Abstract
Thickened oil-in-water emulsions are useful model foods in rat studies due to their high acceptance and similarity to foods consumed by humans. Previous work from this laboratory used oil-in-water emulsions thickened with a biopolymer blend containing starch. Intake and effects of baclofen, a GABA-B agonist that decreases fat intake and drug self-administration, were reported, but the contribution of starch was not assessed. In the present study, intake and effects of baclofen were assessed in rats using emulsions prepared with two fat types (32% vegetable shortening, 32% corn oil) and thickened with three biopolymer blends. One biopolymer blend contained starch and the other two did not. Daily 1-h intake of the vegetable shortening emulsion containing starch was significantly greater than the other emulsions. When starch was added to the emulsions originally containing no starch, intake significantly increased. Baclofen generally reduced intake of all emulsions regardless of starch content and stimulated intake of chow. However, effects were more often significant for vegetable shortening emulsions. This report: 1) demonstrates that products used to prepare thickened oil-in-water emulsions have significant effects on rat ingestive behavior, and 2) confirms the ability of baclofen to reduce consumption of fatty foods, while simultaneously stimulating intake of chow.
Keywords: fat emulsions, baclofen, GABA-B receptors, rats, food intake, ingestive behavior
INTRODUCTION
In humans, most fats generally are not consumed in pure form. Rather, fats are usually consumed as components of complex foodstuffs in the form of oil-in-water emulsions that have been thickened with biopolymer blends. For example, gravy is an emulsion thickened with starch polymers while low-fat salad dressing typically uses a mixture of modified starch and xanthan gum. Like humans, rats avidly consume fatty foods. Others have reported that rats prefer vegetable shortening (solid) over corn oil (liquid) when provided in pure form, but show no preference when provided as oil-in-water emulsions thickened with gelatin (Lucas et al., 1987). In work from our group, vegetable shortening emulsions thickened with a biopolymer blend containing starch were also highly acceptable to rats (Rao et al., 2008). However, the contribution of the starch to the acceptability of the emulsions was not assessed in that report.
Starch intake in rats is complex and varies with concentration and the context in which it is presented. For example, rats are able to detect very low concentrations and various forms of starch (Ramirez, 1991a; Ramirez, 1991b; Sclafani, 1987), prefer some cooked starches over raw starch (Ramirez, 1992), prefer either starch, sucrose, or glucose depending upon the concentrations of each (Ramirez, 1993), and learn to avoid some starches while accepting others (Ramirez, 1991a). Therefore, the focus of this report is the possible contribution of starch to our previous findings. In addition to the solid fat emulsion study mentioned above (Rao et al., 2008), we also have assessed intake of fat/sucrose mixtures made with commercially available powdered sugar containing corn starch (Wong et al., 2009). Thus, the fat emulsion study and the fat/sucrose mixture study had in common the presence of low levels of starch as a component of the fatty optional food. Intakes were higher in both studies than has been reported for pure shortening. This was particularly dramatic in the Rao et al. (2008) fat emulsion study in which 1-h intake of the 18% emulsion approached ~10 grams on average, an amount approximating the rat stomach capacity (Bull and Pitts, 1971). It is possible that the presence of starch contributed to the high intakes in the Wong et al. (2009), and Rao et al. (2008) studies.
The effects of baclofen on emulsion intake were also assessed in the present study. Baclofen is a GABA-B agonist that has been shown to reduce drug self-administration (e.g., Brebner et al., 2000) and solid vegetable shortening (fat) intake (Buda-Levin et al., 2005; Corwin & Wojnicki, 2009). In addition, baclofen has shown promise clinically in the treatment of binge eating (Broft et al., 2007; Corwin et al., 2010). In contrast to its effects on fat intake, baclofen stimulates consumption of foods containing complex carbohydrates (e.g. Ebenezer, 1995; Ebenezer & Pringle, 1992). In the Wong et al. (2009) and Rao et al. (2008) studies described above, differential effects of baclofen were reported with the potency of baclofen being somewhat lower than had previously been reported for 100% vegetable shortening. It is possible that the presence of starch contributed to the reduced potency of baclofen in these studies. Furthermore, the effects of baclofen on consumption of different types of fats have never been directly compared.
The present study, therefore, was designed to assess the contribution of starch to the consumption of thichened fat emulsions, and to additionally assess the ability of baclofen to reduce the intake of emusified fat made with and without starch. Emulsions made with two different types of fat (vegetable shortening and corn oil) and three different biopolymer thickening agents were used in the present study. One of these biopolymer thickeners contained starch, and the other two did not.
METHODS
Animals
Sixty male Sprague Dawley rats (Harlan, Indianapolis, IN), 60 days of age and weighing 290–323 g (310 ± 1.0 g) at the start of the study were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled environment placed on a 12:12 light:dark cycle. All rats were maintained on a nutritionally complete commercial laboratory rodent chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond IN; percent of energy as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g), with chow and tap water available ad libitum throughout all parts of the study. The Pennsylvania State University Institutional Animal Care and Use Committee approved all procedures.
After five days of adaptation to the vivarium, chow intake was measured for three consecutive days and body weights were determined on the fourth after which overnight access to Crisco (Crisco™, J.M. Smucker Co., Orrville, OH; 9.17 kcal/g) was provided.
Prior to the start of the experiment, six groups of 10 rats each were matched by body weight, average amount of chow consumed during three consecutive 24-h periods prior to the overnight access to Crisco, and amount of overnight Crisco consumed during the overnight access session [F(5,59)<1, NS for all three measures].
Emulsions
Six different emulsions were prepared: two different oil types × 3 different biopolymer blends used as thickening agents. Three of the six emulsions contained 32% w/v vegetable shortening (“V” groups; Crisco™, J.M. Smucker Co., Orrville, OH; 9.17 kcal/g), while the other three emulsions contained 32% w/v corn oil (“C” groups; Mazola™, ACH Food Companies, Memphis, TN). Three different biopolymer blends supplied by the same manufacturer (TIC Gums, Belcamp MD) were used to thicken the emulsions. TIC Pretested® Ticaloid® 103 S-Mayo Powder (abbreviated “103” in this paper) was a blend of corn-based starch, microcrystalline cellulose, agar and xanthan gum; TIC Pretested® Pre-Hydrated® Ticalose® CMC 6000 Powder (abbreviated “6000” in this paper) was a blend comprised primarily of cellulose gum; TIC Pretested® Aragum® 3173 Powder (abbreviated “3173” in this paper) was a blend of xanthan gum, propylene glycol alginate and guar gum. Emulsion composition is shown in Table 1 and nutritional information for the biopolymer blends is provided in Table 2.
Table 1.
Emulsion Composition
| Experiment 1 | ||||||
|---|---|---|---|---|---|---|
| V103 | C103 | V6000 | C6000 | V3173 | C3173 | |
| Fat (%) | 32 | 32 | 32 | 32 | 32 | 32 |
| Energy density (kcal/g) | 2.93 | 2.83 | 2.93 | 2.83 | 2.93 | 2.83 |
| Corn-based starch in product (%) | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Biopolymer (% w/v) | 5.75 | 6.25 | 1.25 | 1.75 | 2.25 | 2.75 |
| Experiment 2 | ||||||
| V103 | C103 | V6000 | C6000 | V3173 | C3173 | |
| Fat (%) | 32 | 32 | 32 | 32 | 32 | 32 |
| Energy density (kcal/g) | 2.93 | 2.83 | 3.13 | 3.05 | 3.13 | 3.05 |
| Corn-based starch in product (w/v %) | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Additional corn starch (w/v %) | 0.0 | 0.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Biopolymer (% w/v) | 5.75 | 6.25 | 1.25 | 1.75 | 2.25 | 2.75 |
Table 2.
Biopolymer Blend Nutritional Information (per 100 g)
| 103 S-Mayo | CMC 6000 | Aragum 3173 | |
|---|---|---|---|
| Fat | 0 | 0 | 0 |
| Protein (g) | 0 | 0 | 1 |
| Total Carbohydrate (g) | 87 | 80 | 79 |
| Soluble dietary fiber (g) | 11 | 80 | 79 |
| Insoluble dietary fiber (g) | 14 | 0 | 0 |
| Complex carbohydrates (g) | 62 | 0 | 0 |
| Sodium (mg) | 270 | 7943 | 1461 |
| Potassium (mg) | 45 | 19 | 1139 |
| Calcium (mg) | 19 | 9 | 0 |
| Water and Ash (g) | 12.7 | 12 | 17.4 |
Oil-in-water emulsions at the oil concentrations used in this study have a texture similar to cream that is largely independent of the physical properties of the fat. Thus, emulsons made with a solid fat such as Crisco have a texture similar to emulsions formed with a liquid oil such as vegetable oil. In the present study, the viscosity of the emulsion was increased to produce a consistency similar to pudding at room temperature by the addition of the water-soluble biopolymer blends described above. However, in order to maintain similar consistencies across the emulsions slightly different amounts of the different biopolymer blends were used (Table 1).
Each fat was heated to 85°C, mixed into a 1% w/v bovine sodium caseinate (Sigma Aldrich, St Louis, MO; 3.88 kcal/g) solution, then coarsely homogenized (Omni 5001, Omni International, Kennesaw, GA) for 1 minute. The caseinate acts as an emulsifier and allows the formation of a kinetically stable emulsion. Once the liquid emulsions were homogenized, the appropriate percentage of each biopolymer blend was added to the liquid emulsion, mixed with a hand blender for 2 minutes, heated to 80–85°C for 2 hrs and then refrigerated. The fat content of the final emulsions was 32%. All emulsions were allowed to reach room temperature prior to their presentation to the rats. Emulsions remaining in the jar after feeding were discarded after each session and new batches of emulsions were prepared weekly. The emulsions were physically stable over the course of the experiment with no creaming or visible phase separation.
Procedure
Experiment 1A
After matching the six groups, all rats were given overnight access to their assigned emulsion to prevent neophobia during subsequent emulsion intake. Three days later all rats were given daily (7 days/week) 1-h access to their assigned emulsion 2.5 h prior to the start of the dark cycle for five weeks. Emulsions were provided in glass jars (2.5″ diameter) clipped to the front of the home cage.
Experiment 1B
During the 6th week all rats received a saline injection to adapt them to the injection procedure. During weeks 7 and 8 the GABAB agonist baclofen (Tocris, Ellisville, MO) was administered intraperitoneally (0.0, 0.6, 1.0, 1.8 mg/kg); all rats received all doses, with doses assigned to each rat using a uniform Latin square. Injections were given on Mondays and Fridays, i.e., at least 3 days intervened between injections. The intake of simultaneously available emulsion and chow was assessed.
Experiment 2A
To determine the influence of starch on emulsion intake and on the efficacy of baclofen in reducing emulsion intake, 4% w/v granular corn starch (National Starch, Bridgewater NJ) was added to the V6000, C6000, V3173 and C3173 emulsions (Table 1). The starch was added at the time the biopolymer blends were added to the emulsions as described above. Note that because the products were subsequently cooked to 85°C in an excess of water the starch granules were fully gelatinized prior to delivery to the rats. No starch was added to the 103 Mayo emulsions as the biopolymer blend already provided about 4% corn-based starch (w/v) to those emulsions. After the addition of starch to the 6000 and 3173 emulsions, the energy densities of the vegetable shortening and corn oil emulsions made with these products were 3.13 kcal/g and 3.05 kcal/g, respectively. For the next two weeks the same rats that were used in Experiment 1 received their respective emulsions as described above.
Experiment 2B
After two weeks, baclofen (0.0 and 1.8 mg/kg, i.p.) was again administered to all rats with chow and emulsion simultaneously available. The two injections were counterbalanced within each group and administered on Monday and Friday. The 1.8 mg/kg dose was chosen based upon the results of Experiment 1B.
Statistics
SAS v9.1 (SAS Institute, Cary, NC) was used to analyze all data. Home cage intakes of emulsions during week 5 of Experiment 1A (see Figure 1) were analyzed using a 2-way ANOVA (2 fat types × 3 biopolymer blends) followed by pre-planned LS means comparisons with a Bonferroni correction applied. For the LS means comparisons, alpha was set at 0.01 (.05/5 comparisons per mean). Home cage intakes of emulsions during weeks 1 and 2 of Experiment 2A compared to week 5 of Experiment 1A (Figure 2) were analyzed using a 3-way ANOVA (2 fat types × 3 biopolymer blends × time) followed by a 1-way ANOVA within each group, with time as the repeated measure. Significant differences among weeks were assessed using Tukey’s HSD with alpha set at 0.05. Intake data across weeks were body mass adjusted using Heusner’s equation (Heusner, 1985; intake in kcal/body mass in g 0.67) in order to account for weight gain across the study.
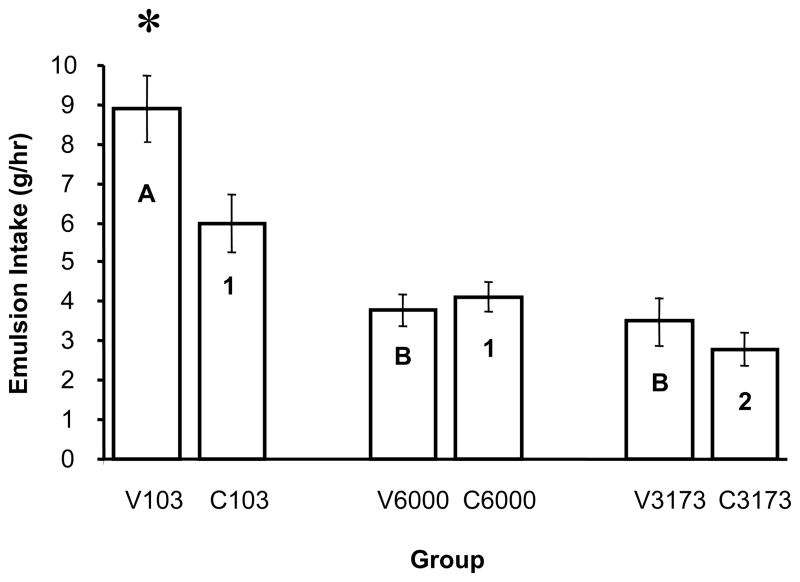
Fig. 1.
Average 1-hr emulsion intake during the 5th week of emulsion access during Experiment 1A. Different letters indicate significant differences among the vegetable shortening groups and different numbers indicate significant differences among the corn oil groups. The asterisk indicates that the V103 group consumed significantly more than all other groups.
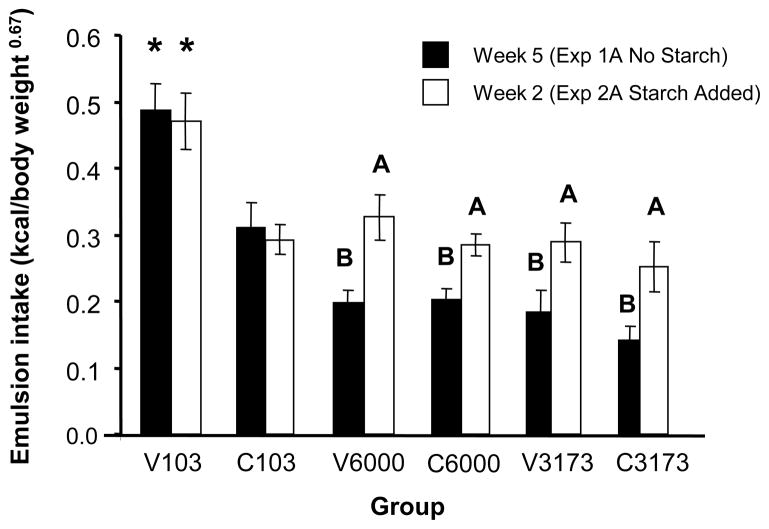
Fig. 2.
Average 1-hr emulsion intake normalized to body weight for week 5 of Experiment 1A and the second week of Experiment 2A when starch was added to the TIC 6000 and TIC 3173 emulsions. Only week 2 is shown for clarity. See text for details. Different letters indicate significant differences between the presence and absence of starch within each group. Asterisks indicate that the V103 group consumed significantly more than all other groups at each time point.
The effects of baclofen on emulsion and chow intake in Experiment 1B (Figures 3 and 4, left panels, respectively) were determined using 3-way ANOVA with fat type and biopolymer blend as non-repeated measures, and baclofen dose as the repeated measure. Significant differences among doses were assessed via 1-way ANOVA within each group, with baclofen dose as the repeated variable followed by a Dunnett’s test for comparison of each dose to saline. Significant differences between doses in Experiment 2B (Figures 3 and 4, right panels, respectively) were analyzed via paired t-tests.
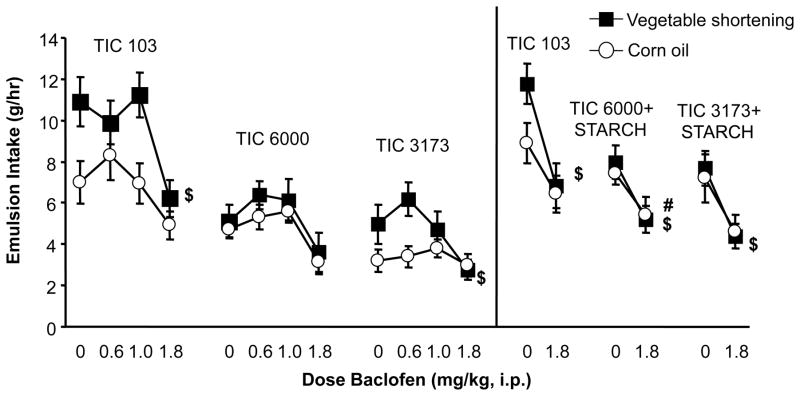
Fig. 3.
Effect of baclofen on 1-hr emulsion intake in Experiment 1B (left panel) and Experiment 2B (right panel). Emulsion intake was assessed during sessions in which chow was also simultaneosly available. $ indicates significant differences between the 1.8 mg/kg dose and saline in the vegetable shortening groups; # indicates significant differences in the corn oil groups.
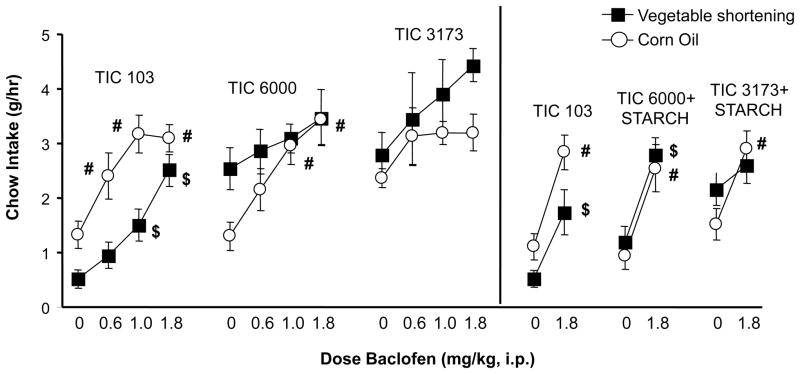
Fig. 4.
Effect of baclofen on 1-hr chow intake in Experiment 1B (left panel) and Experiment 2B (right panel). Chow intake was assessed during sessions in which emulsion was also simultaneosly available. $ indicates significant differences between a given dose and saline in the vegetable shortening groups; # indicates significant differences between a given dose and saline in the corn oil groups.
RESULTS
Emulsion Intake
In Experiment 1A emulsion intake during week 5 was a function of both the type of fat and the biopolymer blend used (Figure 1). There was a main effect of fat type (F(1,54) = 5.16 p 0.0272) due to greater overall intake of the emulsions made with shortening. A main effect of biopolymer blend (F(2,54) = 30.52, p < 0.0001) was because consumption of the 103 emulsions significantly exceeded consumption of the 6000 and 3173 emulsions. There also was a fat by biopolymer blend interaction (F(2,54) = 4.08 p < 0.0224). LS means comparisons revealed that the V103 group consumed significantly more emulsion than any of the other groups (p≤0.001 LSM), the C103 group consumed significantly more emulsion than the V6000, V3173 and C3173 groups (p < 0.009, 0.004, 0.0003 LSM, respectively), but not the C6000 group (p < 0.0293 LSM).
In Experiment 2A the addition of 4% corn starch to the V6000, C6000, V3173, and C3173 emulsions significantly increased intake in all four groups relative to Week 5 of Experiment 1A (Figure 2). There was a main effect of fat type (F(1,54) = 13.45, p < 0.0006) which again was due to greater overall intake of the vegetable shortening emulsions. A main effect of biopolymer blend (F(2,54) = 15.51 p < 0.0001) was because consumption of the 103 emulsions significantly exceeded consumption of the 6000 and 3173 emulsions. There also was a fat by biopolymer blend interaction (F(2,54)= 4.55 p < 0.0149). There was also a main effect of week (F(2,108) = 34.66 p < 0.0001), and a week by biopolymer blend interaction (F(2,108) = 14.07 p < 0.0001). Intake of the 6000 and 3173 emulsions increased significantly within the first week when starch was added and remained elevated thereafter (p < 0.05, Tukey’s HSD). In contrast, intake of the V103 and C103 emulsions did not change during this same period, indicating stable intake across the study in those groups. In addition, intake of the V103 emulsion was significantly greater (range from p < 0.0001 to 0.002, LSM) than any of the other 5 emulsions. Intakes of the V6000, V3173, C6000, C3173 emulsions did not differ from each other after starch was added and also were not statistically different from that of the C103 emulsion.
Effects of Baclofen
When baclofen was administered in Experiment 1B, there were main effects of fat type (F(1,54) = 7.90 p < 0.0069) (shortening > corn oil) and biopolymer blend (F(2,54) = 20.51 p < 0.0001) (103 > 6000, 3173), but no interaction between them. There also was a main effect of baclofen dose (F(3,162) = 31.22 p<0.0001), a dose by biopolymer blend interaction (F(6,162) = 2.16 p 0.0490), and a dose by fat type by biopolymer blend interaction (F(6,162) = 2.29 p < 0.0377). The 1.8 mg/kg baclofen dose significantly decreased emulsion intake relative to saline in the V103 and V3173 groups (p < 0.05, Dunnetts; Figure 3, left panel).
When baclofen was administered in Experiment 2B there was a main effect of dose (F(1,54)= 44.31 p<0.0001), as well as biopolymer blend (F(2,54)= 7.67 p < 0.0012; Figure 3, right panel). The main effect of biopolymer blend was due to generally higher intake of the 103 emulsions. Baclofen significantly reduced 1-h emulsion intake in all of the vegetable shortening groups [paired t-tests: V103 (p < 0.0045), V6000 (p < 0.0099), and V3173 group (p < 0.0083)] and the C6000 group (paired t-test: p < 0.0401) (Figure 3, right panel). There was a marginally non-significant decrease in the C103 (p< 0.0688) and C3173 (p < 0.0892) groups.
Chow intake
In contrast to its effects on emulsion intake, baclofen stimulated intake of the simultaneously available chow. In Experiment 1B there was a main effect of biopolymer blend (F(2,54) = 14.78 p< 0.0001) due to the low chow intakes in the 103 emulsion groups. There was a fat type by biopolymer blend interaction (F(2,54)= 7.76 p < 0.0011), but no main effect of fat type. There also was a main effect of dose (F(3,162)= 22.11 p < 0.0001). Baclofen increased 1-h chow intake in all groups, although this effect did not always achieve significance. Significant stimulation of chow intake relative to saline occurred in the V103, C103, and C6000 groups (p < 0.05, Dunnetts; Figure 4, left panel).
Baclofen also significantly increased 1-h chow intake in Experiment 2B. There was a main effect of biopolymer blend (F(2,54)= 5.13 p < 0.0091) due to the relatively low chow intakes in the 103 emulsion groups coupled with high intakes in the 3173 groups. There also was a main effect of dose (F(1,54)= 59.56 p < 0.0001) and a fat type by biopolymer blend interaction (F(2,54)= 3.43 p < 0.0395). Baclofen significantly stimulated chow intake in the V103, C103, V6000, C6000, and C3173 groups (paired t-tests p < 0.025) (Figure 4, right panel).
In Experiment 1B total intake (chow plus emulsion) was unaffected by baclofen at doses that reduced emulsion intake in all groups except one (data not shown). Specifically, baclofen reduced total intake in the V103 group at the same dose that reduced emulsion intake (1.8 mg/kg). In Experiment 2B similar results were obtained, i.e., baclofen significantly reduced total intake in the V103 group and also in the V3173 group (data not shown).
Body Weight
Terminal body weight and body weight gain did not differ among the groups [terminal body weight: ANOVA F(5,54) = 0.38, p ns; body weight change: ANOVA F(5, 54) = 0.36, p ns].
DISCUSSION
Several new findings are reported. First, emulsion intake was a function of the oil type and biopolymer blend with or without the addition of starch. In Experiments 1A and 2A the V103 group consumed significantly more emulsion than any of the other groups, an amount comparable to that previously reported for the emulsions made with V103 (Rao et al., 2008). Second, the addition of starch to the 6000 and 3173 biopolymer blends significantly increased the amount of emulsion consumed by the vegetable shortening and corn oil groups when compared to intakes in the absence of starch. Intakes of the V103 and C103 did not change between Experiment 1A and 2A indicating that the intakes were stable and the increase in emulsion intake in the other 4 groups was due to the addition of starch rather than some other factor such as time or experience. However, with or without starch, there were no differences between oil groups within the 6000 and 3173 biopolymer blends, i.e., V6000 vs. C6000 and V3173 vs. C3173. Third, the effect of baclofen on emulsion intake was a function of both the oil type and the presence of starch in the emulsion.
The absence of a difference in emulsion intake (acceptance) between vegetable shortening and corn oil with the 6000 and 3173 biopolymer blends is similar to the results of another study that examined preference. Lucas et al. (1987) found that while rats consumed more vegetable shortening than corn oil when presented in pure form, there were no differences in intake when presented in emulsified solid gel form. Although the fat concentration in both studies was comparable (35% in Lucas et al., (1987), 32% here) the emulsifiers were different (0.6% Emplex in Lucas et al. (1987), 1% w/v bovine sodium caseinate in the presents study) and the thickeners were different (2% gelatin in Lucas et al. (1987), the biopolymer blends Ticalose® CMC 6000 Powder and Aragum® 3173 Powder in the present study). Thus, vegetable shortening and corn oil are equally acceptable to rats when formulated in these three different ways.
In contrast, different intakes were obtained between emulsions made with the 103 biopolymer blend that contained starch, i.e., the V103 group consumed significantly more emulsion than the C103 group did. The reasons for this are not clear. If post-ingestive effects of the different oil types were to account for the different intakes with the 103 biopolymer blend, then one would expect similar results with the other biopolymer blends. However, this did not occur. The greater intake of the V103 emulsion also cannot be explained by the presence of starch as intakes of V6000 and C6000 were comparable, as were intakes of V3173 and C3173, after starch was added in Experiment 2. One possibility is that the rheological properties of the 103 provided a different mouthfeel to the vegetable shortening emulsion, which enhanced its acceptability. Regardless, it is clear that the thickener that is used to solidify fat emulsions can have impressive effects on fat intake.
The increase in intake as a function of the addition of 4% starch to the emulsion is not surprising. Corn starch is readily accepted by rats, either alone (Ramirez, 1991a) or when mixed into high-fat diets (Chun et al., 2010). Furthermore, the concentration used in the present study (4%) is within the acceptance range reported by others (Ramirez, 1991a,c, 1992, Sclafani et al., 1988). When allowed to choose, rats prefer corn starch to cellulose (Alphacel) at concentrations of 0.5% and above. They are also are able to detect as little as 0.5% starch from several species of plants and choose the higher of two concentrations in a choice procedure (Ramirez, 1991a). Finally, when given a choice between corn amylopectin and corn amylose, rats prefer amylopectin, the main component of cornstarch (Ramirez, 1991d). The above studies support the results of the present study, i.e., the composition of cornstarch and the concentration used in the present study would be expected to enhance intake, as was found in Experiment 2.
Intake of the 6000 and 3173 emulsions increased for both vegetable shortening and corn oil after the addition of starch, achieving amounts comparable to that of the C103 emulsion. However, none of the intakes matched the V103 emulsion. These results may have been due to rats’ responsiveness to different carbohydrates coupled with textural properties conferred when combined with shortening. The responsiveness of rats to different carbohydrates has been demonstrated in several previous reports. For instance, the preference for either starch, sucrose or glucose depends upon the concentration (Ramirez, 1993). Rats prefer maltodextrins to starch suspensions at various concentrations (Ramirez, 1991b). In brief two-bottle choice tests rats preferred solutions containing 4–8 glucose polymers (maltooligosaccharide) to solutions containing shorter (glucose, maltose) or longer (maltopolysaccharide) polymers (Sclafani, 1987). Rats are also able to discriminate among various forms of carbohydrate. For example, rats trained to avoid starch did not avoid Polycose or sucrose, rats trained to avoid Polycose did not avoid starch or sucrose, and rats trained to avoid sucrose did not avoid starch or Polycose (Ramirez, 1991c). It is possible that the different emulsion intakes in the present study were due to differences in the structural components of the starches used. Specifically, the starch in the 103 emulsion is a proprietary “corn-based” starch, while the starch added to the 6000 and 3173 emulsions in Experiment 2 is a granular non-proprietary corn starch. It is possible that the proprieary corn-based starch in combination with shortening rendered the V103 emulsion particularly acceptable to the rats. However, the possibility that other components of the biopolymer blends, such as the gums, may have conferred a different “mouth feel” in combination with shortening cannot be ruled out.
Baclofen effects
Baclofen generally reduced intake of all of the emulsions at 1.8 mg/kg, although these effects did not always achieve statistical significance. The potency of baclofen was similar whether a thickener with or without starch was used. Interestingly, significant intake reductions were achieved in five of the six tests made with vegetable shortening (V6000 in Experiment 1B being the exception). In contrast, a significant intake reduction was achieved in only one of the six tests made with corn oil, i.e, the C6000 emulsion with starch added. These results replicate those reported by Rao et al. (2008) for vegetable shortening and suggest that baclofen may be somewhat more specific for vegetable shortening than corn oil regardless of the presence or absence of starch. Hydrogenated oils such as vegetable shortening are commonly used in the preparation of energy-dense foods such as cakes, cookies, and pies. Baclofen may prove useful clinically to help reduce the consumption of these types of foods. Indeed, recent reports suggest the usefulness of baclofen to reduce binge eating, although the specific foods consumed were not reported (Broft et al., 2007; Corwin et al., 2010).
In contrast to its effects on emulsion intake, baclofen generally stimulated intake of the simultaneously available chow, although again, these effects did not always achieve statistical significance. The fact that baclofen increased chow intake demonstrates that the reductions in fat intake induced by baclofen were not due to general behavioral suppression. The stimulation of chow reported here is consistent with other reports in which baclofen stimulated food intake in non-food-deprived rats regardless of whether baclofen was administered chronically (Patel & Ebenezer, 2008), acutely (Buda-Levin et al., 2005; Corwin & Wojnicki, 2009; Ebenezer & Pringle, 1992), or whether the food was solid or liquid (Ebenezer, 1995).
Although overall behavioral suppression cannot explain the present results, reductions in fat intake occurred during sessions in which chow intake was simultaneously stimulated for several of the groups in this study. The possibility exists that the act of eating chow interfered with the act of eating shortening. However, such interference cannot explain all instances in which shortening intake was reduced. For instance, baclofen significantly reduced intake of the V3173 emulsion in both Experiments 1B and 2B, while having no significant stimulatory effect on consumption of chow (although chow intakes clearly tended to increase). In addition, overall total consumption (chow and emulsion) was reduced in the V103 groups in both Experiments 1B and 2B (data not shown), indicating that reductions in shortening intake were greater than the stimulatory effects on chow, at least for that group. It appears, therefore, that baclofen can have independent effects on consumption of shortening and chow, and that effects on one do not necessarily explain effects on the other.
It is not clear why in the same sessions baclofen significantly decreased fat intake when a carbohydrate, i.e., starch, was added to a fat emulsion (either the 6000 or 3173 biopolymer blends), yet increased intake of a high carbohydrate food, i.e., chow, in the same sessions. One possibility could be the change in baseline emulsion intake that resulted from the addition of starch. Given rats’ affinity for starchy food, the addition of starch to the emulsions made the emulsions more acceptable as measured by intake, and thus the increased efficacy of baclofen may have been due to the increased baseline intakes. Others have reported differential effects of drugs on food intake depending upon the baseline consumption. For example Sills & Vaccarino (1991) demonstrated that a 2.0 ng dose of CCK significantly decreased feeding in high baseline feeders, but had no effect on low baseline feeders. In the same study a 0.125 mg/kg dose of amphetamine increased feeding of low baseline intake rats and decreased feeding in rats with higher baselines. The effect of a drug on feeding has also been shown to depend on the deprivation state of the animal. Dobrzanski & Doggert (1976) showed that doses of fenfluramine and (+)-amphetamine that decreased food intake in free feeding mice were increased two-fold to decrease food intake in food-deprived mice. Furthermore, amphetamine decreased night-time food intake when food intake levels were high, but increased daytime food intake when food intake levels were low.
A similar argument can be made for baclofen. For instance, in many of the studies in which baclofen stimulated intake, baseline consumption was relatively low as when rats were not food-deprived (Ebenezer & Pringle, 1992; Ebenezer, 1995, Patel & Ebenezer, 2008). However, in studies in which baclofen has been reported to reduce intake, baseline consumption was high even though the rats were not food-deprived (Buda-Levin et. al., 2005; Corwin & Wojnicki, 2009). It appears that, under non-food-deprived conditions, baclofen may reduce food intake when consumption is high (emulsions in the present study), but stimulate intake when intakes are low (chow in the present study). In addition, baclofen has been shown to decrease self-reported bingeing bouts in human clinical trials (Broft et. al., 2007; Corwin et al., 2010). It is important to note, however, that while the baseline argument has appeal, effects do not always hold if the food contains sucrose or if rats are food-deprived. Specifically, baclofen only appears to reduce consumption of fatty foods made with relatively low levels of sucrose in rats. When sucrose is included in the food at concentrations comparable to the starch concentrations used here (3–4%) baclofen reduces intake (Wong et al., 2009). However, when larger amounts of sucrose are included or if liquid sucrose solutions are used, baclofen either has no effect on or stimulates intake even though baseline intakes are already high (Berner et. al., 2009; Corwin and Wojnicki, 2009; Wong et al., 2009). Likewise, when rats are food-deprived and given chow to eat, baseline intakes are high, but baclofen does not reduce intake (Ebenezer & Patel, 2011). The effects of baclofen, therefore, appear to be baseline dependent (high intakes), as well as dependent upon the composition of the food (high-fat) and the deprivation state of the animal (non-deprived). This may account for the reported ability of baclofen to reduce binge eating (Broft et al., 2007; Corwin et al., 2010), a condition in which high intakes of fatty foods occur even in the absence of hunger (APA, 2000).
We have been interested in the development and use of thickened fat emulsions in rat studies due to their usefulness as model foods intended to mimic foods common to human diets. The development of such emulsions would allow for the systematic evaluation of the effects of varying concentrations of fats on brain and behavior as well as the effects of potential therapeutics on intake. The present results demonstrate the importance of the products used in the preparation of such emulsions, with intakes varying as a function of the thickeners and fat types used. In addition, the present results are consistent with many other reports demonstrating the stimulatory effect of starch on rat ingestive behavior. Finally, this report confirms other reports of the ability of baclofen to reduce consumption of fatty foods, while simultaneously stimulating intake of chow.
HIGHLIGHTS.
Emulsion intake was a function of oil type and biopolymer blend.
Addition of starch to biopolymer blends increased emulsion intake.
Baclofen generally reduced emulsion intake, depending upon oil and biopolymer.
Baclofen stimulated intake of chow.
Acknowledgments
The authors would express their gratitude for the invaluable technical assitance of Tim Andon at TIC Gums without whose assistance the present study would not have been possible. We also expresss our gratitude to TIC Gums for their generous donations. Support for this study provided by MH67943 (RLC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA (American Psychiatric Association) Diagnostic and Statistical Manual of Mental Disorders. 4. Washington: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Berner LA, Bocarsly ME, Hoebel BG, Avena NM. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behavioral Pharmacology. 2009;20(7):631–34. doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DCS. Effect of baclofen on cocaine self administration in rats reinforced under fixed-ratio 1 and progressive ratio schedules. Psychopharmacology. 2000;148:314–21. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Attia E, Walsh BT. Baclofen for binge eating: an open-label Trial. International Journal of Eating Disorders. 2007;40:687–91. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- Buda-Levin A, Wojnicki FHE, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiology & Behavior. 2005;86:176–184. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull LS, Pitts GC. Gastric capacity and energy absorption in the force-fed rat. Journal of Nutrition. 1971;101:593–596. doi: 10.1093/jn/101.5.593. [DOI] [PubMed] [Google Scholar]
- Chun MR, Lee YJ, Kim KH, Kim YW, Park SY, Lee KM, Kim JY, Park YK. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. Journal of Korean Medical Sciences. 2010;25:1053–59. doi: 10.3346/jkms.2010.25.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RLW, Wojnicki FHE. Baclofen, raclopride and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behavioral Pharmacology. 2009;20(5–6):537–48. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Boan J, Peters K, Walsh BT, Ulbrecht J. Baclofen reduces binge frequency. Appetite. 2010;54:641. [Google Scholar]
- Dobrzanski S, Doggett NS. The effects of (+)-amphetamine and fenfluramine on feedin in starved and satiated mice. Psychopharmacology. 1976;48:283–86. doi: 10.1007/BF00496862. [DOI] [PubMed] [Google Scholar]
- Ebenzer IS, Patel SM. Effects of intraperitoneal administration of the GABA B receptor agonist baclofen on food intake in rats measured under differend feeding conditions. European Journal of Pharmacology. 2011;25:653:58–62. doi: 10.1016/j.ejphar.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS, Pringle AK. The effect of systemic administration of baclofen on food intake in rats. Neuropharmacology. 1992;31(1):39–42. doi: 10.1016/0028-3908(92)90158-l. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Intraperitoneal administration of baclofen increases consumption of both solid and liquid diets in rats. European Journal of Pharmacology. 1995;273:183–185. doi: 10.1016/0014-2999(94)00707-e. [DOI] [PubMed] [Google Scholar]
- Heusner AA. Body size and energy metabolism. Annual Review of Nutrition. 1985;5:267–93. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiology & Behavior. 1987;45:937–46. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- Patel SM, Ebenezer IS. The effects of chronic intraperitoneal administration of the GABA-B receptor agonist baclofen on food intake in rats. European Journal of Pharmacology. 2008;593:68–72. doi: 10.1016/j.ejphar.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Rao RE, Wojnicki FHE, Coupland J, Ghosh S, Corwin RLW. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacology Biochemistry & Behavior. 2008;89(4):581–90. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez I. Chemoreception for an insoluble nonvolital substance: starch taste? American Journal of Physiology-Regulatory Integrative Comparative Physiology. 1991a;260(29):R192–R199. doi: 10.1152/ajpregu.1991.260.1.R192. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Does starch taste like polycose. Physiology & Behavior. 1991b;50:389–92. doi: 10.1016/0031-9384(91)90083-z. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Thresholds for starch and Polycose are lower than for sucrose in rats. Physiology & Behavior. 1991c;50:699–703. doi: 10.1016/0031-9384(91)90005-9. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Starch flavor: apparent discrimination between amlyopectin and amylose by rats. Physiology & Behavior. 1991d;50:1181–86. doi: 10.1016/0031-9384(91)90580-h. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Is starch flavor unitary? Evidence from studies of cooked starch. Physiology & Behavior. 1992;52:535–40. doi: 10.1016/0031-9384(92)90343-z. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Relative preference for starch and sugar in rats. Physiology & Behavior. 1993;54:1195–1200. doi: 10.1016/0031-9384(93)90348-j. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neuroscience and Biobehavioral Reviews. 1987;11:131–53. [PubMed] [Google Scholar]
- Sclafani A, Vigorito M, Pfeiffer CL. Starch-induced overeating and overweight rats: influence of starch type and form. Physiology & Behavior. 1988;42:409–415. doi: 10.1016/0031-9384(88)90169-2. [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. Facilitation and inhibition of feeding by a single dose of amphetamine: relationship to baseline intake and acumbens cholecystokinin. Psychopharmacology. 1991;105:329–334. doi: 10.1007/BF02244426. [DOI] [PubMed] [Google Scholar]
- Wong KJ, Wojnicki FHE, Corwin RLW. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacology Biochemistry & Behavior. 2009;92(3):528–36. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]