Abstract
Cytolethal distending toxin (CDT) from Actinobacillus actinomycetemcomitans is a G2/M cell-cycle-specific growth-inhibitory toxin that leads to target cell distension followed by cell death. To determine the mechanisms by which A. actinomycetemcomitans CDT acts as an immunosuppressive factor, we examined the effects of highly purified CDT holotoxin on human T lymphocytes. Purified CDT was cytolethal toward normal peripheral T lymphocytes that were activated by in vitro stimulation with phytohemagglutinin. In addition, purified CDT showed cytolethal activity against Jurkat and MOLT-4 cells, which are known to be sensitive and resistant, respectively, to Fas-mediated apoptosis. Death in these cell lines was accompanied by the biochemical features of apoptosis, including membrane conformational changes, intranucleosomal DNA cleavage, and an increase in caspase activity in the cells. Pretreatment of Jurkat cells with the general caspase inhibitor z-VAD-fmk mostly suppressed CDT-induced apoptosis. Furthermore, specific inhibitors of caspase-2 and -7 showed significant inhibitory effects on CDT-induced apoptosis in Jurkat cells, and these inhibitory effects were fully associated with reduced activity of caspase-2 or -7 in the CDT-treated Jurkat cells. These results strongly suggest that CDT possesses the ability to induce human T-cell apoptosis through activation of caspase-2 and -7.
Bacterial infections in mammals evoke a series of immune reactions to bacterial antigens in the infected host, but immune responses are occasionally suppressed or shut down by some bacterial products, such as toxins. Suppression or inactivation of the host immune response is considered to be a bacterial strategy to evade host immune mechanisms. Actinobacillus actinomycetemcomitans is a gram-negative rod-shaped pathogen implicated in the pathogenesis of juvenile and adult periodontitis (38). Previous studies demonstrated that A. actinomycetemcomitans produces a factor(s) that is immunosuppressive for human T and B cells (25). It was recently established that A. actinomycetemcomitans produces a new member of the cytolethal distending toxin (CDT) family which was previously unrecognized as a virulence factor of A. actinomycetemcomitans (40). CDT belongs to the family of toxins with cell-cycle-specific inhibitory activities which block the progression of cells from G2 to M phase (28). CDT-poisoned cells undergo cell distension and nucleus swelling and eventually die. CDT was found to form a complex of three subunits, CDTA, -B, and -C (9, 14, 21, 24, 31, 40), and the subunits were determined to be tandemly encoded by the cdtA, cdtB, and cdtC genes at the chromosomal cdt loci. A. actinomycetemcomitans CDTA, -B, and -C are translated as approximately 25-, 32-, and 21-kDa proteins, respectively, and are secreted into the periplasm (40). After cleavage of their 15- to 21-amino-acid signal sequences at the N terminus, they become 23-, 29-, and 19-kDa proteins, respectively (31, 36, 40). CDTA goes through another processing step to become an 18- to 19-kDa form, designated CDTA′, and forms a complex with CDTB and CDTC to become a holotoxin (40).
In 1999, Shenker et al. purified the immunosuppressive factor of A. actinomycetemcomitans that could affect human T cells and demonstrated that the factor was one of the subunit proteins of CDT, CDTB (34, 36). Their group also demonstrated that a crude CDT preparation of A. actinomycetemcomitans induced cell cycle arrest at the G2 phase in human peripheral blood cells (37). Furthermore, the CDT preparation was shown to induce apoptotic cell death in peripheral blood lymphocytes along with activation of caspase-3, -8, and -9 (35). Despite those findings, whether these caspases are really involved in CDT-induced apoptosis remains virtually unknown.
For this study, we studied the immunosuppressive effect of highly purified A. actinomycetemcomitans CDT on normal human T lymphocytes and made an in-depth characterization of the cytolethal effect by using the T-cell leukemia cell lines Jurkat and MOLT-4, which are sensitive and resistant, respectively, to Fas-mediated apoptosis. We herein demonstrate that CDT induces apoptosis in these cells and that caspase-2 and -7 play important roles in the signaling pathway of CDT-induced cell death, which is distinct from Fas-mediated apoptosis.
MATERIALS AND METHODS
Purification of A. actinomycetemcomitans CDT.
CDT holotoxin was prepared by using the pQE 60 (C-terminal histidine tag) protein expression system in M15 Escherichia coli (Qiagen, Tokyo, Japan). Briefly, for construction of pQEcdtABC, the A. actinomycetemcomitans cdtABC gene was isolated from A. actinomycetemcomitans genomic DNA by PCR amplification with specific primers that contained several restriction enzyme sites for subcloning into vectors, as follows: QIA-U, 5′-AGGTACCATGGAAAAGTTT-3′, which corresponds to the cdtA starting site with an NcoI restriction enzyme site; QIC-L, 5′-AAAGATCTGCTACCCTGA-3′, which corresponds to the end of the cdtC gene, with the stop codon replaced with a BglII site (restriction sites are shown in italics). The amplified cdtABC gene was ligated into pQE60 in frame at the NcoI and BglII sites, so that the C-terminal CDTC was tagged with six histidine residues. The expression of the cdtABC gene was induced by adding isopropyl-β-d-1-thiogalactopyranoside (final concentration of 1 mM; Sigma) at an optical density at 660 nm of 0.5 to 0.7. After induction for 4 h, the culture supernatant was harvested by centrifugation at 5,000 × g for 5 min, and crude proteins were precipitated with ammonium sulfate (final concentration, 80%) by gentle stirring for at least 4 h. The precipitates were recovered by centrifugation at 15,000 × g for 20 min, dissolved in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4), and dialyzed overnight against PBS. Ni-chelated agarose beads were added into the dialyzed solution and gently shaken for at least 1 h, followed by column chromatography. The column was washed with washing buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 20 mM imidazole) and eluted with elution buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 250 mM imidazole). The eluted CDT holotoxin was dialyzed against PBS and concentrated with Centricon 10 concentrators (Millipore, Bedford, Mass.).
Preparation of cells and culture conditions.
Peripheral blood mononuclear cells were obtained from healthy volunteers with their informed consent. Twenty to forty milliliters of heparinized venous blood was diluted with an equal volume of PBS with 1% heparin and layered over Ficoll-Hypaque lymphocyte separation medium (ICN Biomedical Inc., Aurora, Ohio). Density gradient centrifugation was performed at 400 × g for 30 min, and mononuclear cells were harvested from the plasma-lymphocyte separation medium interface. Collected cells were washed twice with Earle's balanced salt solution (Nissui, Tokyo, Japan) containing 2.5% fetal calf serum (FCS) (Intergen Co., Purchase, N.Y.). The number of recovered cells was counted and diluted to 106 cells/ml in RPMI 1640 containing 10% FCS, 100 U of penicillin G/ml, and 100 μg of streptomycin/ml. The isolated lymphocytes were incubated with CDT (100 ng/ml) and cultured at 37°C in 5% CO2, with or without stimulation on day 1 by phytohemagglutinin (PHA) (Difco Lab., Detroit, MI) diluted 1:1,600, and the cell population was monitored for 96 h. A thymic T-cell leukemia cell line, MOLT-4, and a peripheral T-cell leukemia cell line, Jurkat, were maintained in RPMI 1640 containing 10% FCS and 25 μg of kanamycin/ml at 37°C in 5% CO2. The cells (106 cells/ml) were left untreated or were treated with CDT (100 ng/ml) and cultured under similar conditions. In some experiments, Jurkat cells were similarly treated with 100 ng of anti-CD95 (anti-Fas) monoclonal antibody (Ab) CH11 (BD PharMingen, San Diego, Calif.) per ml.
Flow cytometry.
Conformational changes of the membrane by phosphatidylserine translocation and membrane hole formation were observed by counting the percentages of cells that were stained with fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) in a FACScan flow cytometer (BD Biosciences, San Jose, Calif.). Briefly, CDT-treated cells (5 × 105 to 10 × 105) were collected by centrifugation at 350 × g for 2 min and were washed three times with 500 μl of PBS with 1% FCS. The washed cells were resuspended in 180 μl of PBS with 1% FCS, and 0.5 μl of FITC-labeled annexin V and 1 μl of PI, from the MEBCYTO apoptosis kit (MBL, Nagoya, Japan) were added to the cell suspension. After the reaction for 5 min at room temperature, 10,000 cells were analyzed in the FACScan instrument. The data obtained were processed by quadrant population analysis, using CellQuest software (BD Biosciences). The living cell population was determined by counting cells that were negative for both annexin V and PI (distributed in the lower left of the quadrant).
Caspase assay.
CDT-treated cells were harvested and washed with PBS. PBS-washed cells were lysed with lysis buffer (10 mM Tris-Cl [pH 7.4], 25 mM NaCl, 0.25% Triton X-100, 1 mM EDTA) and centrifuged at 15,000 × g for 10 min. The supernatant was diluted with the lysis buffer and the protein concentration was adjusted to 1 mg/ml. Ten micrograms of total protein was incubated in 200 μl of caspase buffer (50 mM Tris-Cl [pH 7.2], 100 mM NaCl, 1 mM EDTA, 10% sucrose, 0.1% CHAPS, and 5 mM dithiothreitol) with a 50 μM concentration (each) of various fluorogenic substrate peptides. The peptides include Ac-DEVD-7-amino-4-methyl cumarine (AMC) for caspase-3, -7, and -8, Ac-DQTD-AMC for caspase-7 and -3, Ac-IETD-AMC for caspase-8, -6, and granzyme, Ac-LEHD-AMC for caspase-9, and Ac-VDVAD-AMC for caspase-2 (Peptide Institute Inc., Osaka, Japan).
The reaction mixture was incubated at 37°C for 60 min, and the release of 7-amino-4-methylcumarin was measured by use of a spectrophotometer (Shimazu RF-540), with excitation at 380 nm and emission at 460 nm. One unit (U) was defined as 5.2 pmol of substrate cleaved per min per mg of protein.
Various caspase inhibitors were used at a concentration of 100 μM. They were Ac-VAD-fmk as a general caspase inhibitor, Ac-WEHD-CHO for caspase-1, Ac-DEVD-CHO for caspase-3, -7, and -8, Ac-DMQD-CHO for caspase-3, Ac-LEHD-CHO for caspase-9, Ac-IETD-CHO for caspase-8 and -6, Ac-DQTD-CHO for caspase-7 and -3, and Ac-VDVAD-CHO for caspase-2 (Peptide Institute Inc.).
Electron microscopy.
Cells were fixed with 2.5% glutaraldehyde for 2 h and rinsed in 0.1 M cacodylate buffer (pH 7.4) for 12 h. After postfixation with 1% osmium tetroxide for 30 min, cells were stained with 2% uranyl acetate for 30 min and dehydrated in graded alcohol, which was then replaced by propylene oxide. After these steps, the cell suspension was spun down at 8,000 × g for 5 min and the supernatant was discarded. The cell pellets were embedded in epoxy resin. Thin sections were stained in 2% uranyl acetate and lead citrate and were observed in a Hitachi H500 electron microscope.
Preparation of cytosolic and mitochondrial fractions.
CDT-treated cells were washed twice with PBS and resuspended in isotonic buffer (10 mM HEPES [pH 7.3], 0.3 M mannitol, 0.1% bovine serum albumin). Digitonin was added to the cell suspension at a concentration of 0.1 mM, and the cells were incubated for 5 min on ice. After the samples were centrifuged at 8,500 × g for 5 min at 4°C, the supernatant was used as the cytosolic fraction. The pellet was resuspended in sonication buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.5% Tween 20). The samples were sonicated with an ultrasonic disrupter (UD200 TOMY) for 20 s at output level 4. After centrifugation at 10,000 × g for 5 min at 4°C, the supernatant was collected and used as the mitochondrial fractions.
Antibodies.
Antibodies against CDTA, -B, and -C that were previously obtained were used under conditions that were described elsewhere (40). Anti-cytochrome c Ab (BD PharMingen) was used according to the instructions provided by the supplier.
RESULTS
Cytolethal effects of highly purified CDT on human peripheral lymphocytes.
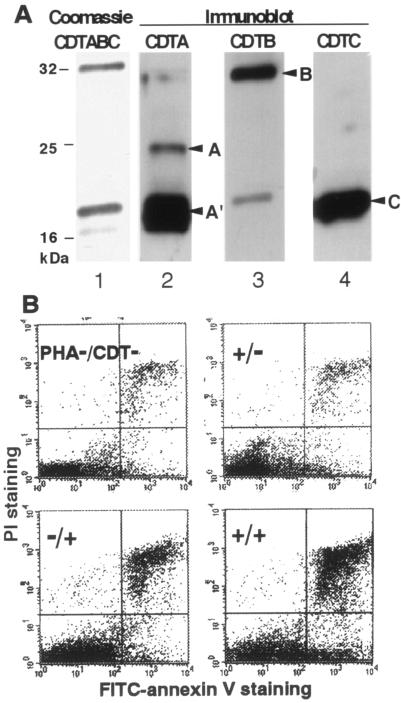
A. actinomycetemcomitans CDT was purified from the culture supernatant of E. coli carrying the A. actinomycetemcomitans cdtABC genes. The secreted CDT complex was purified through a Ni chelation column, using the affinity of six histidine (His) residues tagged to the C terminus of CDTC (Fig. 1A). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting detected CDTA′, CDTB, and CDTC tagged with six histidine residues in the medium fraction, and premature CDTA was also found in a small amount, suggesting that most of the CDT complex consisted of CDTA′, CDTB, and CDTC. Cell distension and the G2/M blocking activity of purified CDT were confirmed in HeLa cells, and the purified CDT complex was used for experiments.
FIG. 1.
Cytolethal effect of A. actinomycetemcomitans CDT on human peripheral lymphocytes. (A) Purified CDTABC complex in the medium fraction from E. coli M15 carrying A. actinomycetemcomitans cdtABC. Lane 1, Coomassie staining of CDTABC complex; lanes 2 to 4, immunoblots for the detection of each subunit by use of antiserum against CDTA, CDTB, and CDTC, respectively. Arrowheads: A, CDTA (premature form); A′, CDTA′ (mature form of CDTA); B, mature CDTB; C, mature CDTC tagged with six histidine residues. (B) Flow cytometry analysis. Lymphocytes were stained with FITC-labeled annexin V and PI and analyzed in a FACScan flow cytometer. Lymphocytes prepared from peripheral blood obtained from healthy human volunteers were treated with several combinations of PHA (1:1,600) and CDT (100 ng/ml). A representative result of the quadrant analysis of annexin V- and PI-stained lymphocytes on day 2 is shown.
To address whether CDT can induce cell death of human peripheral lymphocytes, we added purified CDT to peripheral blood mononuclear cell cultures in the presence or absence of PHA stimulation. Flow cytometry analysis with FITC-annexin V and PI revealed that treatment of normal lymphocytes with a combination of CDT and PHA increased the percentage of dead cells (the sum of annexin V+ PI+ and annexin V+ PI− fractions) (Fig. 1B). It should be noted that CDT did not kill lymphocytes very much unless they were activated with PHA. This is in good agreement with the previously suggested activity of CDT, which is cell-cycle-dependent cytotoxicity against HeLa cells (1), and also suggests that CDT can act as an immunosuppressive toxin by killing activated lymphocytes.
CDT induced apoptosis in Jurkat and MOLT-4 cells.
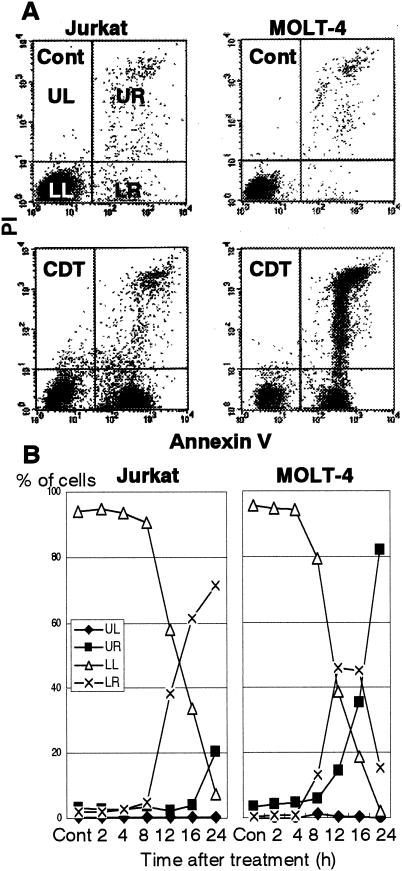
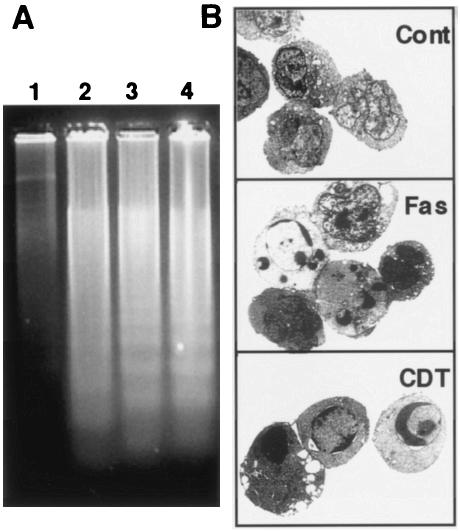
In order to obtain further insights into the cytolethal effect of CDT in T lymphocytes, we used two cell lines, Jurkat and MOLT-4, and monitored population changes in four panels (upper left, upper right, lower left, and lower right [UL, UR, LL, and LR, respectively]) after CDT treatment (Fig. 2). For both cell lines, CDT treatment increased the percentage of dead cells (Fig. 2A). Flow cytometry analysis revealed that the percentage of annexin V+ PI− Jurkat cells (distributed in the LR panel) started to increase 8 h after CDT treatment and continued to increase until 24 h after treatment (Fig. 2B). MOLT-4 showed a somewhat different pattern from that of Jurkat. The percentage of annexin V+ PI− MOLT-4 cells (LR) started to increase 4 h after CDT treatment and reached a plateau 12 to 16 h after the treatment (Fig. 2B). After that, the annexin V+ PI− population (LR) decreased after 16 h. Concomitantly with the increase and decrease of the annexin V+ PI− population (LR), the annexin V+ PI+ population (UR) started to increase after 8 h and kept increasing until 24 h. In both cell lines, the increase of the annexin V+ PI− cell population (LR) in the early stage after treatment strongly suggested that CDT poisoning was able to induce apoptosis in cells that are sensitive to Fas-mediated apoptosis as well as in those that are resistant to Fas-mediated apoptosis. We further investigated the apoptotic characteristics of CDT-poisoned cells, including chromosomal DNA fragmentation and chromatin condensation. As shown in Fig. 3A, electrophoretic analysis of the chromosomal DNA of Jurkat cells showed a typical DNA ladder formation after 16 h of treatment with CDT, which is similar to those observed after treatment with anti-Fas Ab or irradiation. Electron microscopic observation of CDT-poisoned Jurkat cells revealed chromatin condensation, which is associated with cells undergoing apoptosis (Fig. 3B). Similar apoptotic characteristics were also apparent for CDT-treated MOLT-4 cells (data not shown). There was no necrotic change, such as swelling of the cell body and mitochondria or collapse of the plasma and nuclear membranes, in these cell lines.
FIG. 2.
Cytolethal kinetics on CDT-treated cell lines. T-cell leukemia cell lines Jurkat and MOLT-4 were treated with CDT (100 ng/ml) for various times. Cells stained with FITC-labeled annexin V and PI were analyzed by flow cytometry. (A) Representative results for cells with or without treatment of CDT at 16 h. Cont, control. (B) Kinetics of cell death measured at indicated times after CDT treatment. The percentages of cell populations in the UL quadrant (annexin V− PI+, ♦), the UR quadrant (annexin V+ PI+, ▪), the LL quadrant (annexin V− PI−, ▵), and the LR quadrant (annexin V+ PI−, ×) are indicated.
FIG. 3.
Apoptotic DNA fragmentation and morphological change in CDT-treated lymphocytes. (A) DNA ladder formation in CDT-treated cells. Jurkat cells were treated with anti-Fas Ab (100 ng/ml) (lane 2), X-ray irradiation (10 Gy) (lane 3), or CDT (100 ng/ml) (lane 4) for 16 h, and chromosomal DNAs were prepared. The extracted DNAs were separated by agarose gel electrophoresis and visualized by staining with ethidium bromide. Lane 1, DNA from untreated Jurkat cells. (B) Ultrastructure of CDT- or anti-Fas Ab-treated lymphocytes. Jurkat cells were treated with CDT (100 ng/ml) for 16 h and subjected to electron microscopic observation as described in Materials and Methods. Cont, control.
Intracellular caspase activities.
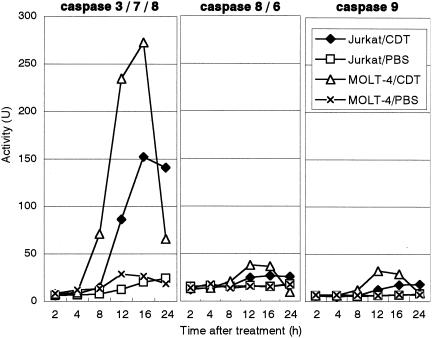
Apoptosis generally involves the activation of cysteine proteases, or caspases. The activity of sets of the major caspases, caspase-3, -7, and -8, caspase-8 and -6, and caspase-9, in Jurkat and MOLT-4 cells was monitored after CDT treatment. In Jurkat cells, the activity of caspase-3, -7, and -8 started to increase 8 h after treatment of the cells with CDT and significantly increased until 16 to 24 h (Fig. 4). On the other hand, the activity of the caspase-8 and -6 set and caspase-9 slightly increased upon treatment with CDT. Interestingly, caspase activity in MOLT-4 cells started to increase earlier and occurred at a higher level than in Jurkat cells between 4 and 16 h after treatment. After 12 to 16 h, caspase activity in MOLT-4 cells went down in parallel with the appearance of the annexin V+ PI− cell population (Fig. 4, Fig. 2B).
FIG. 4.
Caspase activity in CDT-treated lymphocytes. The total protein (10 μg) was extracted from CDT-treated Jurkat or MOLT-4 cells at the indicated times. Caspase activity was measured by incubation of the extract with a fluorogenic substrate for caspase-3, -7, and -8 (left), caspase-8 and -6 (middle), or caspase-9 (right). After incubation, the released 7-amino-4-methylcumarine was measured in spectrophotometer, with excitation at 380 nm and emission at 460 nm. ♦, CDT-treated Jurkat cells; □, PBS-treated Jurkat cells (control); ▵, CDT-treated MOLT-4; ×, PBS-treated MOLT-4 (control). The experiments were repeated at least three times, and similar results were obtained. Representative results are shown.
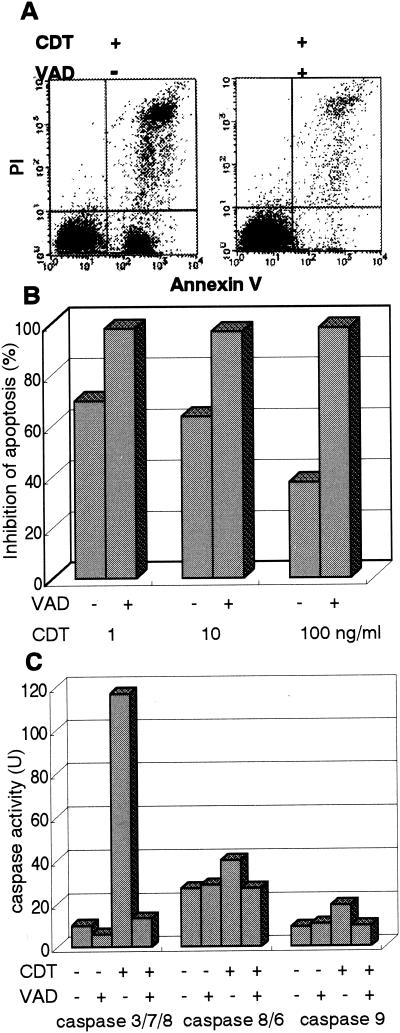
Cells retained the phenotype of living cells (annexinV− PI−) when the cells were pretreated with a general caspase inhibitor, z-VAD-fmk (100 μM), indicating that z-VAD-fmk is able to nearly completely inhibit CDT-induced apoptosis (Fig. 5A and B). It also turned out that the CDT-induced elevation of caspase activity could be blocked by z-VAD-fmk (Fig. 5C). These results indicated that CDT-induced apoptotic cell death in Jurkat and MOLT-4 cells was mostly dependent on the activation of a caspase(s) until at least 24 h after treatment.
FIG. 5.
Effect of general caspase inhibitor on CDT-induced apoptosis. (A) Jurkat cells were preincubated with z-VAD-fmk (100 μM) for 30 min and then were treated with CDT (1, 10, or 100 ng/ml) for 16 h. Cells were stained with FITC-annexin V and PI and analyzed by flow cytometry. The flow cytometry pattern represents CDT (100 ng/ml)-treated lymphocytes with (+) or without (−) z-VAD-fmk. Inhibition of apoptosis was calculated as the relative percentage of living cells, or the population in the LL quadrant (annexin V− PI− population). (B) z-VAD-fmk inhibits apoptosis in the cells treated with various concentrations of CDT. (C) Effect of z-VAD-fmk on caspase-3, -7, and -8, caspase-8 and -6, and caspase-9 activity induced by CDTB. Jurkat cells were preincubated with z-VAD-fmk (100 μM) for 30 min and then were treated with CDT (100 ng/ml). Caspase activity was measured as described in Materials and Methods.
Signaling pathway of caspases.
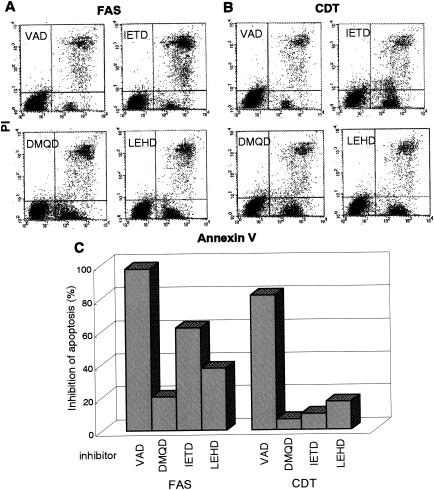
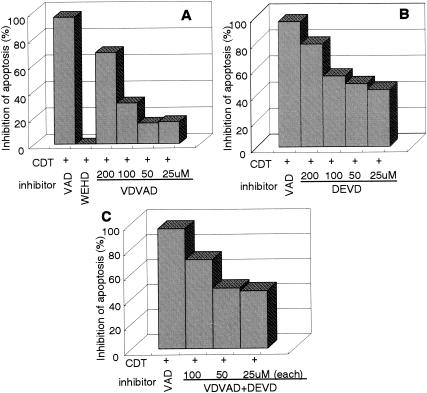
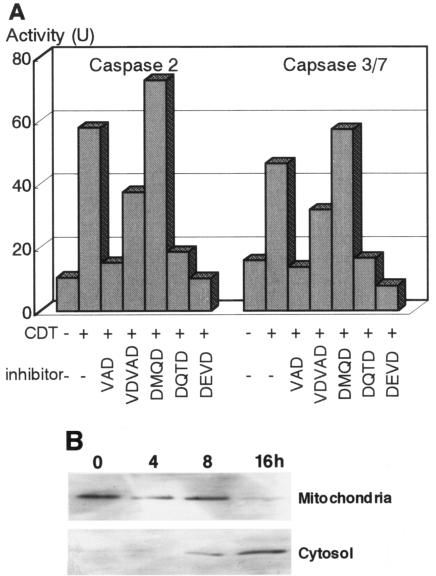
Caspases can be classified into two categories as follows: initiator caspases, including caspase-2, -8, and -9, which are present upstream of the signaling pathway of apoptosis, and effector caspases, which play roles in the cleavage of many regulatory proteins (3). In order to determine the signaling pathway of the caspase(s) in CDT-induced apoptosis, we added a variety of caspase inhibitors and monitored their inhibitory effects on CDT cytotoxicity and apoptotic features in Jurkat cells by using flow cytometry with annexin V-PI double staining. For Fas-mediated apoptosis, caspase-8 was confirmed to be a critical initiator caspase for receptor-mediated apoptosis signaling in Jurkat cells, since the addition of Ac-IETD-CHO, an inhibitor of caspase-8 and -6, significantly inhibited the death of Jurkat cells by an anti-Fas Ab (Fig. 6A and C). On the other hand, inhibitors for caspase-3 (Ac-DMQD-CHO), caspase-8 and -6 (Ac-IETD-CHO), and caspase-9 (Ac-LEHD-CHO) failed to inhibit CDT-induced apoptosis of Jurkat cells (Fig. 6B and C), suggesting that CDT-induced apoptosis might use a different signaling pathway from that of Fas-mediated apoptosis. To determine which caspase(s) among those we tested is actually involved in CDT-induced apoptosis, we analyzed the effects of inhibitors of caspase-1 (Ac-WEHD-CHO), caspase-2 (Ac-VDVAD-CHO), and caspase-3, -7, and -8 (Ac-DEVD-CHO) on CDT-induced apoptosis. The inhibitor of caspase-1 (Ac-WEHD-CHO) had no inhibitory effect on CDT-induced apoptosis of Jurkat cells at concentrations up to 200 μM. On the other hand, CDT-induced apoptosis was dose dependently inhibited by the inhibitor of caspase-2 (VDVAD) or that of caspase-3, -7, and -8 (DEVD) (Fig. 7A and B). However, the combination of inhibitors of caspase-2 (VDVAD) and of caspase-3, -7, and -8 (DEVD) did not show any multiplier effect (Fig. 7C). Together with the fact that inhibitors of caspase-3 (Ac-DMQD-CHO) and caspase-8 and -6 (Ac-IETD-CHO) failed to prevent CDT-induced apoptosis (Fig. 6), our results strongly suggested that caspase-2 and -7 were mainly involved in the activation of this caspase-dependent apoptotic cascade. We therefore measured the activities of caspase-2 and -7 after CDT treatment for 16 h. To measure caspase-7 activity, we used Ac-DQTD-AMC (substrate for caspase-3 and -7) as a substrate because no caspase-7-specific substrate was available. As shown in Fig. 8A, CDT significantly induced the activation of caspase-2. CDT also induced caspase-3 and -7 activity, but the caspase-3-specific inhibitor Ac-DMQD-CHO did not inhibit the activity at all (Fig. 8A). This clearly indicated that the CDT treatment activated caspase-7. These data suggest that caspase-2 and caspase-7 are really involved in the pathway of CDT-induced apoptotic cell death. It is noteworthy that Ac-VDVAD-CHO (caspase-2 inhibitor) showed an inhibitory effect on caspase-3 and -7 activity. Similarly, Ac-DQTD-CHO (the caspase-3 and -7 inhibitor) clearly inhibited the effect on caspase-2. These results suggest that the caspase-2 and -7 pathways of CDT-induced apoptosis are tightly linked to each other, and they are quite consistent with the results of the experiment on the combination effect of inhibitors of caspase-2 and caspase-3, -7, and -8 (Fig. 7C). The incomplete inhibition of caspase-2 activity by Ac-VDVAD-CHO (caspase-2 inhibitor) implies that another molecule with proteolytic activity similar to that of caspase-2 may be involved in CDT-induced cell death.
FIG. 6.
Effect of various caspase inhibitors on CDT-induced apoptosis. Jurkat cells were preincubated with the indicated inhibitors (100 μM) for 30 min and then were treated with anti-Fas Ab (100 ng/ml) (A) or CDT (100 ng/ml) (B). After 16 h, cells were stained with FITC-annexin V and PI and analyzed by FACScan. The inhibitors used were VAD (general caspase inhibitor), DMQD (caspase-3 inhibitor), IETD (caspase-8 and -6 inhibitor), and LEHD (caspase-9 inhibitor). (A and B) Representative flow cytometry patterns. (C) Effect of caspase inhibitors on apoptosis induced by anti-Fas Ab (left) or CDT (right). Inhibition of apoptosis was defined as the relative percentage of normal living cells (annexin V− PI−).
FIG. 7.
Dose-dependent inhibitory effects of caspase inhibitors on CDT-induced apoptosis. Jurkat cells were preincubated with various concentrations of caspase inhibitors (25, 50, 100, and 200 μM) for 30 min and then were treated with CDT (100 ng/ml). The inhibitors used were WEHD (caspase-1 inhibitor), VDVAD (caspase-2 inhibitor), and DEVD (caspase-3, -7, and -8 inhibitor). After 16 h, cells were stained with FITC-annexin V and PI and analyzed by flow cytometry. Panels show the relative inhibition of CDT-induced apoptosis with VDVAD (A), DEVD (B), and VDVAD and DEVD (C). Inhibition of apoptosis was defined as the relative percentage of living cells (annexin V− PI−). VAD (general caspase inhibitor) and WEHD were used at concentrations of 100 and 200 μM, respectively.
FIG. 8.
Elevation of caspase-2 or -7 activity and cytochrome c release by CDT treatment. (A) Jurkat cells were preincubated with the indicated inhibitor (100 μM) for 30 min and then were treated with CDT (100 ng/ml) for 16 h. The inhibitors used were VAD (general caspase inhibitor), VDVAD (caspase-2 inhibitor), DMQD (caspase-3 inhibitor), DQTD (caspase-3 and -7 inhibitor), and DEVD (caspase-3, -7, and -8 inhibitor). Caspase activity was measured as described in Materials and Methods. (B) Cytosol and mitochondrial fractions were extracted from Jurkat cells that were treated with CDT (100 ng/ml) for 0, 4, 8, and 16 h and were subsequently immunoblotted with an anti-cytochrome c Ab followed by a horseradish peroxidase-conjugated secondary Ab. The bands of cytochrome c were visualized by ECL.
To determine whether the mitochondrial pathway is really involved in CDT-induced apoptosis, we analyzed the release of cytochrome c from mitochondria in CDT-treated cells. As shown in Fig. 8B, the immunoblotting assay revealed that cytochrome c was detectable in the cytosol at 8 h, and more apparently so at 16 h, after CDT treatment of Jurkat cells. The appearance of cytochrome c accorded well with the time course of caspase activation, suggesting that the mitochondrial pathway is also involved in CDT-induced apoptosis.
DISCUSSION
In order to investigate CDT-induced apoptosis, we first attempted to establish a cell line model of it. Cytological and biological characterizations of CDT-treated Jurkat and MOLT-4 cells satisfied the apoptosis criteria, such as an increase in membrane conformational changes detected by an increase in the annexin V-positive cell population, intranucleosomal DNA fragmentation, chromatin condensation, and an increase in caspase activity (Fig. 1 to 4). It is noteworthy that the elevation of caspase activity in Jurkat and MOLT-4 cells showed some considerable differences in time course and in activation pattern: the culmination of induced caspase activity was higher in CDT-treated MOLT-4 cells than in similarly treated Jurkat cells. This may suggest that Jurkat and MOLT-4 cells took different apoptotic pathways after exposure to CDT. In this context, it should be noted that Jurkat, but not MOLT-4, cells are deficient in p53 (10). p53 is implicated in the G2/M block in CDT-treated keratinocytes and fibroblasts (7). The phosphorylation of p53 and other phosphorylation signals could play some role in CDT-induced apoptosis, and the lack of p53 might alter the pathway of apoptosis of Jurkat cells from that of MOLT-4 cells.
We tried to obtain further insights into the understanding of the signaling pathway of CDT-induced apoptosis, especially regarding the caspase cascade(s). Caspases are members of the aspartate-specific cysteine protease family which play a critical role in apoptosis (6, 39). They are composed of two major subfamilies, initiator caspases and effector caspases, based on the presence or absence of a large prodomain in the amino-terminal region (33). Initiator caspases generally act upstream of the proteolytic cascade, while effector caspases act downstream and are involved in the cleavage of specific cellular substrate proteins (41). Once processed, the substrates induce morphological changes characteristic of the apoptotic process (8, 11). The long prodomains of the initiator caspases trigger and/or facilitate the activation of proenzymes through interactions with adaptor molecules (13). Caspase-2, -8, -9, and -10 generally act as initiator caspases upstream of the cascade of effector caspases with small prodomains, such as caspase-3, -6, and -7 (26). Among the caspases, caspase-8, -9, and -10 play a fundamental role in transducing the specific apoptotic signal, and they cleave and activate effector procaspase-3, -6, and -7 (4). Effector caspases, in turn, cleave various proteins, leading to morphological and biochemical features characteristic of apoptosis. Recently, it has become clear that caspase-9 is involved in the apoptotic pathway that relys on mitochondrial dysfunction (15). Caspase-8 and -10 are involved in the apoptosis pathway mediated by death receptors (2).
Our results indicated that inhibitors of caspase-2 and -7 showed inhibitory effects on CDT-induced apoptosis. Several bacterial toxins are known to induce apoptosis through caspase-dependent pathways, although the exact molecular mechanism of the signaling cascade has not been well characterized. For instance, Shiga toxin and Shiga-like toxin have been demonstrated to activate caspase-2, -3, -6, -8, and -9 (5, 17, 18). It was suggested that these toxins use the caspase cascade involved in Fas-mediated apoptosis. Other toxins, such as E. coli heat-labile enterotoxin (32), Clostridium difficile toxin B (29), diphtheria toxin (19), and Mannheimia haemolytica leukotoxin (23), were shown to induce caspase-3. However, a detailed caspase cascade induced by bacterial toxins has not been well established. To our knowledge, this is the first report that a bacterial toxin preferentially utilizes caspase-2 and -7 in the signaling pathway for apoptosis. In recent studies, caspase-2 was implicated in the release of cytochrome c from mitochondria in stress-induced apoptotic pathways (16, 22, 27, 30). One such stress-inducing agent is a topoisomerase II poison, etoposide, that induces double-stranded DNA breaks in cells. Robertson et al. (30) demonstrated that etoposide-induced DNA damage induces activation of caspase-2 and hence results in cytochrome c release from mitochondria and subsequent apoptosis. It is interesting that a possible mechanism by which CDT can induce cytopathic effects involves DNA strand breaks induced by its putative DNase activity (12, 20). Such CDT-induced DNA damage may trigger the mitochondrial cascade including caspase-2 and -7. A recent report has indicated the requirement of caspase-2 for the initiation of stress-induced apoptosis prior to mitochondrial permeabilization (22). In our case, CDT-induced DNA damage may directly activate caspase-2 and then induce the mitochondrial cascade, probably followed by caspase-7 activation. Caspase-7 is a late signal transducer and one of the members of the apoptosome complex which is activated by mitochondrial stress (3). Both caspase-2 and -7 are involved in stress-induced cascades, suggesting that CDT-induced apoptosis is related to the mitochondrial pathway. Our present results indicate that CDT can induce mitochondrial membrane permeabilization, resulting in the release of cytochrome c, and that this mitochondrial pathway is highly involved in CDT-induced apoptosis. This is quite in agreement with an experiment showing that Bcl-2 overexpression reduces apoptosis in a CDT-treated human B lymphoblastoid cell line, JY (35).
Fas ligation on the cell surface induces apoptosis through the receptor-mediated signaling pathway, which involves caspase-8 as an initiation signal (2). The fact that caspase-8 inhibitor blocked Fas-mediated apoptosis in Jurkat cells (Fig. 6) indicated that the Fas-dependent apoptotic pathway was active in this cell line. In contrast, no inhibitory effect of caspase-8 inhibitor on CDT-induced apoptosis was observed in Jurkat cells, suggesting that the cytotoxic effect of CDT does not require the activation of death receptors on the cell surface.
Since CDT is able to induce apoptosis of activated T cells, this toxin may play an important role in that the bacteria evade T-cell immune responses in the periodontal pocket. It is conceivable that CDT produced by this pathogen exacerbates local inflammation by inducing apoptotic cell death of T lymphocytes that are responsible for the clearance of bacteria from the periodontal pocket. Further studies on the effect of CDT on the caspase network should unveil the CDT-related signal transduction pathway in T-lymphocyte apoptosis that may lead to the suppression of immune responses to the pathogen.
Acknowledgments
We thank Kei Nakachi, Donald G. MacPhee, and Seishi Kyoizumi at the Department of Radiobiology and Molecular Epidemiology, Radiation Effects Research Foundation, for helpful suggestions. We thank the Research Facilities, Hiroshima University School of Dentistry and School of Medicine, for the use of facilities.
This study was supported in part by a Grant for Development of Highly Advanced Medical Technology (A) and by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan.
Editor: V. J. DiRita
REFERENCES
- 1.Alby, F., R. Mazars, J. De Rycke, E. Guillou, V. Baldin, J.-M. Darbon, and B. Ducommun. 2001. Study of the cytolethal distending toxin (CDT)-activated cell cycle checkpoint. Involvement of the CHK2 kinase. FEBS Lett. 491:261-265. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Bratton, S. B., M. Macfarlane, K. Cain, and G. M. Cohen. 2000. Protein complexes activate distinct caspase cascades in death receptor and stress induced apoptosis. Exp. Cell Res. 256:27-33. [DOI] [PubMed] [Google Scholar]
- 4.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 5.Ching, J. C., N. L. Jones, P. J. Ceponis, M. A. Karmali, and P. M. Sherman. 2002. Escherichia coli Shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases. Infect. Immun. 70:4669-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 8.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 9.Deng, K., J. L. Latimer, D. A. Lewis, and E. J. Hansen. 2001. Investigation of the interaction among the components of the cytolethal distending toxin of Haemophilus ducreyi. Biochem. Biophys. Res. Commun. 20:609-615. [DOI] [PubMed] [Google Scholar]
- 10.Dreyfus, D. H., M. Nagasawa, C. A. Kelleher, and E. W. Gelfand. 2000. Stable expression of Epstein-Barr virus BZLF-1-encoded ZEBRA protein activates p53-dependent transcription in human Jurkat T-lymphoblastoid cells. Blood 96:625-634. [PubMed] [Google Scholar]
- 11.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 12.Elwell, C. A., K. Chao, K. Patel, and L. A. Dreyfus. 2001. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 69:3418-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fesik, S. W. 2000. Insights into programmed cell death through structural biology. Cell 103:273-282. [DOI] [PubMed] [Google Scholar]
- 14.Frisk, A., M. Labens, C. Johansson, H. Ahmed, L. Svensson, K. Ahlman, and T. Lagergard. 2001. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microb. Pathog. 30:313-324. [DOI] [PubMed] [Google Scholar]
- 15.Green, D. R. 2000. Apoptotic pathways: paper wraps stone blunts scissors. Cell 102:1-4. [DOI] [PubMed] [Google Scholar]
- 16.Guo, Y., S. M. Srinivasula, A. Druilhe, T. Fernandes-Alnemri, and E. S. Alnemri. 2002. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 277:13430-13437. [DOI] [PubMed] [Google Scholar]
- 17.Kiyokawa, N., T. Mori, T. Taguchi, M. Saito, K. Mimori, T. Suzuki, T. Sekino, N. Sato, H. Nakajima, Y. U. Katagiri, T. Takeda, and J. Fujimoto. 2001. Activation of the caspase cascade during Stx-1-induced apoptosis in Burkitt's lymphoma cells. J. Cell. Biochem. 81:128-142. [DOI] [PubMed] [Google Scholar]
- 18.Kojio, S., H. Zhang, M. Ohmura, F. Gondaira, N. Kobayashi, and T. Yamamoto. 2000. Caspase-3 activation and apoptosis induction coupled with the retrograde transport of Shiga toxin: inhibition by brefeldin A. FEMS Immunol. Med. Microbiol. 29:275-281. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu, N., T. Oda, and T. Muramatsu. 1998. Involvement of both caspase-like proteases and serine proteases in apoptotic cell death induced by ricin, modeccin, diphtheria toxin, and pseudomonas toxin. J. Biochem. (Tokyo) 124:1038-1044. [DOI] [PubMed] [Google Scholar]
- 20.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartate complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassus, P., X. Opitz-Araya, and Y. Lazebnik. 2002. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297:1352-1354. [DOI] [PubMed] [Google Scholar]
- 23.Leite, F., S. O'Brien, M. J. Sylte, T. Page, D. Atapattu, and C. J. Czuprynski. 2002. Inflammatory cytokines enhance the interaction of Mannheimia haemolytica leukotoxin with bovine peripheral blood neutrophils in vitro. Infect. Immun. 70:4336-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis, D. A., M. K. Stevens, J. L. Latimer, C. K. Ward, K. Deng, R. Blick, S. R. Lumbley, C. A. Ison, and E. J. Hansen. 2001. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems. Infect. Immun. 69:5626-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangan, D. F., N. S. Taichman, E. T. Lally, and S. M. Wahl. 1991. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect. Immun. 59:3267-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22:299-306. [DOI] [PubMed] [Google Scholar]
- 27.Paroni, G., C. Henderson, C. Schneider, and C. Brancolini. 2002. Caspase-2 can trigger cytochrome c release and apoptosis from the nucleus. J. Biol. Chem. 277:15147-15161. [DOI] [PubMed] [Google Scholar]
- 28.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 29.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, J. D., M. Enoksson, M. Suomela, B. Zhivotovsky, and S. Orrenius. 2002. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 277:29803-29809. [DOI] [PubMed] [Google Scholar]
- 31.Saiki, K., K. Konishi, T. Gomi, T. Nishihara, and M. Yoshikawa. 2001. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 45:497-506. [DOI] [PubMed] [Google Scholar]
- 32.Salmond, R. J., R. S. Pitman, E. Jimi, M. Soriani, T. R. Hirst, S. Ghosh, M. Rincon, and N. A. Williams. 2002. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur. J. Immunol. 32:1737-1747. [DOI] [PubMed] [Google Scholar]
- 33.Salvesen, G. S., and V. M. Dixit. 1999. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96:10964-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shenker, B. J., R. H. Hoffmaster, T. L. Mckay, and D. R. Demuth. 2000. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J. Immunol. 165:2612-2618. [DOI] [PubMed] [Google Scholar]
- 35.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167:435-441. [DOI] [PubMed] [Google Scholar]
- 36.Shenker, B. J., T. McKay, S. Datar, M. Miller, R. Chowhan, and D. Demuth. 1999. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 162:4773-4780. [PubMed] [Google Scholar]
- 37.Shenker, B. J., L. Vitale, and C. King. 1995. Induction of human T cells that coexpress CD4 and CD8 by an immunomodulatory protein produced by Actinobacillus actinomycetemcomitans. Cell. Immunol. 164:36-46. [DOI] [PubMed] [Google Scholar]
- 38.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stennicke, H. R., and G. S. Salvesen. 1997. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 272:25719-25723. [DOI] [PubMed] [Google Scholar]
- 40.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66:5008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]