging.
Footnotes
Supporting Information. Experimental Procedures are provided as online Supporting Information, as are the following figures and tables:
Figure S1. Multiple forms of DR extend RLS in a Sir2- and Fob1-independent manner.
Figure S2. Long-lived mutants extend RLS of sir2Δfob1Δ cells.
Figure S3. rDNA recombination and silencing is not increased in long-lived strains.
Table S1. Summary of lifespan data presented in this study.
Table S2. Percent extension in replicative lifespan resulting from each gene deletion or intervention in the indicated genetic background.
Table S3. Genes examined in this study.
Table S4. Strains used in this study
REFERENCES
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- Baur JA, Chen D, Chini EN, Chua K, Cohen HY, de Cabo R, Deng C, Dimmeler S, Gius D, Guarente LP, Helfand SL, Imai S, Itoh H, Kadowaki T, Koya D, Leeuwenburgh C, McBurney M, Nabeshima Y, Neri C, Oberdoerffer P, Pestell RG, Rogina B, Sadoshima J, Sartorelli V, Serrano M, Sinclair DA, Steegborn C, Tatar M, Tissenbaum HA, Tong Q, Tsubota K, Vaquero A, Verdin E. Dietary restriction: standing up for sirtuins. Science. 2010;329:1012–1013. doi: 10.1126/science.329.5995.1012. author reply 1013–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec N, Larsson L, Grantham J, Nystrom T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes & development. 2007;21:2410–2421. doi: 10.1101/gad.439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell S, Napper A, Curtis R, Distefano PS, Fields S, Bedalov A, Kennedy BK. Substrate specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Riesen M, Morgan A. Calorie restriction reduces rDNA recombination independently of rDNA silencing. Aging Cell. 2009;8:624–632. doi: 10.1111/j.1474-9726.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Jr, Li C, Matecic M, Maqani N, Bryk M, Smith JS. Calorie restriction effects on silencing and recombination at the yeast rDNA. Aging Cell. 2009;8:633–642. doi: 10.1111/j.1474-9726.2009.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.