Summary
During a recent Food and Drug Administration workshop on clinical trials to evaluate new TB drugs, questions were raised regarding the use of bacteriologic endpoints such as treatment failure and relapse as measures of improvement in health status and long term outcome after treatment. FDA scientists asked how patients’ clinical signs and symptoms changed during therapy, noting that while such information is usually collected during clinical trials, it is not often reported. We analyzed data from an internationalphase 3 TB treatment trial that included systematic assessments of symptoms. The percentage of subjects with self-reported symptoms at baseline ranged from 30% for dyspnea to 81% for cough, with 51% reporting fever. During therapy, fever, sweats, and dyspnea decreased most rapidly, with near resolution by the end of therapy. Chest pain and cough resolved more slowly; 13% of subjects reported cough at six months.Symptom resolution during treatment did not differ between those who relapsed and those who did not.Among those with microbiological relapse, symptoms returned with significant increases in the proportion with fever, cough, and chest pain. At the time of relapse, cough was the most frequent symptom, occurring in 75% of subjects who relapsed but only 12% of those who did not. Our data support the continued use of bacteriologic endpoints based on sputum culture assurrogate measures of the relief of symptoms, improvement in health status and favorable long term treatment outcome in TB drug trials.
Keywords: tuberculosis, symptoms, surrogate endpoints
INTRODUCTION
Tuberculosis (TB) is a global health emergency and new drugs and treatment regimens are urgently needed by patients and TB control programs. TB is transmitted mainly by inhalation of cough-generated aerosols. The main goals of treatment from the public health standpoint are to rapidly eliminate the pathogen from respiratory secretions thereby eradicating the infection, curing the patient, and preventing transmission to other uninfected persons in the community.
From a regulatory perspective, the most important outcomes of the treatment of disease are survival, disability and the capacity for functioning in daily life.1These endpoints focus on symptom resolution and patient-reported outcomes. During a recent Food and Drug Administration public workshop on the design of clinical trials for new tuberculosis drugs, FDA scientists and others raised important questions regarding the use of surrogate endpoints in TB treatment trials. Specifically they asked the research community for data that captures the relationship between clinical symptoms and trial endpoints.2 Current phase 3 trials of TB treatment rely heavily on microbiological endpoints. The most common are treatment failure, defined as persistent sputum culture positivity, and relapse, defined as sputum culture positivity after completion of successful anti-TB treatment.3 While these provide objective measures for assessing treatment efficacy in a reproducible and efficient manner, they are surrogate endpoints. The ultimate goal of TB treatmentfor patientsis to cure the patient of disease: to restore health and prevent death. With supervised treatment of drug-susceptible TB, death is infrequent, limiting its utility as an endpoint in clinical trials. In regard to restoring health, studies have demonstrated improved quality of life measures after successful TB treatment4, 5, but no contemporary studies have evaluated resolution of symptoms.
Recent TB treatment trials have included assessments of symptoms, weight, and chest radiographs that would allow for correlation with the primary study outcomes. To better characterize the relationship between microbiologically-defined outcomes and clinical symptoms we analyzed data from an international, multicenter phase 3 trial that included systematicserial assessments of symptoms before, during, and after TB treatment.
METHODS
Study Population
Our study sample included 394 patients who participated in a multicenter clinical trial of shortening treatment of drug-susceptible TB conducted in Uganda, Brazil, and the Philippines (ClinicalTrials.gov Identifier NCT00130247) and reported previously.6Patients enrolled were ambulatory HIV-uninfected adults aged 18-60 years with smear positive or negative, culture-confirmed, non-cavitary pulmonary TB, and negative sputum cultures at 2 months. Subjects were treated with 2 months of daily isoniazid (H), rifampin (R), ethambutol, and pyrazinamide followed by HR for 2 months. Patients whose sputum cultures were negative at 2 months were randomly assigned to stop treatment after 4 months or complete a full six months of HR. Therapy was directly observed at least 5 days a week. Subjects were evaluated monthly during treatment, then every 3 months for the first year post-treatment and every 6 months for the second year post-treatment for a total of 30 months. Subjects gave informed consent prior to participation. The protocol was approved by institutional review boards at each site.
Surrogate Endpoints
In the clinical trial, treatment failure was defined as a continued positive sputum culture after 4 months of TB treatment. There were no treatment failures. The primary surrogate endpoint of the original trial was bacteriological or clinical relapse at 30 months. Relapse was defined as recurrent disease with a positive sputum culture after completing treatment.Relapse with the initial M. tuberculosis isolate was confirmed by IS6110 DNA fingerprinting of the patient’s initial M. tuberculosis isolate and isolates obtained at the time of recurrence. The primary study was terminated early due to an increased incidence of relapse in the 4-month arm.6
Assessment and Analysis of Symptoms
At each follow-up visit data was collected including vital signs, weight, physical examination, sputum culture, and self-reported symptoms. These symptoms were reported as present or absent and included fever, sweats, cough, chest pain, sputum production, hemoptysis, and dyspnea on exertion among others. Disease severity was compared with symptoms by analysis of baseline symptom proportions by pre-treatment sputum AFB smear grade7 and extent of disease on chest radiograph8 using the Cochran-Armitage test for trend. Proportions of subjects with fever, cough, chest pain, sweats, and dyspnea were calculated at baseline, 2 months, 4 months, and 6 months. McNemar’sTest for Matched Pairs was used to compare the change in symptoms between baseline and six months. Change in body mass index (BMI) from baseline to six months was compared using a paired t-test.
To compare symptoms between patients who relapsed and those who did not, we used data from the patient’s visit at the time of relapse and the cured patient’s follow-up visit that was closest to the average time of relapse [352 days (SD 142) from the start of treatment]. A two-sided Fisher’s exact test was used to compare symptom proportions between those who relapsed and those who did not relapse.
Time to resolution of symptoms was estimated using Kaplan-Meier curves. A combination of symptoms including fever, cough, sweats, chest pain, and dyspnea was used to assess time to complete resolution of all symptoms among those subjects with at least one symptom at baseline. Time to resolution of fever and cough also were calculated for subjects who reported each symptom at baseline.Patients without resolution of symptoms were censored at their 6 month follow-up visit.
RESULTS
This study included all 394 subjects enrolled in the original trial. The mean age was 31 years, 61% were male, and the mean BMI was 20.5 kg/m2. Eighteen subjects developed recurrent TB during follow-up. Based on IS6110 DNA fingerprint patterns, 16 of 18 (4.1% of all subjects enrolled) relapsed with their initial isolate. Two patients had recurrence due to exogenous infection and were excluded from therelapse analysis.
At baseline 55% of subjects had moderate to far-advanced disease on chest radiograph.8Thirty-four percent had a negative AFB smear, 25%had grade 1-2+, and 41% had grade 3-4+. Increasing extent of disease on chest radiograph and increasing AFB smear grade showed a significant positive trend with the prevalence of fever, cough, and sweats at baseline (Cochran-Armitage, p< 0.005). Chest pain also showed a positive trend with increasing AFB smear grade (Cochran-Armitage, p < 0.05).
The prevalence of symptoms declined rapidly during treatment. The proportion of subjects with symptoms including fever, cough, chest pain, sweats or dyspnea decreased sharply by month 2 of therapy, and continued to decline through its completion (Table 1). Matched comparison by subject between baseline and 6 months reflected a significant decline in all five symptoms (McNemar’s test, p<0.001) and showed that few subjects with symptoms absent at baseline reported symptoms at 6 months (Table 2). Notably, cough persisted after therapy in 13% of subjects who reported this symptom at baseline. BMI significantly increased between baseline and 6 months from 20.5 to 21.5 kg/m2 (paired t-test, p < 0.001).
Table 1.
Proportion of patients with symptoms at baseline and eachfollow-up visit during treatment.
| Time from start of therapy |
||||
|---|---|---|---|---|
| Baseline % (n) |
Month 2 % (n) |
Month 4 % (n) |
Month 6 % (n) |
|
| Fever | 51 (199) | 3.1 (12) | 2 (8) | 1.1 (4) |
| Cough | 81 (317) | 33 (131) | 18 (72) | 13 (50) |
| Chest Pain | 57 (224) | 13 (52) | 8.4 (33) | 6.8 (26) |
| Sweats | 45 (176) | 2.5 (10) | 1 (4) | 0.8 (3) |
| Dyspnea | 30 (119) | 5.8 (23) | 3.6 (14) | 2.6 (10) |
Table 2.
Comparison of the presence or absence of symptoms between baseline and 6 months by subject, using McNemar’s Test.
|
Symptom |
Present at baseline, absent at 6 months % (n) |
Present at baseline, present at 6 months % (n) |
Absent at baseline, absent at 6 months % (n) |
Absent at baseline, present at 6 months % (n) |
p value |
|---|---|---|---|---|---|
| Fever | 51% (193) | 1.1% (4) | 48% (184) | 0% (0) | p<0.001 |
| Cough | 69% (262) | 13% (48) | 18% (70) | 0.5% (2) | p<0.001 |
|
Chest
Pain |
51% (193) | 6.5% (25) | 43% (163) | 0.3% (1) | p<0.001 |
| Sweats | 44% (169) | 0.5% (2) | 56% (209) | 0.3% (1) | p<0.001 |
| Dyspnea | 28% (105) | 2.4% (9) | 70% (267) | 0.3% (1) | p<0.001 |
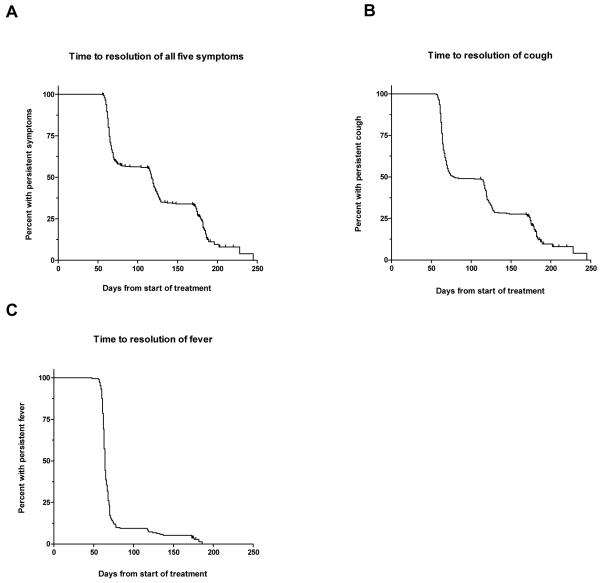
Ninety-one percent(357) of subjects had at least one symptom at baseline (including fever, cough, chest pain, sweats, or dyspnea). The median time to complete resolution of all symptoms was 117 days (Figure: a). Twenty-one percent (75) had at least 1 symptom during the entire 6 months. There was no significant difference in time to resolution of symptoms when stratified by being underweight (BMI < 18.5 kg/m2) at baseline or by relapse status. Separate analysis of time to resolution of cough and fever revealed that cough cleared more slowly (median time to resolution 79 vs 64 days, Figure: b,c).
Figure.
Kaplan Meier curve showing the cumulative percentage of subjects with persistent symptoms during TB treatment; A. Time to resolution of a combination of symptoms including fever, cough, chest pain, sweats and dyspnea among 357 subjects with at least one symptom at baseline; B. Time to resolution of cough among 308 subjects with cough at baseline; C. Time to resolution of fever among 192 subjects with fever at baseline.
Symptoms among the 16 subjects that relapsed were compared with symptoms in subjects that did not relapse. At 6 months 2 subjects (13%) of the 16 that relapsed still had at least one symptom compared with 11.8% among subjects that did not relapse. At the time of relapse, subjects that relapsed were significantly more likely to have fever (31% vs 5.1%, Fisher’s exact, p<0.002), cough (75% vs 12%, Fisher’s exact, p<0.001), and chest pain (25% vs 7.6%, Fisher’s exact, p=0.036) when compared with subjects who did not relapse. The prevalence of sweats or dyspnea was less than 2% at the time of relapse and did not differ between the two groups.
Discussion
In a detailed analysis of data from an international, multicenter phase 3 trial, we found that subjects who successfully completed TB treatment had a rapid decrease in symptoms during therapy. After the first 2 months of treatment the proportion of subjects with symptoms declined by 94% for fever, 59% for cough, 77% for chest pain, 94% for sweats, and 81% for dyspnea. Fever, sweats, and dyspnea decreased most rapidly, with near resolution by the completion of therapy. Chest pain and cough resolved more slowly, and 13% of subjects reported cough even at the end of therapy. While cough was the most frequent symptom prior to beginning therapy, it was also the most common symptom after therapy, which may reflect its limited specificity. In an earlier study, Boon and colleagues reported that only 7% of randomly screened South Africans who reported cough for 2 or more weeks had active TB.9 In addition, TB can cause lung damage such as bronchiectasis, leading to persistence of cough even after completion of successful treatment.10 The percentage of subjects with self-reported symptoms in our study ranged from 30% for dyspnea to 81% for cough. Nearly 20% of subjects did not report a cough and only about 50% reported fever. When a combination of symptoms was analyzed, 91% of subjects had at least one symptom at baseline and the median time to resolution of all symptoms during treatment was 117 days.
Relapse of TB as defined by positive sputum culture after successful treatment was also associated with a significant increase in symptoms. Among those who relapsed, only 2 (13%) had any symptoms at 6 months. Symptoms then returned at the time of relapse with significant increases in the proportion with fever, cough, and chest pain. Cough was the most frequent symptom, occurring in 75% of subjects that relapsed but only 12% of subjects that did not relapse.
Our study has several limitations. The primary study excluded patients with either cavitary pulmonary TB or positive sputum cultures at 2 months. Patients included likely had less severe pulmonary TB than the general population, which is reflected in the lack of subjects with treatment failure and the 4% relapse rate. These selection criteria could have led to an underestimation of the prevalence of symptoms existing in the general population. Still, the patients included in this study represent a significant proportion of patients with pulmonary TB as roughly one-half of patients with TB have non-cavitary disease worldwide and previous studies showeda similar prevalence of symptoms, including a large study conducted in Hong Kong that found the first symptom of TB was cough in 81% of participants.11Strengths of our study include the use of standardized forms for serial collection of data about symptoms during treatment,supervised anti-TB treatment, standardized serial bacteriologic assessment of patients using sensitive liquid as well as solid culture media, and inclusion of patients from South America, subSaharan Africa and Asia.
The surrogate markers for successful treatment of TB and relapse as defined by sputum culture status were closely associated with self-reported symptoms in subjects treated forpulmonary TB. Our study found that microbiologically defined success and failure mirrored the resolution and reappearance of symptoms. In Fleming and DeMets’s commentary on surrogate endpoints they argue that the ideal surrogate is “in the only causal pathway of the disease process, and the intervention’s entire effect on the true clinical outcome is mediated through its effect on the surrogate.”12 In many ways, the microbiological endpoints of sputum culture conversion used in TB trials meet these criteria. Replication of M. tuberculosisin the lungs is the only causal process of active pulmonary disease, and treatment and cure of TB is mediated through the clearance of M. tuberculosis infection demonstrated by culture conversion to negative.While larger studies would be required for validation, our data support the continued use of bacteriologic endpoints based on sputum culture as surrogate markers of long term cure and relief of symptoms in trials of new TB drugs and regimens.
Acknowledgements
The authors thank the staff and patients at the trial sites in Brazil, the Philippines, and Uganda. We also thank W. Henry Boom for his contributions.
Ethical Approval
The protocol for the original trial was approved by institutional review boards at each site. Subjects gave informed consent prior to participation.
Funding
This work was supported by the Tuberculosis Research Unit at Case Western Reserve University, established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under Contract No. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.Temple RJ. Chapter 1. A Regulatory Authority’s Opinion About Surrogate Endpoints. In: Nimmo WS, Tucker GT, editors. Clinical measurement in drug evaluation. J. Wiley; Chichester, West Sussex, England; New York: 1995. pp. 3–22. [Google Scholar]
- 2.Food and Drug Administration . Issues in the Design of Clinical Trials of Antimycobacterial Drugs for Treatment of Tuberculosis. 2009. Public Workshop. [Google Scholar]
- 3.Hopewell P, Cynamon M, Starke J, Iseman M, O’Brien R. Evaluation of new anti-infective drugs for the treatment and prevention of tuberculosis. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15(Suppl 1):S282–95. doi: 10.1093/clind/15.supplement_1.s282. [DOI] [PubMed] [Google Scholar]
- 4.Marra CA, Marra F, Colley L, Moadebi S, Elwood RK, Fitzgerald JM. Health-related quality of life trajectories among adults with tuberculosis: differences between latent and active infection. Chest. 2008;133:396–403. doi: 10.1378/chest.07-1494. [DOI] [PubMed] [Google Scholar]
- 5.Dhuria M, Sharma N, Narender Pal S, Ram Chander J, Saha R, Gopal Krishan I. A study of the impact of tuberculosis on the quality of life and the effect after treatment with DOTS. Asia Pac J Public Health. 2009;21:312–20. doi: 10.1177/1010539509336242. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JL, Hadad DJ, Dietze R, Maciel EL, Sewali B, Gitta P, Okwera A, Mugerwa RD, Alcaneses MR, Quelapio MI, Tupasi TE, Horter L, Debanne SM, Eisenach KD, Boom WH. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180:558–63. doi: 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strong BS, Kubica GP. Isolation and identification of Mycobacterium tuberculosis: A guide for the level II laboratory. U.S. Department of Health and Human Services - Centers for Disease Control and Prevention; Atlanta, Georgia: 1981. [Google Scholar]
- 8.Falk A, O’Connor JB, Pratt PC, Webb WR, Wier JA, Wolinsky E. Diagnostic standards and classification of tuberculosis. 12th ed. National Tuberculosis and Respiratory Disease Association; New York: 1969. Classification of pulmonary tuberculosis; pp. 68–76. [Google Scholar]
- 9.den Boon S, White NW, van Lill SW, Borgdorff MW, Verver S, Lombard CJ, Bateman ED, Irusen E, Enarson DA, Beyers N. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis. 2006;10:876–82. [PubMed] [Google Scholar]
- 10.Salkin D. Tuberculosis as a cause of upper lobe bronchiectasis. Calif Med. 1950;73:577–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Allan WG, Girling DJ, Fayers PM, Fox W. The symptoms of newly diagnosed pulmonary tuberculosis and patients’ attitudes to the disease and to its treatment in Hong Kong. Tubercle. 1979;60:211–23. doi: 10.1016/0041-3879(79)90002-3. [DOI] [PubMed] [Google Scholar]
- 12.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]