Abstract
Normal oxygen level is critical for niches that together with other components of the niche play vital role in regulating stem or tumor cells behavior. Hypoxia plays an important role in normal development and disease progression, including the growth of solid tumors. The hypoxia inducible factors (HIFs) are the key mediators of the cellular response to hypoxia. In this review, we focused on the role of HIFs on bone tumor formation. Further, we also emphasized how hypoxia, stem cells, and its niches regulate the bone tumorigenesis.
Keywords: Hypoxia; Hypoxia inducible factors; Bone tumor, Bone metastasis; Stem cells; Osteosarcoma
1. Introduction
Hypoxia inducible factors (HIFs) are essential for cellular oxygen homeostasis maintenance and hypoxia adaptation when oxygen levels cannot meet the needs of the cell. HIFs is associated with PAS (Per-ARNT-Sim) family of basic helix-loop-helix transcription factors, which bind to DNA as heterodimers, and are composed of an oxygen-dependent α subunit and an oxygen-independent β subunit. The α-subunit has three isoforms, HIF-1α, HIF-2α, and HIF-3α. The β subunit, also called as the aryl hydrocarbon receptor nuclear translocator (ARNT), has only two isoforms referred to as HIF-1β and HIF-2β. Alpha subunit degradation occurs in a posttranslational prolyl hydroxylation manner via the von Hippel-Lindau (VHL)-mediated ubiquitination pathway by binding to the oxygen-dependent degradation domain (ODDD) under normoxia [1-3]. The α-subunit is transported into the nucleus and dimerises with the β subunit when oxygen concentrations become less than 6% [2, 4]. This complex binds to the 5-RCGTG-3 core sequence of the hypoxia responsive element (HRE) within the enhancer promoter region of HIF target genes, facilitating transcription of target genes responsible for adaptations to hypoxia, including anaerobic energy supply, erythropoiesis, angiogenesis, pH regulation, and cell survival [5]. At the cellular level, HIF proteins [6] can influence any biological behavior, including pathological or physiological behavior, under a hypoxic microenvironment. This influence is especially important in the context of tumor growth, as tumors cannot grow beyond several mm3 without angiogenesis due to limited diffusion of O2, glucose, and other nutrients [7]. One major focus in the field of cancer biology is to understand the mechanisms by which hypoxia or HIFs can regulate bone tumor progression and the formation of metastases. Recently, a number of studies have demonstrated a more complex regulatory mechanism for HIFs in tumor progression [8-10], but many aspects of this mechanism remain unresolved. Several unique roles of HIFs in the context of tumor progression will be described in this review (Fig.1). Further, we also discussed how hypoxia, stem cells, and its niches involve in bone tumorigenesis and tumor therapy.
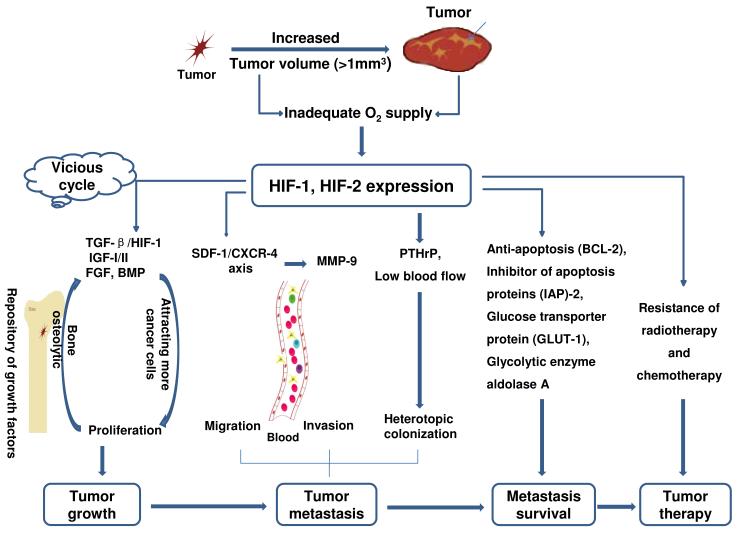
Figure 1.
The role of hypoxia on tumor development. With the fast development of tumor, especially if the tumor volume exceeds several mm3, insufficient angiogenesis and tumor necrosis will be accompanied, which will limit to the diffusion of oxygen, glucose and other nutrients in the tumor, and HIFs will be up-regulated because HIFs play a critical role in mediating tumor adaption to hypoxia. Unfortunately, bone is a repository of several growth factors such as TGF-β, IGF-I/II, FGF and BMP, and those factors are released into the blood and promote tumor growth, which will further cause bone destruction. HIFs can regulate tumor migration, invasion and heterotopic colonization by the mediation of SDF-1/CXCR4, MMP-9 and PTHrP. Additionally, HIFs can also promote tumor cell survival by activating antiapoptotic genes such as the Bcl-2 gene family and inhibitors of apoptosis (IAPs), or by increasing glucose uptake and glycolysis anti-apoptosis.
2. Hypoxia and osteosarcoma
Osteosarcoma is the very common bone cancer in children and adults, characterized by frequent relapse and metastatic disease and show strong resistance to chemotherapy with poor prognosis. It has been established that, when the volume of a tumor exceeds the critical value of 1 mm3 [7], oxygen is unable to diffuse completely throughout the tumor. Because hypoxia exists in almost all solid tumors due to the imbalance between their rapid growth and nutrient and blood supply, the adaptation to hypoxia by tumor cells is particularly important. HIFs, which comprise both a hypoxia-inducible α subunit and a constitutively expressed β subunit, play a critical role in this process. It is associated not only with resistance to therapy and poor survival [11] but also with disease grade, stage and recurrence in the clinic [12-13]. Although HIFs and their downstream genes are up-regulated in osteosarcoma cells cultured under hypoxic conditions in vitro, HIF does not increase tumor cell proliferation or migration but instead enhances apoptosis [14]. This does not correlate with the clinical reports [11, 15-17]. As an explanation for this abnormal phenomenon, some researchers have proposed that the antagonistic effects of HIFs and their downstream genes cancel each other out in vitro [14].
Accumulative evidence suggests that HIF can induce the apoptosis through both direct and indirect mechanisms. It has been demonstrated that HIF promotes tumor cell survival under hypoxic conditions by directly inducing the expression of the proapoptotic genes such as BNIP3 and NIX [18]. The expression of BNIP3 is inhibited by HIF-2, which stabilizes p53 by inactivating the p53 ubiquitin ligase Mdm2 or induces p53 after its binding to the oxygen-dependent degradation domain on HIF-1 that activates p53-mediated cell cycle arrest and results in cellular apoptosis [19]. HIF-1α can also indirectly induce apoptosis and promotes tumor cell survival by activating the anti-apoptotic genes, such as the Bcl-2 gene family and inhibitors of apoptosis (IAPs), or by increasing glucose uptake and glycolysis [20,21].
Hypoxia promotes both HIF-1α and HIF-2α expression and function, as assessed by downstream gene expression, western blot, luciferase assay and ELISA using osteosarcoma models. It is known that hypoxia-induced apoptosis is mediated by HIF-1α. Studies have revealed that expression of hypoxia relevant genes occurs mainly in necrotic areas, which suggest that there is in vivo relationship exists between HIF-1α and apoptosis in osteosarcoma tumors [15]. Interestingly, under hypoxic conditions, HIFs regulate the expression of downstream genes such as Glut-1 and VEGF through binding to their HRE regions; however, there is no HIF transcriptional response under conditions of low glucose, when cellular proliferation is reduced by 45% [14]. The molecular mechanisms associated with this phenomenon are under investigation. Studies have also shown that HIF-1α silencing downregulated the phosphorylation of Akt, which inhibit apoptosis by blocking the activity of proapoptotic factors Bad and caspase-9 that ultimately promotes the cell survival by increasing the phosphorylation of IKKβ, which in turn activates nuclear factor-κB (NF-kβ) [22, 23] Furthermore, mitochondria respond to multiple death stimuli that permeabilize the mitochondrial membrane, which result in release of apoptotic molecules, such as cytochrome c and AIF [24]. These molecules activate Apaf-1 and trigger the caspase machinery that ultimately results in cell death. A recent study suggest that HIF-1α silencing regulated the balance between proapoptotic protein Bax and antiapoptotic proteins Bcl-xL and Bim, as well as dysfunction the mitochondrial activity, which results in caspase-dependent death [25]. Apoptosis induced by inadequate or inappropriate cell-matrix interactions is called anoikis. It is interesting to note that osteosarcoma cells have the properties to detach from matrix components and metastasize as they show anchorage-independent growth and are resistant to anoikis [26]. It has been suggested that the pathways responsible for anoikis include integrin, Rho GTPases, PI3 kinase, and PKB/Akt, together with components of the intrinsic and extrinsic apoptosis pathways [26]. Díaz-Montero et al. [27] have shown that different mediators regulate the resistance to anoikis and apoptosis in osteosarcoma.
3. Hypoxia and chondrosarcoma
Chondrosarcoma is the second most common bone tumor and is typically associated with a poor prognosis due to its insensitivity to radio- and chemotherapy. Wide surgical excision is the only curative treatment. Unlike osteosarcomas, which usually occur in minors, chondrosarcomas generally occur in adults [28]. Because cure of chondrosarcomas has not improved over the past several decades and the classification of the tumor grade is even subjective, identifying an early stage prognostic marker, which potentially improve its treatment is an urgent goal [28].
Mature cartilage contains almost no blood vessels, so cartilage growth occurs in a hypoxic microenvironment [29]. HIF-1, which is the main factor mediating hypoxia response, may have an important role in the prognosis of chondrosarcoma. In fact, Kubo et al. [28] investigated the expression of HIF-1α and HIF-2α in 29 chondrosarcoma specimens and found that HIF-1α protein may be a useful prognosis marker due to its important role in tumor angiogenesis and cellular proliferation. They indicated that HIF-1α protein might be a more objective marker in determining chondrosarcoma patient prognosis than the histological grade of the tumor.
There are several mechanisms responsible for the migration and invasion of chondrosarcoma related to HIF-1α expression and the expression of its downstream genes. The CXCR4/SDF-1 pathway plays a critical role [30]. SDF-1, a downstream target of HIF-1α that is mainly induced by hypoxia, binds to its receptor CXCR4 and indirectly promotes tumor metastasis by mediating tumor cell proliferation and migration. Sun et al. [31] demonstrated that CXCR4 blockade by AMD3100, a clinically approved inhibitor of CXCR4/SDF-1, could inhibit chondrosarcoma invasion and metastasis in vitro. To elucidate the underlying mechanism behind CXCR4/SDF-1-mediated tumor cell invasion, they measured the levels of MMP-1, a critical factor, involved in chondrosarcoma metastasis and found a 9-fold increase in MMP-1 mRNA levels when tumor cells were cultured under hypoxic conditions. These levels could be further increased to 23-fold by SDF-1 stimulation. Further, they found that SDF1 stimulation during hypoxia also increased MMP1 protein expression. Interestingly, CXCR4 inhibitor AMD3100 could block the increased effect on MMP-1 mRNA as well as MMP-1 protein expression during hypoxia, providing further evidence that CXCR4/SDF-1 signaling can induce MMP-1 expression and promote chondrosarcoma cell migration and invasion [31]. Their results suggest blocking the activity of CXCR4 can inhibit the effects of hypoxia on MMP1 expression, which results in inhibition of chondrosarcoma invasion and metastasis [31].
4. Hypoxia and Ewing’s sarcoma
The Ewing’s sarcoma (EWS) family of tumors, characterised by the presence of EWS-ETS gene rearrangements, are the second most frequent bone tumor in teenagers and young adults [32]. They are rare, highly malignant tumors with clinical metastases occurring in approximately one-fourth of all patients [33]. An additional feature of Ewing’s sarcoma is the presence of blood lakes linked by tumor cells. These correlate with poor clinical outcome, whereas outcome does not correlate with variables of angiogenesis [33]. Studies suggest that there is blood flow through these RBC lakes, and this often occurs during adolescence. van der Schaft et al. [33] presents evidence that supports the idea that oxygen tension plays a significant role in tube formation in vitro [34]. Interestingly, the HIF protein, which mediates responses mainly under hypoxic conditions, has a significant effect on Ewing’s sarcoma family tumors (ESFT). Aryee et al. [32] found that the EWS-FLI1 protein, which is characteristic of ESFT, was up-regulated in a HIF-1α-dependent manner and that HIF-1α induced EWS-FLI1 accumulation in a time-dependent dynamic study. Further studies suggesting that the regulation of EWS-FLI1 in hypoxic environments may occur at the posttranscriptional level are supported by the observation that HIF-1α-activated genes, such as VEGF, Aldolase-C, GLUT-1, CA9, and IGFBP3, were increased under hypoxia [35], whereas EWS-FLI1 RNA expression remained unchanged. Hypoxia enhances the malignancy of ESFT mainly through increasing invasive capacity, as demonstrated in a Matrigel-coated transwell insert assay, and colony-formation capacity, as assessed by an anchorage-independent growth assay.
Hypoxia may also contribute to the aggressive metastatic behavior of ESFT. HIF-1α and EWS-FLI1 may function together in both synergistic and antagonistic cross-talk under conditions of hypoxia, and in the future, targeting these functions may prove to be of therapeutic benefit.
5. Hypoxia and breast cancer bone metastases
Most patients with breast cancer die not because of the primary tumor but, instead, due to distal metastasis [36]. Patients with advanced breast cancer are likely to develop bone metastases and develop severe bone pain, pathologic fracture, life-threatening hypercalcemia and other complications due to osteolytic bone destruction [37]. The mechanism underlying tumor cells metastasising directly to bone remains unclear. Tumor cell metastasis to distant organs depends not only on the type of cancer itself but also on the microenvironment of the organs that develop the metastasis [36]. Bone has a unique microenvironment, and the primary functions of this organ are haematopoiesis and weight-bearing. There is a positive feedback mechanism to account for the frequency of bone metastasis. Low blood flow within the red marrow allows tumor cells in the blood circulation to adhere to the bone matrix and the marrow stromal cells [38], while at the same time, facilitating the release of many bone resorbing factors and angiogenic factors that enhance tumor cell development. Unfortunately, bone is also a large repository of growth factors including transforming growth factor β, insulin-like growth factor I and II, fibroblast growth factors, bone morphogenetic proteins and calcium [39]. With the destruction of sclerosis, large amounts of these factors are released into the blood circulation, ultimately attracting more cancer cells circulating within the bloodstream to the sites of tumor cell adhesion and further promoting cancer cell growth. This process ultimately allows distant metastases to thrive.
Of all the growth factors contained within the bone, TGF-β has the most complex functions [40]. While it is considered a growth suppressor early in tumorigenesis, in later stage tumors, has a role increasing the production of PTHrP by breast cancer cells [41], which plays a unique role in breast cancer cells by facilitating their metastasis to bone. In osteoclast bone metastasis of breast cancer, the absorption of bone is mediated by osteoclasts rather than by the tumor cells [42]. Thus, any effect on osteoclast function can play a role in breast cancer metastasis. Parathyroid hormone-related peptide interaction with its receptor, PTHR1, likely stimulates the formation of osteoclasts by the RANKL-OPG pathway through expression of RANKL on the surface of osteoblasts and marrow stromal cells, rather than by acting directly on osteoclast precursors cause increased bone resorption [38, 43]. Additional to the osteolytic bone metastases, NF-kβ-HIF-1α forms a complex, this complex reciprocal regulation and enhanced c-jun expression contributes to the migration, invasion and bone tropism of 1833 (human) and 4T1 (mouse) metastatic breast cancer cells by means of an incomplete epithelial-mesenchymal transition (EMT) [44]. Other important pathways such as hypoxia and TGF-β interaction influence breast cancer bone metastasis [40, 41]. Many genes involved in bone metastasis that are regulated by TGF-β are also regulated by hypoxia, such as CTGF, CXCR4, IL-11 and MMP-1 [45, 46]. These genes influence different steps of the metastasis cascade, such as invasion, homing, angiogenesis, and osteolysis. To study the mechanism underlying the hypoxia and TGF-β interaction, Dunn et al. [40] have developed a model by altering TGF-β expression levels and oxygen supply and have investigated downstream gene expression changes in this model in vitro. They discovered that only VEGF and CXCR4 responded to TGF-β and hypoxia. Additionally, they found significant decreases in osteolysis through gene inhibition of TGF-β or the hypoxia-signaling pathway in vivo. Interestingly, there was no additional reduction in lesion area and survival benefit under the combined inhibition of both the TGF-β and hypoxia pathways, suggesting that these two signalling pathways function in parallel. Neovascularisation and elevated glycolysis, two common characteristics of solid tumors, represent adaptations to a hypoxic microenvironment that are correlated with tumor invasion, metastasis, and lethality [9].
6. Hypoxia and prostate cancer bone metastasis
Unlike breast cancer, where metastasis to bone mainly causes osteolytic lesions, there are two prostate cancer tumor types that commonly metastasise to bone but cause osteoblastic lesions [47]. Patients with metastasised prostate cancer suffer from pain because of pathologic facture and nerve compression due to abnormal bone remodelling. The precise mechanisms involved in prostate cancer osteoblastic bone metastasis are unknown. Several genes, including u-PA [48] and PSA [49], and several growth factors, such as FGF and Endothelin-1, are under investigation for their involvement in this process. In recent years, the unique role of HIF-1α and its target genes, especially VEGF, on prostate cancer bone metastasis is gradually being elucidated [50]. VEGF can affect bone remodelling and facilitate tropism during cancer cell bone metastasis [51]. HIF-1α regulates VEGF transcription via the cyclic AMP-responsive element-binding protein (CREB) pathway under normoxic conditions [51]. Paradoxically, HIF-1α degradation under normoxic conditions occurs via VHL-mediated ubiquitination, and other studies have demonstrated that CREB plays an important role in this process. CREB and HIF-α form a transcription complex in normoxic tumor cells, and coordinated activation of CREB/HIF-1α then directly contributes to the transcription of VEGF in bone metastases. VEGF binds to its receptor, VEGFR-1, and promotes tumor progression by inducing EMT and acquisition of invasive phenotypes. The CREB pathway, however, regulates gene expression in a highly tissue and cell type-specific manner. Additional investigations have shown that VEGF can induce initial differentiation of osteoblasts and can potentially influence the ability of prostate cancer to develop osteoblastic lesions [50, 51]. Similar to bone morphogenetic proteins (BMP) and Endothelin-1 that directly stimulate differentiation of osteoblast precursors to mature mineral-producing osteoblast, VEGF also has an effect on osteoblast differentiation but is not sufficient to promote full osteoblastic differentiation because BMPs, endothelin-1, Wnts, and urokinase plasminogen activator remain absent. This consistent with the hypothesis that VEGF promotes prostate cancer-mediated osteoblastic activity [50].
7. Hypoxia and bone tumor therapy
Doctors are making great strides in preventing bone tumor progression and bone metastasis development. Currently, there are several approaches have been applied in the clinic to treat bone tumors and bone metastases. Most of these emphasise the prevention of stimulating osteoclasts and blockade the bone resorption or decrease PTHrP concentrations in the blood. The drugs applied include Osteoprotegerin, RANK-Fc [52, 53], bisphosphonates [54], PTHrP antibodies [42], and vitamin-D analogues. Other drugs focus on targeting cytokines stored in the bone, such as TGF-β, by preventing their release once the bone has been destroyed.
We find the adaptation to tumor hypoxia is important in both tumor development and metastasis [11]. HIF-1α, which mainly functions under hypoxia, plays a critical role in tumor cell migration and invasion. The antiangiogenic effects produced by pharmacologic inhibition of the HIF target VEGF also underscore the importance of this protein for tumor angiogenesis. In addition, HIFs play a major role in drug-resistance and radiation-resistance [11], which are significant problems in the treatment of cancer. Blockade of the HIF pathway may provide therapeutic advantage in patients suffering from these conditions.
8. HIFs as targets for cancer therapy
Hypoxia is common to all solid tumors and tumor hypoxia has great significance in clinical studies. Hypoxia-inducible factor 1 (HIF-1) is a central mediator of cellular responses to low oxygen and has recently become an attractive target for solid tumor therapy because of frequently reported association between HIF-1α overexpression and poor outcome in clinical series. HIF-1α has been rated top in the list of targets for cancer therapy as it plays a vital role in regulating tumor survival and growth under hypoxic condition. In recent years, several small molecules have been identified, which can specifically target the HIF pathway [55-58]. However, these molecules work indirectly, show pleiotropic effect as well as have poor pharmacologic properties, which suggest that development of HIF inhibitors would be essential [59]. Recently, Narita et al. [60] identified the novel small molecule KC7F2, which act as a potent HIF-1α translation inhibitor and can be used in the development of antitumor agent for clinical cancer therapy. This molecule show enhanced cytotoxicity in several cancer cells during hypoxia and inhibit the HIF transcriptional activity by down-regulating the protein HIF-1α subunit, which ultimately reduce the translation of its mRNA [60]. In addition, it has been found that KC7F2 significantly repressed the phosphorylation of 4EBP-1 that suggests that it can inhibit the HIF-1α protein synthesis [60]. It has been found that mTOR up-regulation elevates the HIF-1α protein expression via translation initiation factors including 4E binding protein 1 (4EBP1) and p70 S6 kinase (S6K) and these factors could be novel targets in HIF-1α inhibitor development [60].
There are several gene therapy strategies targeting tumor hypoxia [61] has been developed, which include hypoxia-responsive promoters combined with Gene-Directed Enzyme Prodrug Therapies (GDEPT) [61], hypoxia-specific replication of adenovirus [62], and anaerobic bacteria-mediated delivery systems [63]. Recently, it has been found that oxygen-dependent degradation (ODD) - protein transduction domain (PTD) fusion protein, a vital tool for gene delivery, can be used to target HIF-1-active microenvironment, which ultimately results in cancer therapy [61]. Based on the above studies, it is clear that targeting HIF-1 by combined treatment of HIF-1 inhibitors, together with upstream and downstream pathways, chemotherapy and radiotherapy would be novel and efficient strategy for cancer therapy in clinic [64].
9. Hypoxia, stem cell and bone tumor
Among all bone cancer, the osteosarcoma is the most common and leading cause of death in children and adolescents. However, how these bone cancers develop is currently unknown. The conventional therapy in which osteosarcoma can be treated by combining chemotherapy with anti-angiogenic therapy. However, we need to develop a novel therapy such as cell therapy, which can kill specifically to cancer stem cells, main culprit in metastasis of tumor. Recently, it has been lots of attention in the field of cancer stem cells (CSC). CSC have been identified in several diverse tumors including, brain, skin, leukemia, breast, neck, colon, head and neck, renal, liver, prostate and pancreatic tumors [65-72]. It is also followed that CSC plays major roles in drug resistance, tumor recurrence, and metastasis. Recent studies suggest that osteosarcoma contains cancer stem cells [73-76]. CSC is also reported in Ewing’s sarcoma [77]. Levings et al. [78] have shown that Osteosarcoma stem cell can activate the Oct-4 gene promoter. Tang et al. [79] and Mohseny et al. [80] have shown that MSCs or osteoprogenitor cells because of disruption in the osteoblast differentiation pathway develop Osteosarcoma.
It is suggested that in hematopoietic niches, cancer stem cells reside to the bone marrow, via receptors such as CXCR-4, in a area where high levels of stromal cell derived factor 1 (SDF-1) is present and this region is regulated by oxygen tension [81]. Osteoblasts are critical in these niches. To eliminate and avoid bone cancer, it is fundamental to destroy cancer stem cells, which home in the osteoblast niche, as well as block the expression or activity of other factors, which regulate or accelerate reentry of these cells in cell cycle. It has been reported that RANK receptor is expressed in cancer cells and its ligand expressed in marrow stromal and immature osteoblast cells. Further, it is known that cancer cells secrete osteoblastic factors (ET-1, AM, VEGF, PDGF, CCNs-bone formation) as well as osteolytic factors (RANK L, PTHrP -bone destruction) [82]. It has been shown that PTHrP is critical in bone metastasis in breast cancer and prostate cancer, and is regulated by RANK L [12].
Recently, several investigations have shown that hypoxia regulate the sub-population of CSC and maintain the normal tissue or non-stem cell tissue in a stem cell state [83-86]. It has also found that hypoxic areas within a tumor might serves as niches for cancer stem cells and hypoxia conditions help in reprogramming of cells in the generation of iPS colonies [87]. Most of metastatic tumor contains CSC and show chemotherapy resistance, and it is possible that tumors may develop from mutation in normal stem cell, which transform to become CSC or tumors develop during hypoxia condition from non-stem cells population (Fig. 2). Further, recent studies suggest that HIF is highly expressed in CSC in several tumors and blocking HIF-1α or HIF-2α activity results in dramatic decrease in CSC proliferation and self-renewal [88-91]. These findings suggest that oxygen tension and microenvironment are critical in cancer development and that targeting hypoxia microenvironment would be novel strategy to eliminate CSC population. Several recent reports suggest that hypoxia and stem cell mediated therapy is a viable in eliminating CSC, which in turn provides therapy for several types of malignancies including bone tumor [88-91].
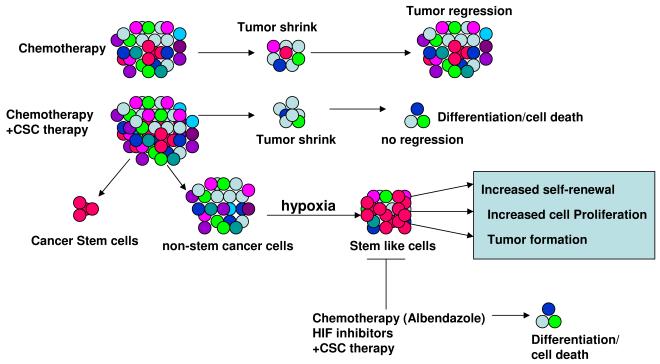
Figure 2.
Cancer stem cell (CSC) model as a strategy in the treatment of bone cancer. The response of bone tumor stem cell to chemotherapy only shrink’s tumor tissues and the tumor develop after some time due to presence of cancer stem cell, which self-renew and differentiate to form the tumor. However, chemotherapy together with CSCs-targeted therapy can kill not only most of the tumor cells but also can kill the CSCs and ultimately the non-CSC tumor will be differentiated with no proliferating ability. Hypoxia can induce the non-CSC tumor to become stem cell type cells, which have self-renewing and proliferating ability and can form tumor. However, chemotherapy (inhibitor such as Albendazole) together with CSC targeted therapy can block these stem cell-like cells and their ability to form the tumor.
9. Concluding remarks
In conclusion, exploration of the mechanisms by which hypoxia influences bone tumors could ultimately contribute to the development of bone tumor prevention and therapeutic strategies. Furthermore, to understand the biology of the bone tumor it is critical to identify prognostic factors and new effective agents combined with stem cell therapy to improve the treatment and ultimately cure the bone cancer [92, 93]. To explore our understanding of CSC behavior and the way these CSC re-enter in the cell cycle is critical in future understanding of bone cancer and cancer in general.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 30801164), Innovation Program of Shanghai Municipal Education Commission (No.09YZ107) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- [1].Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J. Cell Sci. 2009;122:1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- [2].Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- [3].Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nature Rev. Mol. Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- [4].Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Amer. J. Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- [5].Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008;15:686–90. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- [7].Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem. Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- [8].Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J. Mol. Med. 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- [9].Zhong H, De Marzo AM, Laughner E, Lim M, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- [10].Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr. Opin. Genet. Dev. 2001;11:293–9. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- [11].Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Can Metast Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- [12].Miyazawa M, Yasuda M, Fujita M, Hirasawa T, et al. Association of hypoxia-inducible factor-1 expression with histology in epithelial ovarian tumors: a quantitative analysis of HIF-1. Arch. Gynecol. Obstet. 2009;279:789–96. doi: 10.1007/s00404-008-0816-z. [DOI] [PubMed] [Google Scholar]
- [13].Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Knowles HJ, Schaefer KL, Dirksen U, Athanasou NA. Hypoxia and hypoglycaemia in Ewing’s sarcoma and osteosarcoma: regulation and phenotypic effects of Hypoxia-Inducible Factor. BMC Cancer. 2010;10:372. doi: 10.1186/1471-2407-10-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang QC, Zeng BF, Dong Y, Shi ZM, Jiang ZM, Huang J. Overexpression of hypoxia-inducible factor-1alpha in human osteosarcoma: correlation with clinicopathological parameters and survival outcome. Jap. J. Clin. Oncol. 2007;37:127–34. doi: 10.1093/jjco/hyl137. [DOI] [PubMed] [Google Scholar]
- [16].Mizobuchi H, Garcia-Castellano JM, Philip S, Healey JH, Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin. Orthop. Relat. Res. 2008;466:2052–9. doi: 10.1007/s11999-008-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mayes PA, Campbell L, Ricci MS, Plastaras JP, Dicker DT, El-Deiry WS. Modulation of TRAIL-induced tumor cell apoptosis in a hypoxic environment. Cancer Biol. Ther. 2005;4:1068–74. doi: 10.4161/cbt.4.10.2255. [DOI] [PubMed] [Google Scholar]
- [18].Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- [19].Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J. Biol. Chem. 2003;278:13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- [20].Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J. Biol. Chem. 2001;276:43407–12. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- [21].Kilic M, Kasperczyk H, Fulda S, Debatin KM. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027–38. doi: 10.1038/sj.onc.1210008. [DOI] [PubMed] [Google Scholar]
- [22].Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- [23].Hansen AE, Kristensen AT, Law I, Jørgensen JT, Engelholm SA. Hypoxia-inducible factors - regulation, role and comparative aspects in tumourigenesis. Vet. Comp. Oncol. 2011;9:16–37. doi: 10.1111/j.1476-5829.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- [24].Gross A, Yin XM, Wang K, Wei MC, J, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- [25].Xu K, Ding Q, Fang Z, Zheng J, Gao P, Lu Y, Zhang Y. Silencing of HIF-1α suppresses tumorigenicity of renal cell carcinoma through induction of apoptosis. Cancer Gene Ther. 2010;17:212–222. doi: 10.1038/cgt.2009.66. [DOI] [PubMed] [Google Scholar]
- [26].Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011:959248. doi: 10.1155/2011/959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Díaz-Montero CM, McIntyre BW. Acquisition of anoikis resistance in human osteosarcoma cells does not alter sensitivity to chemotherapeutic agents. BMC Cancer. 2005;5:39. doi: 10.1186/1471-2407-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kubo T, Sugita T, Shimose S, Matsuo T, Arihiro K, Ochi M. Expression of hypoxia-inducible factor-1alpha and its relationship to tumour angiogenesis and cell proliferation in cartilage tumours. J. Bone Joint Surg. Br. 2008;90:364–70. doi: 10.1302/0301-620X.90B3.19806. [DOI] [PubMed] [Google Scholar]
- [29].Domm C, Schunke M, Christesen K, Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthr. Cartil. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- [30].Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Met Rev. 2006;25:573–87. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- [31].Sun X, Wei L, Chen Q, Terek RM. CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion through ERK signaling and increased MMP1 expression. Mol Can. 2010;9:17. doi: 10.1186/1476-4598-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aryee DN, Niedan S, Kauer M, Schwentner R, Bennani-Baiti IM, Ban J, et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Cancer Res. 2010;70:4015–23. doi: 10.1158/0008-5472.CAN-09-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65:11520–8. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- [34].Ratcliffe PJ, O’Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J. Exp. Biol. 1998;201:1153–62. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- [35].Kauer M, Ban J, Kofler R, Walker B, Davis S, Meltzer P, et al. A molecular function map of Ewing’s sarcoma. PLoS One. 2009;4:e5415. doi: 10.1371/journal.pone.0005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu X, Yan CH, Yuan M, Wei Y, Hu G, Kang Y. In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer Res. 2010;70:3905–14. doi: 10.1158/0008-5472.CAN-09-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roodman GD. Mechanisms of bone metastasis. New Eng. J. Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- [38].van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Lowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J. Bone Min. Res. 2001;16:1077–91. doi: 10.1359/jbmr.2001.16.6.1077. [DOI] [PubMed] [Google Scholar]
- [39].Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 1986;261:12665–74. [PubMed] [Google Scholar]
- [40].Dunn LK, Mohammad KS, Fournier PG, McKenna CR, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yin JJ, Selander K, Chirgwin JM, Dallas M, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR. Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan Elect Microsc. 1986;IV:1537–54. [PubMed] [Google Scholar]
- [43].Yates AJ, Gutierrez GE, Smolens P, et al. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J. Clin. Invest. 1988;81:932–8. doi: 10.1172/JCI113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bendinelli P, Matteucci E, Maroni P, Desiderio MA. NF-kappaB activation, dependent on acetylation/deacetylation, contributes to HIF-1 activity and migration of bone metastatic breast carcinoma cells. Mol. Cancer Res. 2009;7:1328–41. doi: 10.1158/1541-7786.MCR-08-0548. [DOI] [PubMed] [Google Scholar]
- [45].Duivenvoorden WC, Hirte HW, Singh G. Transforming growth factor beta1 acts as an inducer of matrix metalloproteinase expression and activity in human bone-metastasizing cancer cells. Clin Exp. Metast. 1999;17:27–34. doi: 10.1023/a:1026404227624. [DOI] [PubMed] [Google Scholar]
- [46].Kang Y, Siegel PM, Shu W, Drobnjak M, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- [47].Roudier MP, Vesselle H, True LD, Higano CS, et al. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin. Exper. Metast. 2003;20:171–80. doi: 10.1023/a:1022627421000. [DOI] [PubMed] [Google Scholar]
- [48].Achbarou A, Kaiser S, Tremblay G, Ste-Marie LG, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994;54:2372–7. [PubMed] [Google Scholar]
- [49].Cramer SD, Chen Z, Peehl DM. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 1996;156:526–31. doi: 10.1097/00005392-199608000-00076. [DOI] [PubMed] [Google Scholar]
- [50].Kitagawa Y, Dai J, Zhang J, Keller JM, et al. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res. 2005;65:10921–9. doi: 10.1158/0008-5472.CAN-05-1809. [DOI] [PubMed] [Google Scholar]
- [51].Mohamedali KA, Li ZG, Starbuck MW, et al. Inhibition of prostate cancer osteoblastic progression with VEGF121/rGel, a single agent targeting osteoblasts, osteoclasts, and tumor neovasculature. Clin. Cancer Res. 2011;17:2328–38. doi: 10.1158/1078-0432.CCR-10-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wu D, Zhau HE, Huang WC, Iqbal S, et al. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene. 2007;26:5070–7. doi: 10.1038/sj.onc.1210316. [DOI] [PubMed] [Google Scholar]
- [53].Body JJ, Greipp P, Coleman RE, Facon T, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–92. doi: 10.1002/cncr.11138. 2003. [DOI] [PubMed] [Google Scholar]
- [54].Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2001;61:2572–8. [PubMed] [Google Scholar]
- [55].Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- [56].Tan C, de Noronha RG, Roecker AJ, et al. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–12. [PubMed] [Google Scholar]
- [57].Melillo G. Inhibiting hypoxia-inducible factor 1 for cancer therapy. Mol. Cancer Res. 2006;4:601–5. doi: 10.1158/1541-7786.MCR-06-0235. [DOI] [PubMed] [Google Scholar]
- [58].Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nature Rev. Drug Discov. 2003;2:803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- [59].Belozerov VE, Van Meir EG. Inhibitors of hypoxia-inducible factor-1 signaling. Curr. Opin. Investig. Drugs. 2006;7:1067–76. [PubMed] [Google Scholar]
- [60].Narita T, Yin S, Gelin C, et al. Identification of a novel small molecule HIF-1α translation inhibitor. Clin. Cancer Res. 2009;15:6128–36. doi: 10.1158/1078-0432.CCR-08-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv. Drug Deliv. Rev. 2009;61:623–32. doi: 10.1016/j.addr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [62].Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- [63].Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- [64].Wang R, Zhou S, Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr. Med. Chem. 2011;18:3168–89. doi: 10.2174/092986711796391606. [DOI] [PubMed] [Google Scholar]
- [65].Sasaki A, Boyce BF, Story B, Wright KR, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–7. [PubMed] [Google Scholar]
- [66].Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- [67].Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- [68].Bae KM, Su Z, Frye C, McClellan S, et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J. Urol. 2010;183:2045–53. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Nat. Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- [70].Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [71].Murase M, Kano M, Tsukahara T, Takahashi A, et al. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Brit. J. Cancer. 2009;101:1425–32. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J. Orthopaed. Surg Res. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Di Fiore R, Santulli A, Ferrante RD, et al. Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J. Cell Physiol. 2009;219:301–13. doi: 10.1002/jcp.21667. [DOI] [PubMed] [Google Scholar]
- [75].Wang L, Park P, Lin CY. Characterization of stem cell attributes in human osteosarcoma cell lines. Cancer Biol. Ther. 2009;8:543–552. doi: 10.4161/cbt.8.6.7695. [DOI] [PubMed] [Google Scholar]
- [76].Gillette JM, Nielsen-Preiss SM. Cancer stem cells: Seeds of growth in osteosarcoma. Cancer Biol. Ther. 2009;8:553–554. doi: 10.4161/cbt.8.6.8142. [DOI] [PubMed] [Google Scholar]
- [77].Suva ML, Riggi N, Stehle JC, et al. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–81. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- [78].Levings PP, McGarry SV, Currie TP, et al. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–55. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin. Orthop. Relat. Res. 2008;466:2114–30. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mohseny AB, Szuhai K, Romeo S, et al. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J. Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- [81].Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Nat. Acad. Sci.USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006;12:6213s–6s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- [83].Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Nat. Acad. Sci.USA. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].McCord AM, Jamal M, Shankavaram UT, et al. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol. Cancer Res. 2009;7:489–97. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. J. Cell Mol. Med. 2009;13:4319–28. doi: 10.1111/j.1582-4934.2009.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [88].Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer. 2010;102:789–95. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li Z, Bao S, Wu Q, Wang H, Eyler C, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Singh SR. Stem Cell, Regenerative Medicine and Cancer. Nova Science Publishers; New York: 2011. [Google Scholar]
- [93].Gibbs CP, Jr, Levings PP, Ghivizzani SC. Evidence for the osteosarcoma stem cell. Curr Orthop. Pract. 2011;22:322–326. doi: 10.1097/BCO.0b013e318221aee8. [DOI] [PMC free article] [PubMed] [Google Scholar]