Abstract
OBJECTIVE
Greater chest compression fraction (CCF, or proportion of CPR time spent providing compressions) is associated with better survival for out-of-hospital cardiac arrest OOHCA) patients in ventricular fibrillation (VF). We evaluated the effect of CCF on return of spontaneous circulation (ROSC) in OOHCA patients with non-VF ECG rhythms in the Resuscitation Outcomes Consortium Epistry.
METHODS
This prospective cohort study included OOHCA patients if: not witnessed by EMS, no automated external defibrillator (AED) shock prior to EMS arrival, received > 1 minute of CPR with CPR process measures available, and initial non-VF rhythm. We reviewed the first minutes of electronic CPR records following defibrillator application, measuring the proportion of compressions/min during the resuscitation.
RESULTS
Demographics of 2,103 adult patients from 10 U.S. and Canadian centers were: mean age 67.8; male 61.2%; public location 10.6%; bystander witnessed 32.9%; bystander CPR 35.4%; median interval from 911 to defibrillator turned on 8min:27sec; initial rhythm asystole 64.0%, PEA 28.0%, other non-shockable 8.0%; median compression rate 110/min; median CCF 71%; ROSC 24.2%; survival to hospital discharge 2.0%. The estimated linear effect on adjusted odds ratio with 95% confidence interval (OR; 95%CI) of ROSC for each 10% increase in CCF was (1.05; 0.99, 1.12). Adjusted (OR; 95%CI) of ROSC for each CCF category were: 0–40% reference group); 41–60% (1.14; 0.72, 1.81); 61–80% (1.42; 0.92, 2.20); and 81–100% (1.48; 0.94, 2.32).
CONCLUSIONS
This is the first study to demonstrate that increased CCF among non-VF OOHCA patients is associated with a trend toward increased likelihood of ROSC.
Keywords: cardiopulmonary resuscitation, heart arrest, resuscitation
INTRODUCTION
Background
Survival to hospital discharge for out-of-hospital cardiac arrest (OOHCA) varies by community, but rarely exceeds 8%.1, 2 Bystander cardiopulmonary resuscitation (CPR) and defibrillation are two of only a few modifiable factors clearly associated with increased survival for OOHCA,3 and the importance of good quality CPR is increasingly being recognized.4–8 There is compelling evidence from animal studies suggesting that frequent and prolonged CPR interruptions have a detrimental effect on survival and neurological outcomes.9–11 Human studies also demonstrate that experienced CPR providers stop CPR frequently during out-of-hospital 12 and in-hospital resuscitation.13 The 2005 emergency cardiovascular care guidelines that recommended changing the chest compression to ventilation ratio from 15:2 to 30:2 did thereby reduce interruptions to chest compressions.14 However, a growing number of reports suggest the benefit of chest compression-only CPR, avoiding interruptions for ventilation.15–19
Importance
Compression fraction is defined as the proportion of CPR time spent providing chest compressions. A recent study by Christenson et al. evaluated the incremental benefit of higher chest compression fraction on survival to hospital discharge for OOHCA patients with an initial ECG rhythm of ventricular fibrillation or tachycardia (VF/VT).20 These authors described the relationship between increasing chest compression fraction and survival to hospital discharge, the highest survival (29%) being observed in the group of patients where 61% to 80% of CPR time was spent doing chest compressions. Survival is more common among patients with an observed initial rhythm of VF/VT compared with all other initial cardiac rhythms, but this VF/VT group generally represents less than 30% of all cardiac arrest patients.1, 2
Goal of this investigation
The objective of the present study was to estimate the independent effect of chest compression fraction on return of spontaneous circulation (ROSC) in a cohort of OOHCA patients with an initial rhythm other than VF/VT.
METHODS
Study Design
This was an observational cohort study of OOHCA patients prospectively enrolled in the Resuscitation Outcomes Consortium (ROC) Cardiac Arrest Epistry. ROC is a clinical network of 11 regional centers distributed across North America conducting research in the fields of OOHCA and serious traumatic injury.21 The ROC cardiac arrest epidemiological registry or “Epistry” has been collecting population-based prospective data on OOHCA from more than 260 EMS agencies since December, 2005.22 Information collected by the ROC Epistry is subject to standardized operational definitions, and all data are managed by a central data coordinating center. In addition to collecting all the elements suggested in the Utstein template,23 ten of the eleven ROC regional centers at the time of this study also collected digital electronic recordings of rhythm and chest compression for some events, otherwise known as CPR process data.
Setting
Centers collecting this information and included in the present report are Ottawa, ON; Toronto, ON; and Vancouver, BC in Canada; and Seattle/King County, WA; Pittsburgh, PA; Portland, OR; Dallas, TX; Iowa City, IA; Milwaukee, WI; and Birmingham, AL in the United States. One region (San Diego, CA) was excluded from this analysis because they self-reported incomplete data.
Selection of participants
From December, 2005 to June 2007, patients eligible for this study included those adults (≥18 years old) who experienced OOHCA before EMS arrival, who had a first recorded rhythm other than VF/VT, who had cardiac arrest of suspected cardiac etiology for which resuscitation was attempted, and who had at least 1 minute of digitally recorded CPR process data completed before or during the minute when rhythm was first analyzed. The initial rhythm was determined to be other than VF/VT if the initial automated external defibrillator did not recommend a shock, or if the EMS provider interpreted the initial rhythm to be other than VF/VT. All rhythm diagnoses were later confirmed by research staff in cases where an ECG existed. We excluded patients who received a shock from an automated external defibrillator prior to EMS arrival, those who were enrolled in a concurrent ROC clinical trial, and those for which information on ROSC was missing.
ROC received approval from 74 US Institutional Review Boards and 34 Canadian Research Ethics Boards as well as 26 EMS Services Institutional Review Boards to establish and conduct research activities with the Epistry database.22
Methods of measurement
We used digital CPR recordings available from PhysioControl (Medtronic, Redmond, WA) defibrillators (n=1766), Zoll (Chelmsford, MA) defibrillators (n=181), Philips (Andover, MA) defibrillators (n=118), and Laerdal (Stavanger, Norway) defibrillators (n=9) following electrode application onto the patient’s chest, and measured the presence and frequency of chest compressions. This was accomplished indirectly, either by changes in thoracic impedance recorded from external defibrillation electrodes 24 or via an accelerometer interface between the rescuer and the patient’s chest using commercially available defibrillators. For Cardiac Science (Bothell, WA) defibrillators (n=29), presence and frequency of chest compressions was hand calculated using variations in the electronic ECG with confirmation via voice recording. Each case included up to a maximum of 5 minutes. Chest compression fraction was defined as the proportion of resuscitation time without spontaneous circulation during which chest compressions were administered, averaged over all available minutes for each patient. This was calculated by analytic software which permitted identification of all interruptions greater than two seconds (Philips, ZOLL devices) or three seconds (PhysioControl devices), which were considered time with no chest compressions. Trained research staff reviewed the automated calculation of chest compression fraction at each site prior to entering chest compression fraction values.
Outcome measures
The low survival rate from non-VF/VT cardiac arrest (only 42 in the whole cohort) precluded selection of survival as a primary outcome for this analysis. Accordingly, the prospectively selected primary outcome measure was out-of-hospital ROSC, which we determined to be present if there was any palpable pulse in any vessel for any length of time.
Primary data analysis
All statistical analyses were performed with a commercially available statistical package (SAS, version 9.2, Cary, NC; R, version 2.1.1, Vienna, Austria). Summary results are presented as mean ± standard deviation (SD) or median with inter-quartile range (IQR). We performed a multivariable linear-regression analysis to estimate the effect of a 10% change in chest compression fraction on ROSC. As an exploratory analysis, we fit a penalized cubic smoothing spline curve to further characterize the nature of the relationship between chest compression fraction and ROSC.25
Secondary data analysis
As a secondary analysis, we categorized chest compression fraction (from 0 to 100%) into four groups based on the average chest compression fraction delivered to the patient over all minutes with available data: 0–40%, 41–60%, 61–80%, and 81–100%. Because there were too few subjects in the 0–20% group to analyze, we combined the 0–20% and the 20–40% groups for analyses. Collectively, these groups corresponded to receiving cardiopulmonary resuscitation, on average, for 0–24, 25–36, 37–48, and 49–60 seconds per minute, respectively, over all analyzed minutes of data.
Potential confounding variables identified a priori included: age, gender, location of cardiac arrest (public place or private residence), bystander-witnessed cardiac arrest, bystander CPR, chest compression rate, and the time interval from receipt of the emergency call to emergency medical services arrival at the scene. We calculated descriptive statistics and used logistic regression to estimate the unadjusted and adjusted odds ratio of ROSC for each category of chest compression fraction relative to the lowest category (0–40%). The adjusted model included ROC site as a covariate to control for unmeasured confounders at the research site level.
RESULTS
Characteristics of study subjects
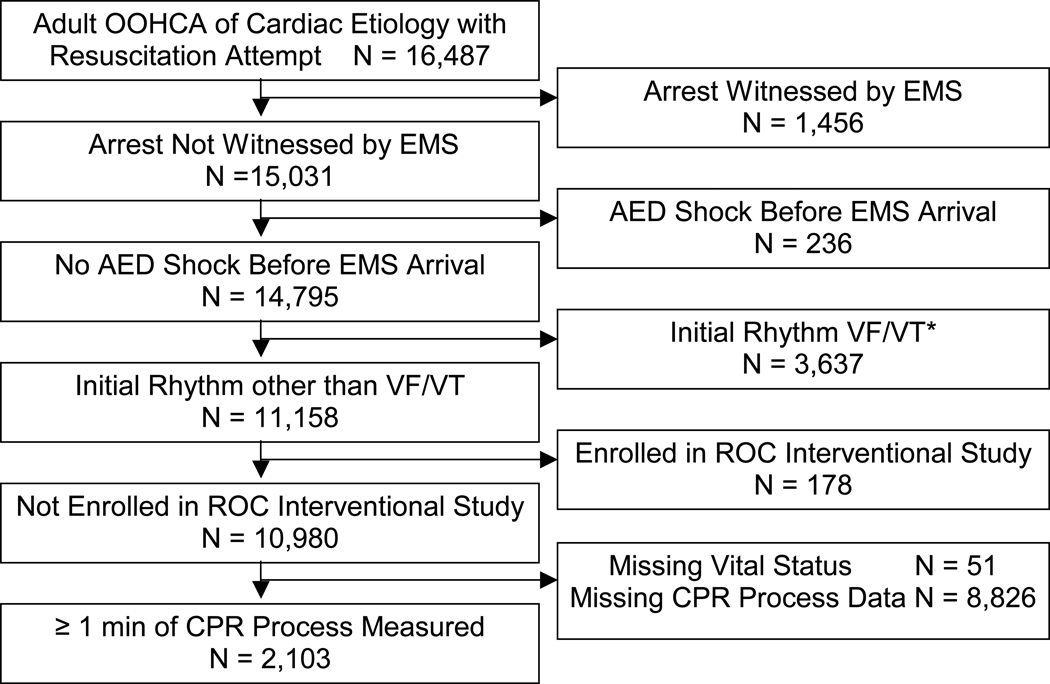
Among the 16,487 cardiac arrest cases of suspected cardiac etiology for which resuscitation was attempted, 14,795 were not witnessed by EMS and did not receive a shock before EMS arrival. Of these, 11,158 had an initial rhythm other than VF/VT and 2,332 had CPR process measures, making them eligible for analysis. Outcome data were missing for 51 patients and 178 had been enrolled in an interventional study, leaving 2,103 for the available data set (Figure 1).
Figure 1.
Study cohort and exclusions.
OOHCA indicates out-of-hospital cardiac arrest; EMS, emergency medical services; AED, automated external defibrillator; VF/VT, ventricular fibrillation or tachycardia; ROC, Resuscitation Outcomes Consortium; and CPR, cardiopulmonary resuscitation.
*Only cases with initial rhythm of VF/VT among those not witnessed by EMS and for which no shock was delivered by an AED before EMS arrival.
Overall patient and system characteristics for included cases are presented in Table 1, alongside those for cases with an initial rhythm other than VF/VT, but with missing chest compression fraction data. Two sites with preexisting capacity to download these electronic data contributed 58.8% of the included cases. Analyzed patients appeared to have a higher proportion of bystander CPR, of out-of-hospital shock delivered, and ROSC, but a lower proportion of advanced care paramedics first on scene, less epinephrine use, and lower survival compared with those with missing chest compression fraction data. The mean age of included cases was 67.8 years, 61.2% were male, 10.6% arrested in a public location, 32.9% were witnessed, and 35.4% received bystander CPR. The non-VF/VT initial rhythms observed in our cohort included asystole (64.0%), PEA (28.0%), and rhythms reported as “non-shockable” but without an ECG (8.0%). Overall, 509 patients (24.2%) had ROSC, and 42 (2.0%) survived to hospital discharge.
Table 1.
Patient and System Characteristics Comparing Cases Included in the Analyses to those Excluded Because They Had No Available CPR Process Measures
| Included Cases (n=2,103) |
Excluded Cases (n=8,826) |
|
|---|---|---|
| Age – mean (SD) | 67.8 (16.5) | 68.1 (16.4) |
| Male – n (%) | 1,286 (61.2%) | 5,201 (58.9%) |
| Public location – n (%) | 223 (10.6%) | 935 (10.6%) |
| Bystander witnessed – n (%) | 691 (32.9%) | 3,058 (34.6%) |
| Bystander CPR – n (%) | 744 (35.4%) | 2,560 (29.0%) |
| No. of contributing agencies * – mean (SD) Site code – n (%) |
1.7 (0.5) | 1.6 (0.5) |
| 216 | 0 (0%) | 437 (5.0%) |
| 307 | 798 (37.9%) | 509 (5.8%) |
| 315 | 48 (2.3%) | 684 (7.7%) |
| 389 | 222 (10.6%) | 2330 (26.4%) |
| 434 | 129 (6.1%) | 619 (7.0%) |
| 477 | 49 (2.3%) | 412 (4.7%) |
| 663 | 187 (8.9%) | 285 (3.2%) |
| 671 | 439 (20.9%) | 434 (4.9%) |
| 791 | 172 (8.2%) | 1750 (19.8%) |
| 864 | 2 (0.1%) | 233 (2.6%) |
| 925 | 57 (2.7%) | 1133 (12.8%) |
| ALS first on scene – n (%) | 371 (17.6%) | 4,083 (47%) |
| ALS on scene – n (%) | 1,851 (88.0%) | 8,247 (93.4%) |
| Minutes from 9-1-1 call to scene – median (Q1, Q3) |
5.4 (4.1,7.1) | 5.3 (4.0, 7.0) |
| Minutes from 9-1-1 call to first EMS shock assessment – median (Q1, Q3) |
9.6 (7.9, 12.2) | 9.7 (7.6, 12.5) |
| Epinephrine use noted – n (%) | 1,517 (72.1%) | 6,806 (77.1%) |
| Any out-of-hospital shock – n (%) | 437 (20.8%) | 1,432 (16.2%) |
| Any out-of-hospital ROSC – n (%) | 509 (24.2%) | 1584 (17.9%) |
| Survived to hospital discharge – n (%) | 42 (2.0%) | 236 (2.7%) |
SD indicates standard deviation; CPR, cardiopulmonary resuscitation; ALS, Advanced Life Support; Q1, Q3, the 1st and 3rd quartiles; EMS, emergency medical services; and ROSC, return of spontaneous circulation.
Information was only available about the first four EMS units at the scene.
Main results
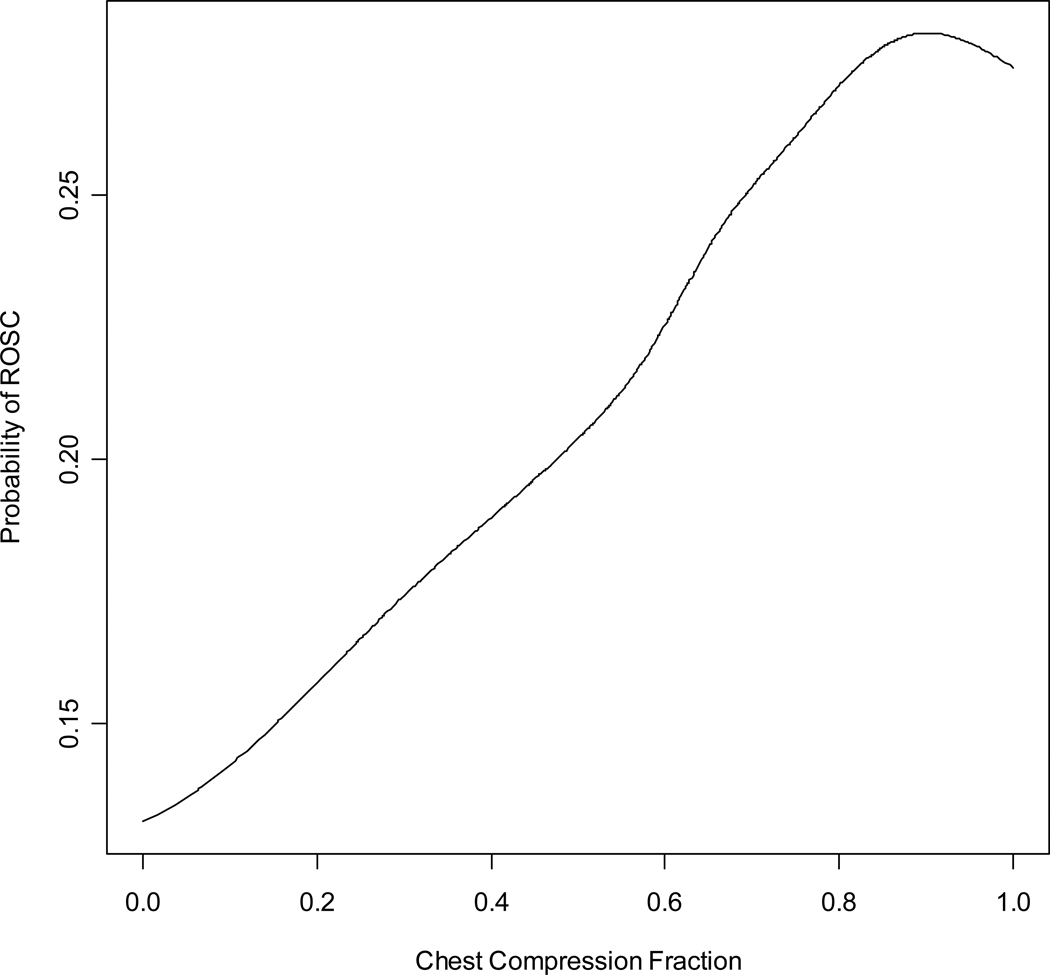
Unadjusted and adjusted odds ratios of ROSC for the preselected factors potentially associated with ROSC are presented in Table 2. The effect of an increasing chest compression fraction on ROSC remained positive (although no longer statistically significant), even after adjusting for factors known to possibly be associated with ROSC including age, gender, location of arrest, bystander witness status, bystander CPR, chest compression rate, time interval from receipt of the emergency call to emergency medical services arrival at the scene, and ROC study site. The adjusted odds ratio of ROSC for each linear increase in chest compression fraction of 10% was 1.05 (95% confidence interval 0.99 to 1.12). We fitted a smoothing spline curve to visually explore the unadjusted relationship between chest compression fraction and ROSC changes over the range of chest compression fractions (Figure 2).
Table 2.
Predictors of Out-of-hospital Return of Spontaneous Circulation
| Unadjusted OR (95% CI) |
Adjusted OR (95% CI)* |
|
|---|---|---|
| OR (95% CI) of ROSC per 10% linear increase in CCF |
1.11 (1.05, 1.18) | 1.05 (0.99, 1.12) |
| OR (95% CI) of ROSC by chest compression category |
||
| 0–40% | reference | reference |
| 41–60% | 1.12 (0.73, 1.71) | 1.14 (0.72, 1.81) |
| 61–80% | 1.53 (1.03,2.27) | 1.42 (0.92,2.20) |
| 81–100% | 1.83 (1.23,2.72) | 1.48 (0.94,2.32) |
| OR (95% CI) of ROSC associated with possible predictors |
||
| Age (per ten year increase) | 1.04 (1.97, 1.10) | 0.99 (0.93, 1.06) |
| Male | 0.89 (0.73, 1.10) | 0.81 (0.65, 1.00) |
| Public location | 1.43 (1.06, 1.94) | 1.26 (0.90, 1.75) |
| Bystander witnessed | 2.55 (2.08, 3.13) | 2.72 (2.18, 3.39) |
| Bystander CPR | 1.16 (0.94, 1.42) | 0.84 (0.67, 1.06) |
| Chest Compression rate (per 10 compressions/minute increase) |
1.01 (0.96, 1.05) | 1.02 (0.97, 1.07) |
| Time from 9-1-1 call to AED turned on (per 1 minute increase) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
OR indicates odds ratio; CI, confidence interval; ROSC, return of spontaneous circulation; CCF, chest compression fraction; CPR, cardiopulmonary resuscitation; and AED, automatic external defibrillator.
Adjusted for age, sex, public location, bystander witnessed, bystander CPR, chest compression fraction, chest compression rate, time from 911 call to AED turned on, and ROC site.
Figure 2.
Smoothing spline representing the unadjusted incremental probability of return of spontaneous circulation corresponding to a linear increase in chest compression fraction.
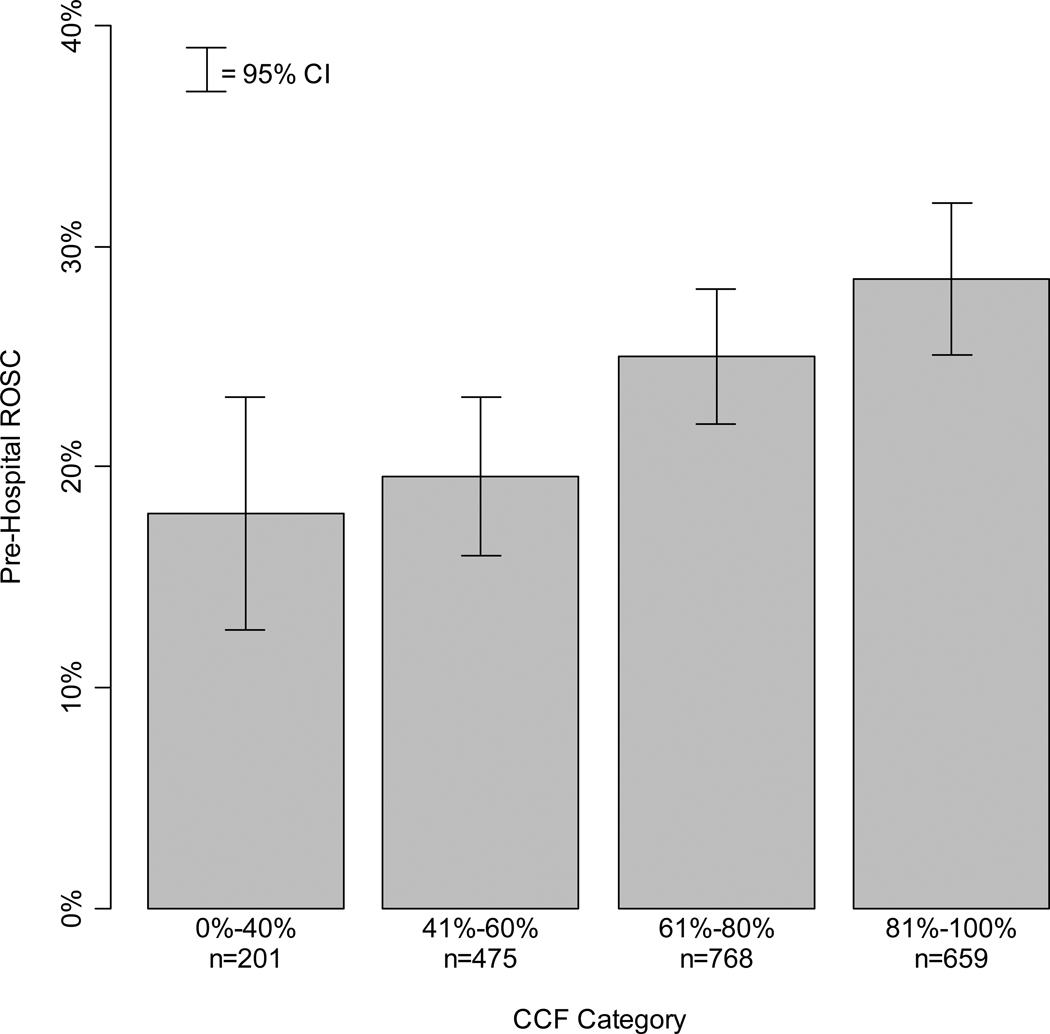
Patient and system characteristics by chest compression fraction category are presented in Table 3. The percentage of patients with ROSC was 17.9%, 19.6%, 25.0%, and 28.5%, respectively, in the four categories of increasing chest compression fraction. The number of survivors in each category was too small to analyze in this cohort of patients with initial rhythm other than VF/VT. We did not identify any imbalance in the distribution of patient and system characteristics among the four chest compression fraction categories, with the exception of advanced life support personnel first on scene which appeared to decrease with increasing chest compression fraction, and with median chest compression rate which appeared to increase with chest compression fraction. The association between chest compression fraction category and the probability of ROSC is illustrated in Figure 3.
Table 3.
Patient and System Characteristics by Chest Compression Fraction Category
| Average Chest Compression Fraction First Five Minutes of CPR Process Data |
|||||
|---|---|---|---|---|---|
| Total (n=2103) |
0–40% (n=201) |
41–60% (n=475) |
61–80% (n=768) |
81–100% (n=659) |
|
| Age – mean (SD) | 67.8 (16.5) | 68.6 (15.4) | 68.0 (16.4) | 67.8 (16.3) | 67.4 (17.1) |
| Male – n (%) | 1,286 (61.2%) | 128 (63.7%) | 301 (63.4%) | 449 (58.5%) | 408 (61.9%) |
| Public location – n (%) | 223 (10.6%) | 25 (12.4%) | 41 (8.6%) | 75 (9.8%) | 82 (12.4%) |
| Bystander witnessed – n (%) | 691 (32.9%) | 77 (38.3%) | 153 (32.2%) | 251 (32.7%) | 210 (31.9%) |
| Bystander CPR – n (%) | 744 (35.4%) | 55 (27.4%) | 149 (31.4%) | 285 (37.1%) | 255 (38.7%) |
| ALS first on scene – n (%) | 371 (17.6%) | 59 (29.4%) | 119 (25.1%) | 122 (15.9%) | 71 (10.8%) |
| ALS on scene – n (%) | 1,851 (88.0%) | 174 (86.6%) | 419 (88.2%) | 681 (88.7%) | 577 (87.6%) |
| No. of contributing agencies *– mean (SD) | 1.7 (0.5) | 1.8 (0.5) | 1.8 (0.5) | 1.8 (0.5) | 1.6 (0.5) |
| Epinephrine use noted – n (%) | 1,517 (72.1%) | 134 (66.7%) | 341 (71.8%) | 554 (72.1%) | 488 (74.1%) |
| CPR fraction – median (Q1, Q3) | 0.71 (0.55, 0.83) | 0.31 (0.25, 0.36) | 0.52 (0.48, 0.56) | 0.71 (0.65, 0.76) | 0.86 (0.83, 0.90) |
| Chest compression rate – median (Q1,Q3) |
110 (97, 122) | 101 (70, 118) | 108 (95, 122) | 111 (98, 122) | 112 (102, 123) |
| Time from 9-1-1 call to scene – median (Q1, Q3) |
5.4 (4.1, 7.1) | 5.8 (4.0, 7.8) | 5.2 (4.0, 6.9) | 5.4 (4.1, 7.1) | 5.5 (4.2, 7.1) |
| Time from 9-1-1 to AED on – median (Q1,Q3) |
8.5 (6.6, 11.2) | ||||
| Time from 9-1-1 call to first EMS shock assessment - median (Q1, Q3) |
9.6 (7.9, 12.2) | 10.3 (7.9, 14.2) | 9.4 (7.8, 12.1) | 9.6 (7.9, 12.3) | 9.7 (8.0, 11.9) |
| Any out-of-hospital shock – n (%) | 437 (20.8%) | 40 (19.9%) | 98 (20.6%) | 158 (20.6%) | 141 (21.4%) |
| Any out-of-hospital ROSC – n (%) | 509 (24.2%) | 36 (17.9%) | 93 (19.6%) | 192 (25.0%) | 188 (28.5%) |
| Survived to hospital discharge – n (%) | 42 (2.0%) | 8 (4.0%) | 8 (1.7%) | 15 (2.0%) | 11 (1.7%) |
SD indicates standard deviation; CPR, cardiopulmonary resuscitation; ALS, Advanced Lite Support; Q1, Q3, the 1st and 3rd quartiles; EMS, emergency medical services;and ROSC, return of spontaneous circulation.
Information was only available about the first four EMS units at the scene.
Figure 3.
Return of spontaneous circulation for each category of chest compression fraction.
ROSC indicates return of spontaneous circulation; CI, confidence interval; and CCF, chest compression fraction.
LIMITATIONS
This study has a number of limitations. First, because of its observational nature, we could not determine a causal relationship between chest compression fraction and the probability of ROSC. However, we found an association between the two that is consistent with findings from previous animal and human studies demonstrating an incremental outcome benefit when limiting CPR interruptions. Second, the low survival rate from non-VF/VT cardiac arrest precluded selection of survival as a primary outcome for this analysis. More than 12,000 cases would have been required to derive sufficient power to show a 1% increase in survival. Third, a large number of potentially suitable cases were excluded because CPR process measures were not available. Collectively, these cases were overall similar to those included, with the exception of a few factors with limited clinical significance. ROSC appeared significantly lower in the excluded cases – this was true for all study sites, and may represent a relationship between quality of care and availability of CPR process data. The majority of cases were contributed by two sites with pre-existing ability to analyze CPR process data. The other contributing sites employed EMS agencies with various levels of ECG monitoring sophistication. Fourth, selection bias based on differential patient contribution by study site, although possible, may have been limited as the effect of chest compression fraction on ROSC was independent of study sites. Finally, even a very large observational study such as this one marginally lacked the power to maintain statistical significance around the adjusted OR for the effect of CCF on ROSC. That being said, point estimates remained positively polarized, suggesting the incremental benefit of increasing CCF on predicted ROSC.
DISCUSSION
This large multi-center prospective cohort study is the first to suggest that increased chest compression fraction results in a higher likelihood of ROSC in a population of OOHCA patients presenting with initial rhythms other than VF/VT – representing the majority of cardiac arrests in this setting. The observation is independent of other known potential predictors of ROSC.
The positive correlation, demonstrated by the smoothing spline representing the unadjusted incremental probability of ROSC (figure 2), corresponds to a linear increase in chest compression fraction extending to 80% but not further. Possible explanations for this limit include a higher level of fatigue in CPR providers in the 80% to 100% category, a lower number of cases with advanced care paramedics first at scene, less CPR interruptions for other life saving procedures in a population believed to be less likely to survive, or other variables not accounted for in our analyses.
The current findings, together with similar data for shockable rhythm,20 are consistent with experimental and clinical studies on CPR performance. Berg et al. 9 observed that coronary perfusion pressure improved when using a strategy of chest compression alone compared with chest compressions interrupted by rescue breathing in a ventricular fibrillation swine model. Other studies by Sanders et al. 11, Ewy et al. 15, and Kern et al. 17 also used a ventricular fibrillation swine model and observed that survival with good neurological outcome at 24 hours improved when using a strategy of chest compression only CPR. This is in contrast with a study by Dorph et al.18 where ROSC occurred faster and more frequently when using a 30:2 chest compression to ventilation ratio compared with continuous chest compressions. Unlike the other animal models, Dorph’s study used a pig model with a valve hindering passive inhalation to simulate a human airway and excluded gasping as a confounding variable.
Nagao and the SOS-KANTO study group published the results of a multi-center observational study in which 4,068 OOHCA patients were witnessed by bystanders.19 Among that group of patients, the 439 who received chest compression only CPR had better neurological outcomes at 30 days compared with the 712 for which bystanders chose to provide a combination of chest compressions with ventilations. Patients with apnoea, a shockable rhythm, and for which resuscitation was initiated within 4 minutes of collapse appeared to benefit the most from chest compression only CPR. In the multi-center observational study of 506 VF/VT OOHCA patients by Christenson et al. described earlier,20 increased chest compression fraction was independently associated with an increased likelihood of survival to hospital discharge. Bobrow et al. 26 published the results of a multi-center before-after study of 886 OOHCA patients where survival to hospital discharge significantly improved after EMS personnel were instructed to complete 200 uninterrupted chest compressions before and between each rhythm analyses, to use epinephrine early, and delay endotracheal intubation. Overall survival to hospital discharge increased from 1.8% in the before phase to 5.4% in the after phase (odds ratio 3.0; 95% confidence interval 1.1, 8.9). Whether our observations have implications for future changes in guidelines remains to be determined.
In summary, increased chest compression fraction was independently associated with a trend toward an increased likelihood of ROSC in this observational study of OOHCA patients with non-VF/VT initial rhythm. These findings are important since non-VF/VT rhythms constitute the majority of initial cardiac arrest rhythms observed in the out-of-hospital setting. Rescuers should target a chest compression fraction of 80% when attempting resuscitation of patients with an initial non-VF/VT cardiac rhythm.
Acknowledgement
The present multi-center observational cohort study was possible thanks to the same clinical research network (ROC) that collected data for the Christenson study on the effect of chest compression fraction in a cohort of VF/VT OOHCA patients.
Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. Sep 29 2009;120(13):1241–1247.
We would like to acknowledge the professional care provided by first responders and paramedics, the hard labor of research team members at each participating sites, and the diligent supervision provided by the central data coordinating center study personnel, without which many more lives would be lost each day.
Funding Sources
The ROC is supported by a series of cooperative agreements with 10 regional clinical centers and one data Coordinating Center (5U01 HL077863, HL077881, HL077871, HL077872, HL077866, HL077908, HL077867, HL077885, HL077887, HL077873, HL077865) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, and the American Heart Association.
Appendix
Disclosures
-
-
Siobhan Everson-Stewart, Jim Christenson, Douglas Andrusiek, Judy Powell, Tom P. Aufderheide, Robert Berg, and Ian G. Stiell have no conflict of interest to declare.
-
-
Christian Vaillancourt, once received a small (<$25,000) unrestricted grant from Medtronic PhysioControl in 2001 to study to optimum placement of AEDs in the community.
-
-
Sheldon Cheskes, once received a small honorarium from Zoll as a speaker.
-
-
Graham Nichol, has the following conflicts of interests to disclose:
Research Grants
Resuscitation Outcomes Consortium (NIH U01 HL077863-05) 2004–2010; Co-PI
Evaluation of Video Self-Instruction in Compressions-Only CPR (Asmund S. Laerdal Foundation for Acute Medicine) 2007–2010; PI
Randomized Trial of Hemofiltration After Resuscitation from Cardiac Arrest (NHLBI R21 HL093641-01A1) 2009–2011; PI
Randomized Field Trial of Cold Saline IV After Resuscitation from Cardiac Arrest (NHLBI R01 HL089554-03) 2007–2012; Co-I
Resynchronization/Defibrillation for Advanced Heart Failure Trial (RAFT) (200211UCT-110607) 2003–2010; Co-I
Outcome and Cost-Effectiveness of FDG PET in LV Dysfunction (PARR 2)- 5 Year Follow-Up (165202) 2007–2010; Co-I
Novel Methods of Measuring Health Disparities (1RC2HL101759-01) 2009–2011; Co-I Cascade Cardiac Resuscitation System (Medtronic Foundation) 2010–2015; PI
Research Collaborator
Gambro Renal Inc., Lakewood, CO
Sotera Wireless, San Diego, CA
Lifebridge Medizintechnik AG, Ampfing, Germany
Other
Chair, AHA Executive Database Steering Committee;
Chair, Mission:Lifeline EMS Task Force
-
Co-Chair, AHA Resuscitation Science Symposium Planning Committee;
Member, AHA Advanced Cardiac Life Support Subcommittee; Member, AHA Epidemiology and Statistics Committee;
Member, Pacific Mountain Affiliate Board of Directors, American Heart Association Received travel reimbursement, AHA.y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: See Appendix
REFERENCES
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008 Sep 24;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaillancourt C, Stiell IG. Canadian Cardiovascular Outcomes Research Team (CCORT). Cardiac arrest care and emergency medical services in Canada. Canadian Journal of Cardiology. 2004;20(11):1081–1090. [PubMed] [Google Scholar]
- 3.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. New England Journal of Medicine. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 4.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007 Apr;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006 Nov;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Jantti H, Silfvast T, Turpeinen A, Kiviniemi V, Uusaro A. Influence of chest compression rate guidance on the quality of cardiopulmonary resuscitation performed on manikins. Resuscitation. 2009 Apr;80(4):453–457. doi: 10.1016/j.resuscitation.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006 Dec;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Olasveengen TM, Vik E, Kuzovlev A, Sunde K. Effect of implementation of new resuscitation guidelines on quality of cardiopulmonary resuscitation and survival. Resuscitation. 2009 Apr;80(4):407–411. doi: 10.1016/j.resuscitation.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 10.Ewy GA. Cardiac arrest--guideline changes urgently needed. Lancet. 2007 Mar 17;369(9565):882–884. doi: 10.1016/S0140-6736(07)60422-X. [DOI] [PubMed] [Google Scholar]
- 11.Sanders AB, Kern KB, Berg RA, Hilwig RW, Heidenrich J, Ewy GA. Survival and neurologic outcome after cardiopulmonary resuscitation with four different chest compression-ventilation ratios. Annals of Emergency Medicine. 2002;40(6):553–562. doi: 10.1067/mem.2002.129507. [DOI] [PubMed] [Google Scholar]
- 12.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 13.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 14.American Heart Association in collaboration with the International Liaison Committee on Resuscitation. 2005 International Consensus on Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) With Treatment Recommendations. Part 2: Adult Basic Life Support. Circulation. 2005;112(22):III-5–III-16. doi: 10.1016/j.resuscitation.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewy GA, Zuercher M, Hilwig RW, et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest.[see comment] Circulation. 2007 Nov 27;116(22):2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 16.Kellum MJ. Compression-only cardiopulmonary resuscitation for bystanders and first responders. Current Opinion in Critical Care. 2007 Jun;13(3):268–272. doi: 10.1097/MCC.0b013e32814b0524. [DOI] [PubMed] [Google Scholar]
- 17.Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation. 2002 Feb 5;105(5):645–649. doi: 10.1161/hc0502.102963. [DOI] [PubMed] [Google Scholar]
- 18.Dorph E, Wik L, Stromme TA, Eriksen M, Steen PA. Oxygen delivery and return of spontaneous circulation with ventilation:compression ratio 2:30 versus chest compressions only CPR in pigs. Resuscitation. 2004;60(3):309–318. doi: 10.1016/j.resuscitation.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Nagao K The SOS-KANTO study group. Cardiopulmonary resuscitation by bystanders with chest compression only: an observational study. Lancet. 2007;369:920–926. doi: 10.1016/S0140-6736(07)60451-6. [DOI] [PubMed] [Google Scholar]
- 20.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009 Sep 29;120(13):1241–1247. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehospital Emergency Care. 2007 Oct–Dec;11(4):369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 22.Morrison LJ, Nichol G, Rea TD, et al. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008 Aug;78(2):161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004 Nov 23;110(21):3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 24.Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005 Aug 30;112(9):1259–1265. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 25.Chambers JM, Hastie T. Statistical models in S. Pacific Grove, Calif.: Wadsworth & Brooks/Cole Advanced Books & Software; 1992. [Google Scholar]
- 26.Bobrow BJ, Clark LL, Ewy GA, et al. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008 Mar 12;299(10):1158–1165. doi: 10.1001/jama.299.10.1158. [DOI] [PubMed] [Google Scholar]