Abstract
Pain in HIV frequently co-occurs with substance use and depression. The complex associations among patient characteristics, pain, depression, and drug use in HIV suggests a role for testing models that can account for relationships simultaneously, control for HIV status and also test for mediation. Using structural equation modeling (SEM), the current study examined associations among pain, sociodemographics, illicit drug use and depressive symptoms in 921 HIV seropositive and 1,019 HIV seronegative men from the Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of the natural history of HIV infection among gay/bisexual men. Longitudinal repeated measures data collected over a 6 year period were analyzed using predictive path models in which sociodemographics, HIV status and CD4+ cell counts predicted pain which in turn predicted depressive symptoms and illicit drug use. The path models did not differ substantially between HIV seropositive and seronegative men. Analyses using the total sample indicated that pain served both as a mediator and as a predictor of more use of cannabis, cocaine and heroin, as well as more depressive symptoms. HIV seropositive status predicted more use of inhaled nitrites. In this cohort, having lower CD4+ cell counts (predicted by HIV status), being African-American, less educated, and older were all associated with more pain which in turn was associated with more illicit drug use and more depressive symptoms. The results underscore the need for adequate pain management, particularly among vulnerable subgroups of HIV seropositive and HIV seronegative men to reduce the risk of drug use and depression.
Keywords: substance use, depression, HIV, pain, drug use
1. Introduction
Pain is a common occurrence among persons living with HIV—67% of a nationally representative sample of HIV seropositive persons in the United States (US) reported experiencing pain in the past month [16]. Investigation of the correlates of pain in HIV has highlighted the frequent co-occurrence of pain with both psychological problems and illicit drug use [17,18,55]. Pain in HIV seropositive persons is exacerbated when there is a coexisting psychological disorder [22,47,52,57], particularly depression [22,32,33]. Injection drug use (IDU) [15,23,37,60], as well as use of other illicit drugs (not just IDU) have also been associated with increased pain in HIV [58]. Efforts to identify which patients are more vulnerable to pain as well as depression and substance use, have indicated some common clinical and sociodemographic characteristics. For example, more advanced HIV disease has been associated with both increased pain [2,7,8 16,22,28,50,51], and with higher rates of depression (e.g., [38; 21]). Similarly, lower socioeconomic status (SES) has been linked with more pain [58; 41] and also with increased illicit drug use [58] in HIV seropositive persons.
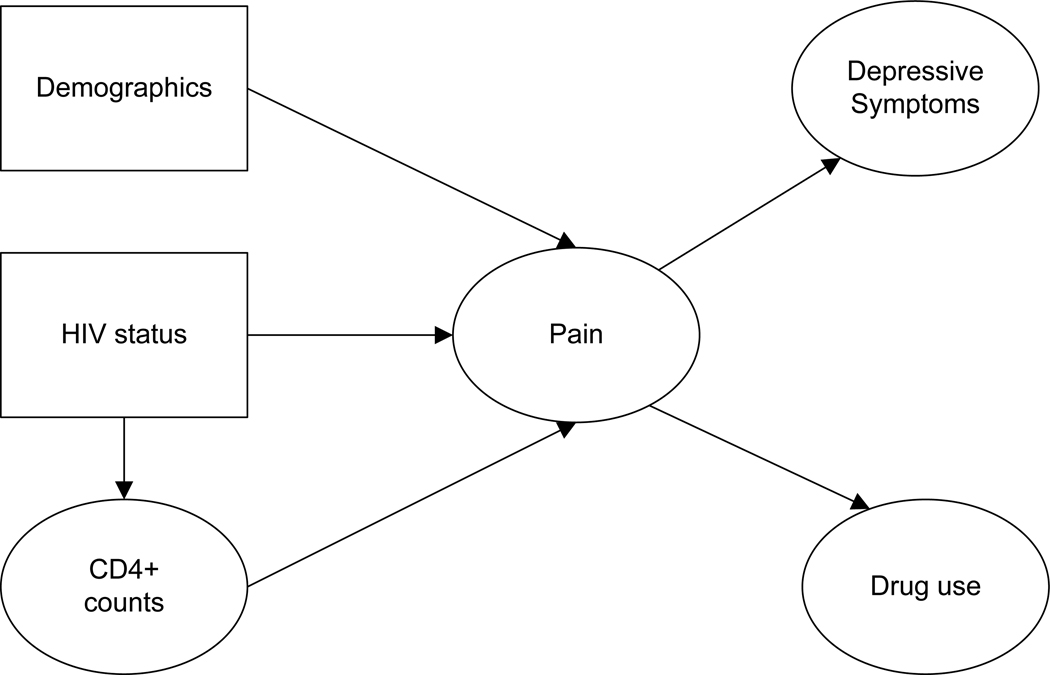
These findings suggest that the observed relationships among clinical/sociodemographic characteristics, pain, depression, and drug use in HIV may be part of a more complex sequence of effects. Yet, previous research has rarely employed models that go beyond simple correlational designs. The goal of a mediational model is to clarify the mechanism through which an initial variable (e.g., SES) affects an outcome (e.g., drug use) via a third explanatory or mediator variable [36]. We propose a conceptual model in which pain acts as a mediator between clinical/sociodemographic characteristics and depressive symptoms/drug use (see Figure 1). Our mediational model tests whether certain patients are more vulnerable to pain, and whether pain in turn leads to depressive symptoms and drug use. If confirmed, our model may assist in the targeting of pain interventions to patient subgroups with the aim of reducing pain, depression and drug use.
Figure 1.
Hypothesized path model for MACS participants
In order to disentangle the associations among the variables in terms of the impact of HIV-positivity on these behaviors, we also tested the proposed model in a control group of HIV seronegative persons. Few prior studies have examined whether the strength of such relationships is altered or moderated by HIV status. HIV status therefore functioned as a moderator variable in our analyses. Inclusion of an HIV seronegative control group may also provide information on the mediating role of pain in non-HIV infected populations (e.g., persons suffering from other health conditions). We used structural equation modeling (SEM) to test our conceptual model in a longitudinal sample of HIV seropositive and seronegative gay/bisexual men enrolled in the Multicenter AIDS Cohort Study (MACS). We hypothesized that HIV seropositivity as well as key clinical and sociodemographic characteristics would predict more pain which would in turn be associated with more depressive symptoms and more drug use.
2. Method
2.1 Participants and procedures
Participants were enrolled in the Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of the natural and treated histories of HIV infection among homosexual and bisexual men in the United States. A total of 6972 men were recruited (4954 in 1984–1985, 668 in 1987–1991, and 1350 in 2001–2003) at four centers: Baltimore/Washington DC; Chicago; Los Angeles; and Pittsburgh. The study design has been described in more detail previously [19,31]. MACS study protocols were approved by the institutional review boards of each of the participating centers, their community partners, and community advisory boards. Informed consent was obtained from all participants.
This study used longitudinal repeated measures data from the MACS and included 1940 recent participants available in the last 12 waves of data collection (1019 HIV− men, 921 HIV+ men). MACS participants return every 6 months for detailed interviews, physical examinations, and collection of blood for laboratory testing and storage in a central repository. The interview includes questions about medical conditions, medical treatments, emotional distress, and various health-related behaviors including illicit drug use (defined below) and pain perceptions. Because of the voluminous quantity of measures in this study, pertinent variables from the 12 study visits (April 1, 2003-March 31, 2009) were combined by twos to yield 6 contiguous study visit means used in the current study. If systematic change over time was detected, we planned to use a latent growth modeling approach. However, because no significant change was detected by latent growth models over the time period among the variables selected for this study (see means reported in Table 1), we used the individual repeated measures as indicators of over-arching latent variables which we felt could better capture the underlying long-term relations among the constructs in the model [14].
Table 1.
Summary statistics and factor loadings of variables in Confirmatory Factor Analyses for HIV seropositive and HIVseronegative men.
| HIV+ N = 921 |
HIV− N = 1019 |
|||
|---|---|---|---|---|
| Variable | Mean / SD | FL* | Mean / SD | FL |
| CD4+ Count** | ||||
| Time 1 | 566.61/278.01 | .82 | 942.49/297.58 | .89 |
| Time 2 | 574.98/285.79 | .89 | 959.34/302.61 | .92 |
| Time 3 | 562.47/278.49 | .94 | 943.43/302.90 | .93 |
| Time 4 | 572.99/281.93 | .95 | 945.40/306.35 | .94 |
| Time 5 | 575.82/270.88 | .91 | 936.72/314.92 | .92 |
| Time 6 | 595.70/278.23 | .85 | 942.89/313.39 | .90 |
| Pain*** | ||||
| Time 1 | 1.90/0.96 | .76 | 1.78/0.81 | .79 |
| Time 2 | 1.95/0.99 | .79 | 1.78/0.81 | .81 |
| Time 3 | 1.91/0.98 | .73 | 1.81/0.91 | .77 |
| Time 4 | 1.93/1.04 | .77 | 1.83/0.92 | .76 |
| Time 5 | 1.92/1.00 | .77 | 1.82/0.93 | .77 |
| Time 6 | 1.95/1.01 | .69 | 1.82/0.87 | .73 |
| Marijuana Use*** | ||||
| Time 1 | 0.84/1.29 | .85 | 0.66/1.16 | .87 |
| Time 2 | 0.84/1.30 | .88 | 0.65/1.15 | .89 |
| Time 3 | 0.85/1.28 | .90 | 0.63/1.15 | .90 |
| Time 4 | 0.81/1.31 | .91 | 0.59/1.14 | .89 |
| Time 5 | 0.83/1.33 | .88 | 0.59/1.16 | .90 |
| Time 6 | 0.81/1.32 | .85 | 0.61/1.16 | .87 |
| Crack Cocaine*** | ||||
| Time 1 | 0.30/0.80 | .70 | 0.16/0.59 | .73 |
| Time 2 | 0.28/0.80 | .80 | 0.17/0.65 | .82 |
| Time 3 | 0.28/0.79 | .77 | 0.16/0.63 | .84 |
| Time 4 | 0.26/0.79 | .86 | 0.14/0.56 | .82 |
| Time 5 | 0.24/0.75 | .84 | 0.15/0.61 | .82 |
| Time 6 | 0.21/0.72 | .70 | 0. 14/0.59 | .75 |
| Other Cocaine*** | ||||
| Time 1 | 0.19/0.64 | .60 | 0.08/0.38 | .64 |
| Time 2 | 0.16/0.59 | .63 | 0.10/0.44 | .64 |
| Time 3 | 0.15/0.57 | .65 | 0.09/0.42 | .63 |
| Time 4 | 0.12/0.50 | .67 | 0.08/0.41 | .60 |
| Time 5 | 0.11/0.48 | .63 | 0.06/0.36 | .56 |
| Time 6 | 0.11/0.48 | .50 | 0.07/0.36 | .54 |
| Heroin | ||||
| Time 1 | 0.07/0.46 | .80 | 0.05/0.39 | .74 |
| Time 2 | 0.07/0.43 | .85 | 0.06/0.40 | .68 |
| Time 3 | 0.06/0.44 | .72 | 0.05/0.37 | .89 |
| Time 4 | 0.05/0.40 | .90 | 0.04/0.31 | .83 |
| Time 5 | 0.04/0.35 | .63 | 0.03/0.27 | .75 |
| Time 6 | 0.03/0.32 | .67 | 0.04/0.34 | .58 |
| Depressive Symptoms *** | ||||
| Time 1 | 11.85/10.91 | .82 | 9.87/9.90 | .84 |
| Time 2 | 11.17/10.36 | .86 | 9.35/9.69 | .88 |
| Time 3 | 10.72/10.12 | .89 | 9.11/9.60 | .87 |
| Time 4 | 10.97/9.64 | .87 | 9.88/10.05 | .88 |
| Time 5 | 10.73/10.01 | .85 | 9.16/9.53 | .88 |
| Time 6 | 10.99/9.72 | .82 | 9.49/9.35 | .82 |
| Inhaled Nitrites *** | ||||
| Time 1 | 0.62/1.06 | .80 | 0.48/0.97 | .84 |
| Time 2 | 0.62/1.06 | .87 | 0.49/0.98 | .88 |
| Time 3 | 0.62/1.07 | .87 | 0.49/0.98 | .91 |
| Time 4 | 0.60/1.07 | .88 | 0.49/1.02 | .91 |
| Time 5 | 0.57/1.03 | .86 | 0.44/0.95 | .91 |
| Time 6 | 0.57/1.03 | .82 | 0.44/0.95 | .86 |
| Demographics | ||||
| Age (years)** | 43.9/8.6 | --- | 47.8/10.2 | --- |
| Black (%)*** | 31.3 | --- | 18.7 | --- |
| White (%)** | 53.8 | --- | 73.8 | --- |
| Education (1–7)** | 5.0/1.5 | --- | 5.5/1.4 | --- |
|
Hard Drug Use Second Order Factor*** |
||||
| Crack | --- | .86 | --- | .89 |
| Other Cocaine | --- | .73 | --- | .69 |
| Heroin | --- | .52 | --- | .44 |
= Factor loading,
= significantly higher for HIV- men,
= significantly higher for HIV+ men.
2.2 Measures
Single item demographic variables
Age, education and ethnicity were included as baseline demographic correlates and predictors. Following the recommendations of Edwards et al [20], we use the term “ethnicity” to describe groups of participants in this study. According to Edwards et al., ethnicity is based on distinguishing behaviors, culture, history, experience, ancestry, and beliefs. Consequently, ethnicity includes what is usually called “race”, as well as characteristics that are of social, psychological, cultural, and political nature. Age was reported in years (the average age for the whole sample was 45.9 years at the start of the first assessment point of this current study). Education was assessed categorically on a 1–7 scale: 1 = 8th grade or less, 2 = 9, 10, or 11th grade, 3 = 12th grade, 4 = At least one year college, but no degree; 5 = Four years of college/got degree, 6 = Some graduate work; 7 = Post-graduate degree. White or Black ethnicity were coded as separate yes/no variables (1 = yes; 0 = no); all other ethnic groups (Hispanics, Asians/Pacific Islanders, Native Americans) were used as the reference group. The sample was 64% White, 25% Black, and 11% Other.
CD4+ Cell Count
T-lymphocyte subset levels were quantified at each study visit using standardized flow cytometry [25,49].
Pain
The bodily pain scale of the Short-Form 36 (SF-36), a widely used and psychometrically verified instrument [27,54], assessed self-reported pain. A latent variable for Pain was indicated by the mean of responses to the 2 scale items: 1) “During the past four weeks, how much did pain interfere with your normal work (including work outside the house and housework)?” Responses ranged from “Not at all” = 1, to “extremely” = 5; 2) How much bodily pain have you had during the past four weeks? Responses ranged from “none” = 1 to “very severe” = 6. Thus, there were 6 repeated measures indicators for Pain (mean of the two items at assessment waves 1 and 2, up to assessment waves 11 and 12). Coefficient alpha for the 2 scales = .93, which indicated that combining the responses was acceptable.
Depressive Symptoms
Depressive symptoms were assessed with the Center for Epidemiological Studies Depression (CES-D) scale [45]. The 20-item self-report instrument is designed to measure depressive symptomatology in the general population. Each item measures the frequency of a symptom during the past week on a 4-point response scale from 0 to 3 ranging from “Rarely or none of the time (Less than 1 day)” to “Most of the time (5–7 days). Examples of CES-D items are “I felt depressed,” and “I felt fearful.” The scale was summed; higher scores indicate more depressive symptoms.
Illicit Drug Use
Drug use was considered illicit if the drugs appeared on the US Drug Enforcement Administration (DEA) list of controlled substances. In the current study, illicit drug use included cannabis (marijuana), heroin, cocaine, including its base form known in the US as crack cocaine, and inhaled nitrites (i.e., butyl or isopropyl nitrites inhaled for recreational purposes; also known as "poppers"). The following drugs are referred to using their vernacular form: marijuana, crack cocaine, heroin. The frequency of participants’ use of illicit drugs since their last study visit was assessed on a 0–4 scale as follows: 0 = None, 1 = Daily, 2 = Weekly, 3 = Monthly, 4 = Less often. Items were rescaled so that responses ranged from 0 = none to 4 = daily.
2.3 Statistical analysis
Confirmatory Factor Analysis
Latent variable structural equation modeling (SEM) was performed using the EQS SEM program [4]. The robust comparative fit index (RCFI), and Satorra-Bentler robust chi-square values (S-B χ2), were used as indicators of fit [4,29] due to the very high multivariate kurtosis in the data set (normalized estimate = 945.12). The RCFI compares the improvement of fit of a hypothesized model to a model of complete independence among the measured variables. The RCFI ranges between 0 and 1; values greater than or equal to .95 indicate a good fit [4,29]. As an additional indicator of fit, we also report the Root Mean Square Error of Approximation (RMSEA). The RMSEA is a measure of fit per degrees of freedom, controlling for sample size; values less than .06 indicate a relatively good fit between the hypothesized model and the observed data [29].
An initial confirmatory factor analysis (CFA) was performed for the entire sample and also separately for each group of men (HIV seropositive and HIV seronegative) with each hypothesized latent construct indicated by its measured indicators. All latent constructs, and the single-item variables were correlated with no imputation of causality or temporal ordering in the CFA. This analysis assessed the adequacy of the proposed factor structure and the relations among the latent and measured variables. This analysis was used in the next stage which compared the HIV-seropositive men and the HIV-seronegative men with multi-sample models. This analysis tested whether the entire sample related similarly to the items and could be used in a single path analysis or, alternatively, needed to be evaluated in two separate path analyses assessing the impact of reported pain on drug use and depressive symptoms. If relations were substantially different, then 2 path models would be warranted. To improve fit, we allowed a few correlated error residuals (auto-correlations only) between the same substances over time if they were suggested by the LaGrange Multiplier (LM) Test [12]. Because associations among the latent variables of Crack Cocaine Use, Other Cocaine Use, and Heroin Use were very high, a second-order factor was hypothesized to account for the substantial relationship among those items [4]. This alternative formulation was tested in the analysis and found to be superior to keeping the primary latent variables separate in terms of overall model fit. This second-order factor is labeled using the vernacular term, “Hard Drug Use.” Thus, Crack Cocaine Use, Other Cocaine Use, and Heroin Use are referred to collectively as Hard Drug Use, as these drugs are listed as Schedule I and II controlled substances in the US by the DEA indicating high abuse potential. In the analyses, Crack Cocaine Use, Other Cocaine Use, and Heroin Use constitute first order latent variable indicators of the second order factor of Hard Drug Use. Marijuana and Inhaled Nitrites Use were kept as separate latent variables in the analyses.
Multisample models
Covariance structure analysis has become the method of choice for assessing the comparability of measures in different groups through the testing of measurement invariance with varying and successive degrees of stringency across groups [53,62]. Using this methodology, one can specify an a priori factor model in two groups and test it for various degrees of invariance using structural modeling. Furthermore, structured means models can be used to assess the equivalency of the latent means of the factors across the groups [4]. Establishing factorial invariance does not necessarily mean that different groups will report the same mean scores on a particular measurement instrument. Even if factor structures are similar across different groups, one group may report significantly higher means than the other group [26,53]. For instance, we would expect CD4+ counts to be substantially higher among the men who are HIV seronegative.
We contrasted the two groups on their factor structures, covariances among the constructs, and also on their latent means. We began by specifying an initial baseline model with no constraints that was used for comparison purposes. We then tested a model in which their factor structures were constrained to equality. Subsequently, we constrained the covariances among the latent variables to equality and tested whether they were significantly different in the two groups. These constrained covariances (correlations) would test whether there are differential associations between reported pain and use of various illicit drugs among the HIV seropositive and HIV seronegative men. In addition, following the test that constrained the measurement model (factor structure) to equality, we assessed differences in latent construct means. The tenability of the successively more stringent set of constraints was assessed with the goodness-of-fit indexes described above, χ2 difference tests, and results from the LM test [12], which in this context identifies constraints that are untenable.
Path model
A predictive path model was tested for the entire sample and for each group separately in which baseline characteristics of age, ethnicity, education, and CD4+ cell counts across the 6 assessment periods predicted Pain which in turn would be associated with Depressive Symptoms, and use of Marijuana, Hard Drugs, and Inhaled Nitrites. This initial model was testing full mediation by the Pain latent variable to assess the amount of variance Pain alone could account for in the outcome variables. Because other variables in the model were expected to impact the outcomes as well (e.g., age, Black ethnicity, etc.), supplementary relationships between the baseline characteristics and the outcome variables were added based on suggestions from the LM test once the contribution of Pain was assessed. Non-significant paths were dropped until only significant paths remained. In the combined sample, HIV status was added as a predictor of CD4+ cell counts and a correlate of the other predictors.
3. Results
3.1 Confirmatory factor analyses
All measured variables loaded significantly (p < 0.001) on their hypothesized latent factors. Fit of the model for the total sample was very good: S-B χ2 (1266, N = 1940) = 2439.24, RCFI = 0.96, RMSEA = 0.024 (90% confidence interval = .023–.026). Fit indexes were good for each separate group as well: HIV+ men: S-B χ2 (1221, N = 921) = 1854.09, RCFI = 0.95, RMSEA = 0.026 (90% confidence interval = .023–.028). HIV- men: S-B χ2 (1221, N = 1019) = 1599.61, RCFI = 0.97, RMSEA = 0.019 (90% confidence interval = .017–.022). Degrees of freedom are higher for the total sample because an additional variable representing HIV+ or negative status was included in that analysis. Table 1 reports summary statistics for the measured variables and factor loadings of the latent variables for each group.
Table 2 reports correlations among all variables in the model stratified by HIV status. Of note, the main variable of interest, Pain, was highly associated with more Depressive Symptoms, and more modestly but significantly with Hard Drug Use in both samples. Pain was significantly associated with Marijuana Use among the HIV seropositive men (.11, p ≤ .01) but not among the HIV seronegative men. Pain was unrelated to CD4+ cell count among HIV seronegative men (.00) but a lower CD4+ cell count was significantly associated with more pain among HIV seropositive men (−.12, p ≤ .001). More pain was reported among older HIV seronegative men and among Black HIV- men. Less education was associated with more pain in both samples (−.09 for negatives, −.14 for positives). In addition, being Black was associated with more Hard Drug Use; being White was associated with more Inhaled Nitrites Use, being Black with less Inhaled Nitrites Use.
Table 2.
Correlations among model variables. Correlations for HIV+ men below diagonal, HIV− men above diagonal.
| Variables | Age | Black | White | Education | CD4+ count |
Pain | Marijuana | Hard Drug Use |
Depressive Symptoms |
Inhaled Nitrites |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | — | −.24*** | .33*** | .19*** | −.02 | .11** | −.19*** | −.21*** | −.18*** | .03 |
| 2. Black | −.15*** | — | −.81*** | −.31*** | .06 | .12** | .10** | .50*** | .21*** | −.15*** |
| 3. White | .40*** | −.73*** | — | .34*** | .03 | −.07 | −.08* | −.45*** | −.20*** | .16*** |
| 4. Education | .24*** | −.23*** | .31*** | — | −.06 | −.09* | −.14*** | −.38*** | −.22*** | .15*** |
| 5. CD4+ count | .04 | −.07* | .09* | .09* | — | .00 | .11** | .06 | −.01 | .06 |
| 6. Pain | .01 | .04 | −.02 | −.14*** | −.12*** | — | .04 | .18** | .47*** | −.06 |
| 7. Marijuana | −.10** | .04 | −.01 | −.08* | −.02 | .11** | — | .29*** | .09* | .17*** |
| 8. Hard Drug Use |
−.12*** | .39*** | −.30*** | −.27*** | −.12** | .08* | .13*** | — | .29*** | −.07* |
| 9. Depressive Symptoms |
−.24*** | .09* | −.18*** | −.16*** | −.14*** | .53*** | .07 | .21*** | — | −.01 |
| 10. Inhaled Nitrites |
.10** | −.25*** | .32*** | .14*** | .08* | −.06 | .21*** | −.07 | −.05 | — |
p ≤ .05;
p ≤ .01;
p ≤ .001. Bold-face italicized type indicates a significant difference, p = ≤ .01
3.2 Multiple group analyses
Constrained factor structure
The unconstrained multisample model served as the baseline (S-B χ2 (2442) = 3422.81, RCFI = 0.96). When the factor structures were constrained to equality, there was no significant decrement in fit in terms of the chi-square difference between the two models (adjusted χ2-square difference (42 df) = 31.15). Thus, we were able to assume equal factor structures for the two groups and to proceed to the next level of stringency by constraining the covariances (correlations) between the constructs to equality.
Constrained covariances
The more constrained model in which the covariances among the latent variables and also with the single item variables were constrained to equality across the groups also did not have a significant increase in its chi-square value (adjusted χ2-difference (87 df) = 81.70). However, some individual covariances differed significantly in the two groups (p ≤ .01). Because we were interested in substantive differences between the groups even if the overall difference between the two groups was non-significant, we report the significant differences in Table 2 (see bold-face, italicized correlations). Most significant differences were associations with sociodemographics rather than differences among the latent variables. Depressive Symptoms had a lower association with Black ethnicity among HIV seropositive men than among HIV seronegative men (.09 vs. .21); being Black was less associated with Hard Drug Use among HIV seropositive men (.39 vs. .50) although overall, Black ethnicity was substantially associated with more Hard Drug Use in both groups than in Whites. White HIV seropositive men had higher associations with Inhaled Nitrites Use than White HIV seronegative men (.32 vs. .16). Hard Drug Use was less negatively associated with age among HIV seropositive men than among HIV seronegative men (−.12 vs. −.21). White HIV seronegative men reported less use of Hard Drugs than White HIV seropositive men (−.45 vs. −.30). There were some significant age differences in the racial distributions of the 2 samples as well. Of most substantive interest, there were no significant group differences concerning the associations with pain, although as reported below and in Table 1, HIV seropositive men reported more pain than HIV seronegative men.
Latent means comparisons
Table 1 reports the results of the latent means comparisons. There were significant differences between the latent means of the two groups in almost all of the variables. Not surprisingly, the HIV seronegative men had significantly higher CD4+ cell count. The HIV seropositive men reported more pain, more marijuana use, more crack cocaine use, more other cocaine use, more depressive symptoms, more inhaled nitrites use, and, overall, more hard drug use. There were no differences in heroin use, which was infrequently reported in both groups. Single-item sociodemographics were also contrasted in this analysis. The HIV seropositive men were proportionally more likely to be Black, less educated, and younger.
3.3 Path model
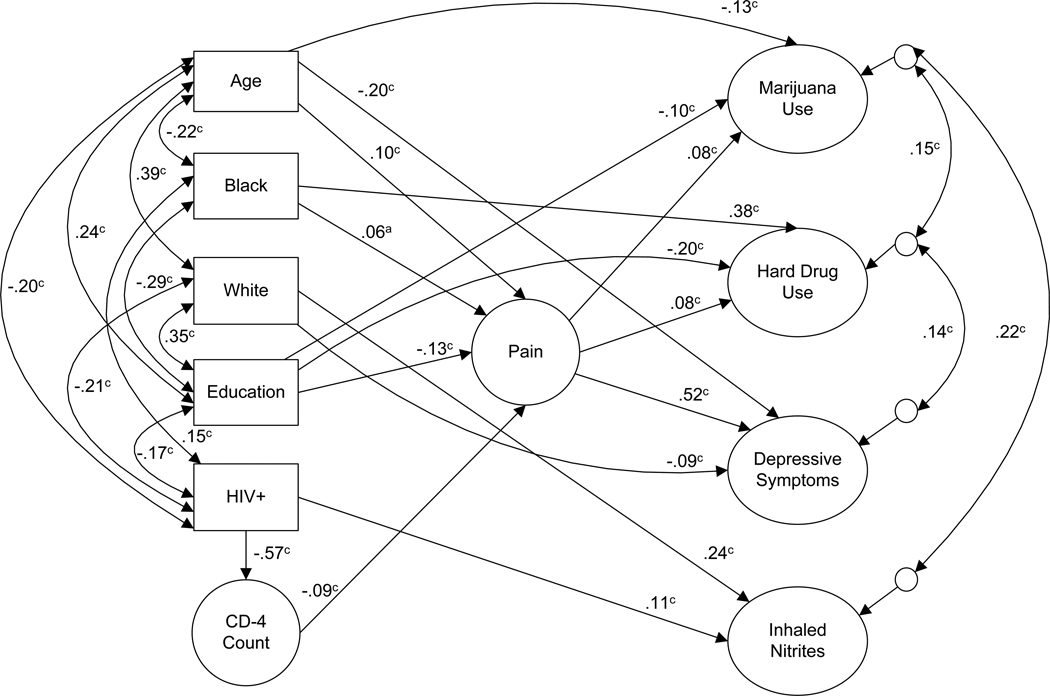
The separate group path models were highly similar. We thus for simplicity and ease of reporting present one path model that combined both groups and also includes their HIV status as a further predictor and correlate. The fully mediated initial path model that only had Pain as a predictor of the outcomes had an acceptable fit: S-B χ2 (1290, N = 1940) = 2527.57, RCFI = .96, RMSEA = 0.025 (90% confidence interval = .023–.026). Although Pain was a significant predictor of the outcomes, it explained only 1% of the variance in Marijuana Use, 2% of Hard Drug Use, and less than 1% of Inhaled Nitrites Use. It explained 27% of the variance in Depressive Symptoms. After model modification in which non-significant paths and covariances were dropped and other significant paths were added based on suggestions from the LM test (i.e., paths from age, Black ethnicity, education, and CD+4 cell count to the outcomes), the fit indexes improved: S-B χ2 (1288, N = 1940) = 2290.08, RCFI = 0.96, RMSEA = 0.022 (90% confidence interval = .021–.024). Figure 2 depicts the results of the path analysis with only significant paths included. This model explained 4% of the variance in Marijuana Use, 33% of the variance in Depressive Symptoms, 24% of the variance in Hard Drug Use, and 6% of the variance in Inhaled Nitrites Use; HIV seropositivity predicted a lower CD4+ count and a higher CD4+ cell count predicted less Pain. Being older, Black and less educated predicted more Pain. Pain was associated with more Marijuana Use, more Hard Drug Use, and considerably more Depressive Symptoms. In this model, in addition to its substantial associations with the other baseline characteristics (see Figure 2), being HIV seropositive predicted more use of Inhaled Nitrites. Black ethnicity predicted more Hard Drug Use; White ethnicity predicted more use of Inhaled Nitrites and fewer Depressive Symptoms. Age predicted fewer Depressive Symptoms. Less education was associated with more Marijuana and Hard Drug Use.
Figure 2.
Path model for MACS participants (N = 1940). Latent constructs are in circles, single items are in rectangles; 1-headed arrows depict standardized regression paths, 2-headed arrows represent correlations (standardized covariances) and correlations among residuals of dependent
There were several significant indirect effects including a significant effect of HIV seropositivity on Pain, mediated through CD4+ cell count (p ≤.001). In addition, a lower CD4+ cell count had a significant effect on more Marijuana and Hard Drug Use (p ≤.05), and greater Depressive Symptoms (p ≤.001) mediated through Pain. Positive HIV status had significant indirect effects on Marijuana Use and Hard Drug Use (p ≤.05), and on Depression (p ≤.001) mediated through its effect on a low CD4+ cell count which in turn predicted more Pain.
4. Discussion
Consistent with the hypothesized conceptual model, our results demonstrated that in a large sample of HIV+ and HIV- gay/bisexual men enrolled in the MACS, pain served as a mediator between key clinical/sociodemographic characteristics and both depressive symptoms and illicit drug use (see Figure 2). Overall, having a lower CD4+ cell count, being older, being Black, and having less education predicted more pain which in turn was associated with more marijuana use, more hard drug use (i.e., use of crack cocaine, other cocaine and heroin), and more depressive symptoms. The final model predicted 33% of the variance in depressive symptoms and 34% of the variance in drug use (combined across all drug categories). Being HIV seropositive predicted a lower CD4+ cell count and more use of inhaled nitrites. The predictive path models we tested indicated that the pattern of associations among the study variables were similar across HIV status. Our latent means comparisons indicated however, that relative to HIV seronegative men, HIV seropositive men reported more pain and more depressive symptoms, as well as more use of a variety of drugs (i.e., marijuana, crack cocaine use, cocaine use, inhaled nitrites) (see Table 1). Our results derive from longitudinal data from four study sites representing major population centers in the US that comprise the MACS. A further strength of this analysis is our use of repeated measures data extended over 6 years which gives us additional confidence in the robustness of our results.
Our findings are consistent with prior work in a US national sample of HIV seropositive individuals which indicated that use of a broad range of illicit drugs (not just IDU) predicted worse illness burden (i.e., lower CD4+ cell count and presence of wasting syndrome) which in turn predicted more pain. This earlier study also found that lower SES and older age predicted more pain, which is consistent with the present results. Our findings are also in line with Miaskowski et al who recently reported that in a community sample of indigent persons living with HIV, both lower education and depression were associated with more severe pain [41]. Additionally, the current findings support previous work in HIV seropositive samples showing strong relationships between pain and depressive symptoms [22,32,33,57] as well as between pain and illicit drug use [15,23,37,60,58].
However, some prior studies did not find associations between pain and IDU [8,9]. Miaskowski et al found no relationship between pain severity and use of a range of illicit drugs; they posited that high overall rates of substance use in their sample of indigent persons made it difficult to detect significant differences [41]. In the present analyses, the amount of variance in drug use explained by pain alone (i.e., without the clinical/sociodemographic variables) was relatively small. One possible reason is that we did not include use of opioid or other prescription drugs as the MACS did not assess the use of these substances during the study visits we analyzed. Earlier, we found strong links between pain and aberrant use of prescription analgesics in a different sample of HIV-infected persons particularly among individuals with a history of problematic illicit drug [58]. Given the recent and growing problem of prescription drug misuse, future work may test whether the present model also applies to use of such medications. It should also be noted that in the current analyses, pain was not associated with use of inhaled nitrites. It may be that use of this recreational drug is more prevalent among higher functioning persons who are not experiencing significant pain.
There are several possible pathways by which pain may increase the risk of depressive symptoms and drug use (see [3] for additional discussion of pain and depression comorbidity). Pain and pain-related disability may lead to disruptions in physical, social, and occupational activities which may in turn lead to social isolation, loss of independence and a subsequent increase in depressive symptoms. Such considerations may be especially relevant for gay/bisexual men, who along with lesbian women are at higher risk of mood and anxiety disorders compared to the general population [6]. Gay men also report greater illicit drug use compared to heterosexual men in the US [13,30], and persistent pain may lead to the use of illicit drugs for pain relief among these men. One study found that nearly one-third of HIV-infected persons reported using marijuana to manage pain and other symptoms [61]. Another investigation found that among HIV-infected patients with peripheral neuropathy, 15% reported using marijuana and 7% reported using street drugs to manage their symptoms [42].
Sociodemographics played a large role in the associations with HIV status as well as the dependent variables (i.e., depressive symptoms and drug use). Many of the strong and substantial mean differences between HIV-infected and HIV-uninfected participants were apparently due more to the sociodemographic correlates of HIV status rather than to HIV status itself. In the current analyses, participants who were HIV seropositive reported more pain than the HIV seronegative men but pain was not significantly and independently predicted by being HIV seropositive once demographic correlates and the association with CD4+ cell count were included. The meaning of being a HIV seropositive participant in the MACS in large part reflects the demographic differences between the two groups – i.e., compared to the HIV seronegative participants, the HIV seropositive participants were more likely to be Black, more poorly educated, and younger. These socioeconomic disparities have been shown to have severe implications for decreased survival and quality of life among HIV-infected individuals [5,39].
Our finding that Blacks reported more pain compared to Whites is consistent with earlier research in an HIV seropositive sample [8], as well as data in the general population indicating more severe pain among ethnic minorities vs. Whites [40,44,46]. However, some investigations in HIV-infected samples have revealed that Blacks reported less pain than Whites [2,16]; others have found no ethnic differences in pain [48,55; 41]. Differences in pain assessment, sample characteristics, and/or stage of HIV disease may account for the divergent results. Our finding that lower education was linked with more pain is consistent with previous work in a US national sample of HIV seropositive people [16,58], as well as research in the general population [24,43,44,55], although an investigation of HIV–infected individuals with comorbid psychological and substance use disorders revealed no differences in pain based on education [55]. The latter finding may be due to restricted range of educational levels in this selected subgroup.
Several limitations to our findings should be mentioned. Because the MACS is comprised of men, we could not test for sex differences. Although we previously found in a US national sample of HIV seroprevalent individuals that after controlling for age and SES, women reported more pain than men regardless of ethnicity, mode of HIV transmission or prior drug use history [56], others have found no sex differences in pain in HIV [2,11,15,16,34,35,55,48]. Differences in sociodemographic characteristics may be partially responsible for sex differences in pain, particularly among ethnic minority women in the US where the seroprevalence is highest [59], and most existing research among HIV seroprevalent samples has not taken this consideration into account. Additionally, the MACS assessment does not differentiate between recreational vs. medically prescribed marijuana use. Other associations in the data could have been tested including path models that used a different directionality. For example, an alternative model might have tested whether depression mediated the association between pain and illicit drug use. However, our interest centered on implications of the mediating effect of pain on substance use and depressive symptoms in these high-risk samples.
The findings underscore the need for pain assessment and treatment aimed at older, less educated, Black gay/bisexual men as such individuals may be more vulnerable to pain. Among HIV-infected men such efforts should target those with more advanced HIV disease. Research in HIV-uninfected populations has found substantial disparities in the quality of pain management for ethnic minorities compared to Whites [1], and less utilization of care for pain among persons of low SES [40]. Early research indicated substantial undertreatment of pain among HIV-infected persons, particularly the less educated and IV-drug users [9,10], but there is a dearth of recent research in this area. The current findings point to the continued need to assess and adequately treat pain in gay/bisexual men with, or at risk for HIV, as doing so may reduce the likelihood of drug use and depressive symptoms in these vulnerable populations.
Acknowledgments
Support for this research was provided by DA026093 awarded to the first author and by DA01070-37 awarded to the second author by the National Institute on Drug Abuse. The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Michael Plankey (Co-Principal Investigator), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Steven M. Wolinsky (Principal Investigator), Sheila Badri, Craig Conover, Maurice O'Gorman, David Ostrow, Frank Palella, Ann Ragin. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Otoniel Martínez-Maza (Co-Principal Investigator), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, John Fahey, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Barbara R. Visscher, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence A. Kingsley (Co-Principal Investigator), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall. . Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Muoz (Co-Principal Investigator), Alison, Abraham, Keri Althoff, Christopher Cox, Gypsyanber D’Souza, Stephen J. Gange, Elizabeth Golub, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041. Website located at http://www.statepi.jhsph.edu/macs/macs.html. The authors would like to thank Lauren Savaglio and Sameer Arora for their technical assistance in preparation of this manuscript. The authors also gratefully acknowledge the statistical assistance of Siavash Jalal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest in this article.
Pain partially mediated the association between sociodemographic/clinical characteristics and illicit drug use/depressive symptoms in a longitudinal cohort of HIV seropositive and HIV seronegative men.
Reference List
- 1.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10:1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Aouizerat BE, Miaskowski CA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Lee KA. Risk factors and symptoms associated with pain in HIV-infected adults. J Assoc Nurses AIDS Care. 2010;21:125–133. doi: 10.1016/j.jana.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 4.Bentler PM. EQS 6 Structural Equations Program Manual. Encino, CA: Multivariate Software, Inc; 2006. [Google Scholar]
- 5.Bing EG, Hays RD, Jacobson LP, Chen B, Gange SJ, Kass NE, Chmiel JS, Zucconi SL. Health-related quality of life among people with HIV disease: results from the Multicenter AIDS Cohort Study. Qual Life Res. 2000;9:55–63. doi: 10.1023/a:1008919227665. [DOI] [PubMed] [Google Scholar]
- 6.Bostwick WB, Boyd CJ, Hughes TL, McCabe SE. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health. 2010;100:468–475. doi: 10.2105/AJPH.2008.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitbart W. Pain management and psychosocial issues in HIV and AIDS. Am J Hosp Palliat Care. 1996;13:20–29. doi: 10.1177/104990919601300108. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart W, McDonald MV, Rosenfeld B, Passik SD, Hewitt D, Thaler H, Portenoy RK. Pain in ambulatory AIDS patients. I: pain characteristics and medical correlates. Pain. 1996;68:315–321. doi: 10.1016/s0304-3959(96)03215-0. [DOI] [PubMed] [Google Scholar]
- 9.Breitbart W, Rosenfeld B, Passik S, Kaim M, Funesti-Esch J, Stein K. A comparison of pain report and adequacy of analgesic therapy in ambulatory AIDS patients with and without a history of substance abuse. Pain. 1997;72:235–243. doi: 10.1016/s0304-3959(97)00039-0. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart W, Rosenfeld BD, Passik SD, McDonald MV, Thaler H, Portenoy RK. The undertreatment of pain in ambulatory AIDS patients. Pain. 1996;65:243–249. doi: 10.1016/0304-3959(95)00217-0. [DOI] [PubMed] [Google Scholar]
- 11.Cervia LD, McGowan JP, Weseley AJ. Clinical and demographic variables related to pain in HIV-infected individuals treated with effective, combination antiretroviral therapy (cART) Pain Med. 2010;11:498–503. doi: 10.1111/j.1526-4637.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 12.Chou C, Bentler P. Model modification in covariance structure modeling: A comparison among likelihood ratio, Lagrange multiplier, and Wald tests. Multivar Behav Res. 1990;25:115–136. doi: 10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 13.Cochran SD, Ackerman D, Mays VM, Ross MW. Prevalence of non-medical drug use and dependence among homosexually active men and women in the US population. Addiction. 2004;99:989–998. doi: 10.1111/j.1360-0443.2004.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran PJ, Bollen KA. The best of both worlds: combining autoregressive and latent curve models. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, D.C: American Psychological Assocation; 2001. pp. 107–135. [Google Scholar]
- 15.Del Borgo C, Izzi I, Chiarotti F, Del Forno A, Moscati AM, Cornacchione E, Fantoni M. Multidimensional aspects of pain in HIV-infected individuals. AIDS Patient Care STDS. 2001;15:95–102. doi: 10.1089/108729101300003690. [DOI] [PubMed] [Google Scholar]
- 16.Dobalian A, Tsao JCI, Duncan RP. Pain and the use of outpatient services among persons with HIV: results from a nationally representative survey. Med Care. 2004;42:129–138. doi: 10.1097/01.mlr.0000108744.45327.d4. [DOI] [PubMed] [Google Scholar]
- 17.Douaihy AB, Stowell KR, Kohnen S, Stoklosa JB, Breitbart WS. Psychiatric aspects of comorbid HIV/AIDS and pain, Part 1. AIDS Read. 2007;17:310–314. [PubMed] [Google Scholar]
- 18.Douaihy AB, Stowell KR, Kohnen S, Stoklosa JB, Breitbart WS. Psychiatric aspects of comorbid HIV/AIDS and pain, Part 2. AIDS Read. 2007;17:350–352. 357–361. [PubMed] [Google Scholar]
- 19.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 20.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 21.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Evans S, Ferrando S, Sewell M, Goggin K, Fishman B, Rabkin J. Pain and depression in HIV illness. Psychosomatics. 1998;39:528–535. doi: 10.1016/S0033-3182(98)71285-X. [DOI] [PubMed] [Google Scholar]
- 23.Fantoni M, Ricci F, Del Borgo C, Izzi I, Damiano F, Moscati AM, Marasca G, Bevilacqua N, Del Forno A. Multicentre study on the prevalence of symptoms and symptomatic treatment in HIV infection. J Pallitat Care. 1997;13:9–13. [PubMed] [Google Scholar]
- 24.Fuentes M, Hart-Johnson T, Green CR. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. J Natl Med Assoc. 2007;99:1160–1169. [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgi JV, Cheng HL, Margolick JB, Bauer KD, Ferbas J, Waxdal M, Schmid I, Hultin LE, Jackson AL, Park L, Taylor JMG. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 26.Handelsman L, Stein JA, Grella CE. Contrasting predictors of readiness for substance abuse treatment in adults and adolescents: a latent variable analysis of DATOS and DATOS-A participants. Drug Alcohol Depend. 2005;80:63–81. doi: 10.1016/j.drugalcdep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 28.Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70:117–123. doi: 10.1016/s0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- 29.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 30.Hughes TL, Michele E. Substance use and abuse in lesbian, gay, bisexual and transgender populations. The Journal of Primary Prevention. 2002;22:263–268. [Google Scholar]
- 31.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 32.Kowal J, Overduin LY, Balfour L, Tasca GA, Corace K, Cameron DW. The role of psychological and behavioral variables in quality of life and the experience of bodily pain among persons living with HIV. J Pain Symptom Manage. 2008;36:247–258. doi: 10.1016/j.jpainsymman.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Lagana L, Chen XH, Koopman C, Classen C, Kimerling R, Spiegel D. Depressive symptomatology in relation to emotional control and chronic pain in persons who are HIV positive. Rehabilitation Psychology. 2002;47:402–414. [Google Scholar]
- 34.Lebovits AH, Smith G, Maignan M, Lefkowitz M. Pain in hospitalized patients with AIDS: analgesic and psychotropic medications. Clin J Pain. 1994;10:156–161. doi: 10.1097/00002508-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the South. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York: Erlbaum; 2008. [Google Scholar]
- 37.Martin C, Pehrsson P, Osterberg A, Sonnerborg A, Hansson P. Pain in ambulatory HIV-infected patients with and without intravenous drug use. Eur J Pain. 1999;3:157–164. doi: 10.1053/eujp.1999.0111. [DOI] [PubMed] [Google Scholar]
- 38.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:2233–2238. [PubMed] [Google Scholar]
- 39.McFarland W, Chen S, Hsu L, Schwarcz S, Katz M. Low socioeconomic status is associated with a higher rate of death in the era of highly active antiretroviral therapy, San Francisco. J Acquir Immune Defic Syndr. 2003;33:96–103. doi: 10.1097/00126334-200305010-00014. [DOI] [PubMed] [Google Scholar]
- 40.Meghani SH, Cho E. Self-reported pain and utilization of pain treatment between minorities and nonminorities in the United States. Public Health Nurs. 2009;26:307–316. doi: 10.1111/j.1525-1446.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 41.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. doi: 10.1016/j.jpain.2011.04.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholas PK, Kemppainen JK, Canaval GE, Corless IB, Sefcik EF, Nokes KM, Bain CA, Kirksey KM, Eller LS, Dole PJ, Hamilton MJ, Coleman CL, Holzemer WL, Reynolds NR, Portillo CJ, Bunch EH, Wantland DJ, Voss J, Phillips R, Tsai YF, Mendez MR, Lindgren TG, Davis SM, Gallagher DM. Symptom management and self-care for peripheral neuropathy in HIV/AIDS. AIDS Care. 2007;19:179–189. doi: 10.1080/09540120600971083. [DOI] [PubMed] [Google Scholar]
- 43.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136:235–238. doi: 10.1016/j.pain.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 46.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: non-Hispanic whites, non-Hispanic blacks, and Hispanics. J Pain. 2007;8:75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenfeld B, Breitbart W, McDonald MV, Passik SD, Thaler H, Portenoy RK. Pain in ambulatory AIDS patients. II: impact of pain on psychological functioning and quality of life. Pain. 1996;68:323–328. doi: 10.1016/s0304-3959(96)03220-4. [DOI] [PubMed] [Google Scholar]
- 48.Rotheram-Borus MJ. Variations in perceived pain associated with emotional distress and social identity in AIDS. AIDS Patient Care STDS. 2000;14:659–665. doi: 10.1089/10872910050206586. [DOI] [PubMed] [Google Scholar]
- 49.Schenker EL, Hultin LE, Bauer KD, Ferbas J, Margolick JB, Giorgi JV. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14:307–317. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 50.Schofferman J, Brody R. Pain in far advanced AIDS. In: Chapman R, Foley K, editors. Advances in Pain Research and Therapy. Vol. 16. New York: Raven Press; 1990. pp. 379–386. [Google Scholar]
- 51.Singer EJ, Zorilla C, Fahy-Chandon B, Chi S, Syndulko K, Tourtellotte WW. Painful symptoms reported by ambulatory HIV-infected men in a longitudinal study. Pain. 1993;54:15–19. doi: 10.1016/0304-3959(93)90094-6. [DOI] [PubMed] [Google Scholar]
- 52.Smith MY, Egert J, Winkel G, Jacobson J. The impact of PTSD on pain experience in persons with HIV/AIDS. Pain. 2002;98:9–17. doi: 10.1016/s0304-3959(01)00431-6. [DOI] [PubMed] [Google Scholar]
- 53.Stein JA, Lee JW, Jones PS. Assessing cross-cultural differences through the use of multiple group invariance analysis. J Pers Assess. 2006;87:249–258. doi: 10.1207/s15327752jpa8703_05. [DOI] [PubMed] [Google Scholar]
- 54.Stewart AL, Ware JE. Measuring functioning and well-being: the medical outcomes study approach. Durham: Duke University Press; 1992. [Google Scholar]
- 55.Tsao JC, Soto T. Pain in persons living with HIV and comorbid psychologic and substance use disorders. Clin J Pain. 2009;25:307–312. doi: 10.1097/AJP.0b013e31819294b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsao JC, Stein JA, Dobalian A. Sex differences in pain and misuse of prescription analgesics among persons with HIV. Pain Med. 2010;11:815–824. doi: 10.1111/j.1526-4637.2010.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao JCI, Dobalian A, Naliboff BD. Panic disorder and pain in a national sample of persons living with HIV. Pain. 2004;109:172–180. doi: 10.1016/j.pain.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Tsao JCI, Dobalian A, Stein JA. Illness burden mediates the relationship between pain and illicit drug use in persons living with HIV. Pain. 2005;119:124–132. doi: 10.1016/j.pain.2005.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Centers for Disease Control and Prevention. [accessed July 11, 2011];HIV in the United States. http://www.cdc.gov/hiv/resources/factsheets/us.htm.
- 60.Vogl D, Rosenfeld B, Breitbart W, Thaler H, Passik S, McDonald M, Portenoy RK. Symptom prevalence, characteristics, and distress in AIDS outpatients. J Pain Symptom Manage. 1999;18:253–262. doi: 10.1016/s0885-3924(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 61.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Yin P, Fan X. Assessing the factor structure invariance of self-concept measurement across ethnic and gender groups: findings from a national sample. Educ Psychol Meas. 2003;63:296–318. [Google Scholar]