Abstract
Background
Prenatal serotonin reuptake inhibitor (SRI) exposure has been related to adverse newborn neurobehavioral outcomes; however these effects have not been compared to those that may arise from prenatal exposure to maternal major depressive disorder (MDD) without SRI treatment. This study examined potential effects of MDD with and without SRI treatment on newborn neurobehavior.
Methods
This was a prospective, naturalistic study. Women were seen at an outpatient research center twice during pregnancy (26–28 and 36–38 weeks gestational age (GA)). Psychiatric diagnoses were assessed using the Structured Clinical Interview for the DSM-IV; medication use was measured with the Timeline Follow-Back instrument. Three groups were established based upon MDD diagnosis and SRI use: Control (N=56), MDD (N=20) or MDD+SRI (N=36). Infants were assessed on a single occasion within 3 weeks of birth with the NICU Network Neurobehavioral Assessment Scale (NNNS). Generalized Linear Modeling was used to examine neurobehavioral outcomes by exposure group and infant age at assessment.
Results
Full-term infants exposed to MDD+SRIs had a lower GA than CON or MDD-exposed infants and, controlling for GA, had lower quality of movement and more central nervous system stress signs. In contrast, MDD-exposed infants had the highest quality of movement scores, while having lower attention scores than CON and MDD+SRI-exposed infants.
Conclusion
MDD+SRI-exposed infants appear to have a different neurobehavioral profile than MDD-exposed infants in the first three weeks after delivery; both groups may have different neurobehavioral profiles with increasing age from birth.
Keywords: infant, motor quality, central nervous system, depression, pregnancy, treatment
INTRODUCTION
The prevalence of Major Depressive Disorder (MDD) during pregnancy is estimated at 3–12%, with 7.5% of pregnant women having a new episode.[1; 2] MDD is associated with altered levels of neurotransmitters and neuro-regulators in the brain and periphery.[3–5] Some evidence suggests that pregnant women with MDD and their infants have increased urinary levels of norepinephrine and cortisol metabolites with decreased dopamine compared to non-depressed pregnant women and their infants.[6; 7] Recent findings suggest that placental mRNA expression of the serotonin transporter gene (SLC6A4) is higher in women with MDD, regardless of antidepressant treatment.[12] The link between placental environment and infant outcomes in women with prenatal MDD has not been established. However, animal and human studies on prenatal stress support the notion that the fetus is affected by placental transfer of altered hormones and neurotransmitters as well as restricted uterine blood flow. [8–11] Prenatal MDD has been associated with earlier gestational age at delivery[21–25] and other adverse obstetrical outcomes.[26] In addition newborns prenatally exposed to maternal depression are reported to have more difficulty with behavioral state regulation, low muscle tone, irritability, and reactivity.[27–30] The implications of these findings for development remains unclear and the additional influence for co-morbid factors such as drug and alcohol abuse, [13] smoking,[14] inadequate sleep,[15] poor nutrition,[16–18] and inadequate prenatal care[19; 20] have not been fully explored.
It is estimated that at least 8% of all pregnant women[31] and 37–39% of those with MDD are treated with the selective serotonin reuptake inhibitor antidepressants (SSRIs) or dual-action serotonin and norepinephrine reuptake inhibitors (SNRIs; SRIs collectively) every year.[32; 33] Fetal SRI exposure has been confirmed by depleted levels of serotonin and its metabolite levels in cord blood.[34; 35] A cluster of symptoms has been observed in 30% of SRI-exposed newborns, including irritability, tremors, jitteriness, alterations in muscle tone, trouble feeding, agitation, respiratory distress, and poor sleep. [36; 37] Additional, less common, symptoms include convulsions, abnormal posturing, and shivering.[38; 39] This cluster of symptoms has been compared with Neonatal Abstinence Syndrome (NAS) which is observed in neonates withdrawing from opiates.[34; 40] However, there is some overlap in these symptoms and those of infants prenatally exposed to maternal MDD, yet there has not been a systematic evaluation in comparison to infants exposed to maternal MDD without pharmacological treatment. Data from a large database study support the notion that withdrawal from SRIs is not the sole reason for such newborn difficulties, as third trimester withdrawal of medication did not prevent newborn symptoms.[41]
Alterations in key CNS systems occur with gestational SRI exposure and continue long after birth, including decreased pain response in newborns and at 2 months of age,[42; 43] and altered HPA stress reactivity with reduced basal cortisol levels in cord blood and at 3 months.[44] Prenatal SRI exposure has been linked to lower GA at birth, higher preterm birth rates [21; 23] and lower APGAR scores.[23; 45] However, in a large study, GA at birth and the preterm birth rate were not different between SRI-exposed and not SRI-exposed when propensity score matching was utilized to account for maternal MDD and other factors related to medication use.[46] Birth weight differences reported in at least one study were found to be related to GA,[47] while other studies failed to find birth weight differences between SRI- and not SRI-exposed infants[48; 49] or between early versus late gestation SRI exposure.[46; 50] Duration of SRI exposure, rather than SRI dosage may be related to adverse newborn outcomes.[46]
Previous research has not compared the neurobehavior of SRI-exposed infants to a non-pharmacologically treated MDD comparison group; resulting in ambiguity of the risks associated with SRI treatment. The current study utilized a comprehensive, standardized tool to detect effects of MDD without pharmacological treatment and SRI-treated MDD on newborn neurobehavior in the first three weeks post-delivery. The main hypothesis is that a differential pattern of neurobehavioral responses would be observed between infants in MDD, MDD+SRI, and non-MDD/non-SRI control groups. Specifically, we hypothesized that MDD exposure would result in lower motor tone and higher levels of irritability while MDD+SRI-exposed infants would have more stress signs (e.g. startles, tremors, back-arching) compared to non-exposed infants. A secondary hypothesis is that more optimal neurobehavioral scores would be observed with lower depressive symptom severity within the MDD and MDD+SRI groups.
MATERIALS and METHODS
Subjects
This was a prospective, naturalistic cohort study. Women were initially phone-screened to determine likely eligibility. Inclusion criteria included: age 18–40, 23–36 weeks gestation, singleton pregnancy, no prenatal illicit drug use, no hypertension or diabetes, alcohol use < 0.5 drinks/day, and < 10 cigarettes/day during pregnancy. Women taking medications that were FDA Class D or X at time of enrollment (2002–2007) or who took benzodiazepines in the third trimester[51] were excluded.
Initially, 189 women enrolled and were evaluated for eligibility; 168 met inclusion criteria through 2 sessions during pregnancy, 148 continued participation through the infant assessment (12% withdrew). However, 36 infants met exclusion criteria for the analyses in this study, resulting in a final N of 112: 12 were born before 37 weeks GA, 8 had abnormalities or serious health conditions, and 16 were unable to be examined within 21 days of birth (e.g. born at outlying hospitals).
Procedures
Procedures were approved by the hospital’s IRB; written informed consent was obtained from all participants prior to assessments. Participants attended two assessments during pregnancy, 24–28 weeks (2ndTRI) and 36–38 weeks (3rdTRI) GA, at which time a semi-structured diagnostic assessment was conducted. Infants were then seen for a newborn assessment once within three weeks after birth.
Measures
Maternal MDD was diagnosed with the Structured Clinical Interview for the DSM-IV (SCID)[52] at enrollment. SCID mood modules were repeated at the 3rd TRI session. Depression severity was determined using the 17-item, interviewer-rated Hamilton Rating Scale for Depression (HRSD) [53] at the 2ndTRI and 3rdTRI sessions.
SRI Exposure was determined using the Timeline Follow Back interview (TLFB) [54], a calendar-based semi-structured interview designed to measure drug and alcohol use with excellent psychometrics across clinical and non-clinical populations,[55] as well as pregnant women.[56] The TLFB has been used reliably to measure medication adherence in clinical trials[57; 58] and was used in this study to obtain type and timing of antidepressant and other medication use , as well as caffeine, tobacco, and alcohol use over pregnancy. For each SRI, mean standard dose was calculated for each trimester and total pregnancy based on the reported dose taken divided by the standard dose defined by the Physician’s Desk Reference. Duration in weeks of SRI use reported by women was also calculated.
Participants were ultimately categorized into the non-SRI/non-MDD control group (CON; N=56) if they did not use SRIs or meet criteria for MDD or any Axis I psychiatric illness during their current pregnancy. Women in the MDD group met full DSM-IV-R criteria for MDD (N=20) for at least 4 weeks during the second and/or third trimester of this pregnancy and were not receiving psychotropic medications at any time prenatally. The MDD+SRI group (N=36) comprised women who met MDD criteria within the previous year and reported taking an SRI medication for at least 4 consecutive weeks during the 2nd and/or 3rd trimesters of this pregnancy.
The Hollingshead index of socioeconomic status (SES) characterized participants’ SES status; categories 4 and 5 were rated as low SES.[59]
Infant Measures
Infants were assessed with the NICU Network Neurobehavioral Scale (NNNS), [60] a validated, comprehensive assessment of infant neurobehavior developed for the National Institute of Child Health and Development NICU Research Network.[61] The NNNS assesses neurological integrity and behavioral function of infants at risk due to exposure to drugs or prematurity and includes: (1) classical neurological items assessing active and passive tone, reflexes, and Central Nervous System (CNS) integrity; (2) behavioral items including state, sensory and interactive responses; and (3) stress/abstinence items observed with withdrawal/discontinuation from opiates and other CNS drugs. NNNS summary scales (Table 2) have been developed using conceptual and statistical aggregation in previous samples; [62] concurrent and predictive validity has been documented in numerous studies, across varying prenatal exposures. [63–66]
Table 2.
NICU Network Neurobehavioral (NNNS) Scores by Exposure Group
| CON | MDD | MDD+SRI | Group Main Effect |
||||
|---|---|---|---|---|---|---|---|
| Description | Scoring Range |
Mean (95% C.I.) | Mean (95% C.I.) | Mean (95% C.I.) | W-χ2 | p | |
| Attention | A measure of the infant's ability to localize and track auditory and visual stimuli; requires a sustained quiet alert state; high scores indicate appropriate/sustained alertness. | 1–9 | 5.84 (5.53,6.16) | 4.36 (3.79,4.92) ***c,d | 5.96 (5.45,6.46) | 21.59 | 0.00 |
| Quality of Movement | A measure of motor quality including smoothness, maturity, modulation of movement as well as startles and tremors; high scores indicate good motor control. | 1–9 | 4.63 (4.47,4.79) | 4.82 (4.59,5.04) | 4.27 (4.02,4.52) *c,**d | 6.09 | 0.05 |
| Self-Regulation | A measure of the infant's responses in motor activity, physiology, and state in response to the demands of the exam; higher scores indicate better regulation. | 1–9 | 5.50 (5.31,5.70) | 5.47 (5.26,5.68) | 5.27 (5.02,5.51) | 0.81 | 0.67 |
| Handling | Mean number of maneuvers needed to keep the infant alert and calm during orientation; scores closer to 1 indicate more help from the examiner was needed. | 0–1 | 0.38 (0.30,0.45) | 0.40 (0.24,0.56) | 0.42 (0.33,0.51) | 1.76 | 0.41 |
| Arousal | Level of arousal and associated motor activity during the exam; high scores indicate high arousal, activity, and fussing and crying | 1–9 | 4.27 (4.08,4.45) | 4.20 (3.86,4.53) | 4.04 (3.77,4.31) | 6.97 | 0.03 |
| Excitability | A measure of high levels of motor, state and physiological reactivity. | 0–15 | 3.55 (3.00,4.10) | 3.17 (2.25,4.09) | 3.67 (2.89,4.44) | 0.63 | 0.73 |
| Lethargy | A measure of low levels of motor, state and physiological reactivity. | 0–15 | 3.02 (2.48,3.56) | 4.24 (3.19,5.29) | 3.34 (2.62,4.07) | 0.01 | 1.00 |
| Stress- Abs.Signs:Total |
Mean number of stress/abstinence signs displayed by the infant across five organ systems; a score closer to 1 indicates a high number of observed stress/abstinence signs. | 0–1 | 0.08 (0.06,0.09) | 0.08 (0.06,0.10) | 0.11 (0.09,0.12) | 4.57 | 0.10 |
| Stress Abs. Signs:CNS |
Mean number of central nervous system related stress/abstinence signs, including startles, tremors, backarching, and abnormal posturing; a score closer to 1 indicates a high number observed. | 0–1 | 0.05 (0.03,0.07) | 0.06 (0.03,0.08) | 0.13 (0.10,0.16) ***c,d | 21.72 | 0.00 |
| Non-Optimal Reflexes | The total number of non-optimal reflex responses elicited. | 0–15 | 1.60 (1.07,2.14) | 1.73 (0.80,2.66) | 2.17 (1.40,2.94) | 1.79 | 0.41 |
| Asymmetrical Reflexes | The total number of asymmetric responses to elicited reflexes. | 0–16 | 0.33 (0.14,0.52) | 0.50 (0.18,0.82) | 0.56 (0.25,0.86) | 0.67 | 0.72 |
| Hypertonia | The number of items in which the infant's response was characterized by high muscle tone (rigidity or lack of relaxation at rest) in the arms, legs, and/or trunk. | 0–10 | 0.05 (−0.01,0.11) | 0.04 (−0.03,0.11) | 0.16 (−0.01,0.33) | 3.97 | 0.05 |
| Hypotonia | The number of items in which the infant's response was characterized by decreased muscle tone (limp, flaccid, or weak) in the arms, legs, and/or trunk. | 0–10 | 0.16 (0.04,0.27) | 0.05 (−0.04,0.14) | 0.23 (0.08,0.38) | 0.47 | 0.49 |
Estimated marginal means (95% Confidence Interval) presented, adjusted for gestational age at birth and age at NNNS assessment; additionally, exposure group X age at NNNS assessment was included in the model to examine influences of infant age on the outcomes for each group; significant relationships with infant age presented in Figures 1–6. Tests of Model Effects using Wald Chi-Square Statistics are shown for exposure group main effect. Pairwise comparisons with sequential Bonferonni corrections for multiple comparisons were used to evaluate exposure group differences; significance from one or both groups indicated in the table:
p<.05,
p<.01,
p<.001,
significant difference from CON group,
significant difference from the clinical comparison group (MDD or MDD+SRI). N=112 total (56 CON, 20 MDD, and 36 MDD+SRI) except for the following variables as infants who were not in the required behavioral state during the assessment were not able to be scored for these variables: Attention, N=47 CON, 14 MDD, 28 MDD+SRI; Quality of Movement and Self-Regulation, N=55 CON, and Handling, N=49 CON, 14 MDD, 30 MDD+SRI; CNS:Central Nervous System
Infant assessments were conducted by certified NNNS examiners on a single occasion between 1 and 21 days after delivery either in the newborn nursery or at the infants’ home. NNNS scores have the potential to change over the first few weeks from birth due to neurobehavioral maturation. Infant age at time of the assessment was therefore used as a predictor in the statistical models to control for this variability on the main effects and, due to previous findings of transient neurobehavioral symptoms in newborns,[40] examine potential relationships between the NNNS outcomes and time from delivery. NNNS examiners obtained inter-rater reliability exceeding 90% agreement and were blind to maternal group status. Incidental breaking of the blind (e.g. participant mentioning medication status) was monitored and occurred in 8.5% of cases; these cases were not significantly different from those without a violation and were retained.
Statistical Analyses
Generalized Linear Modeling (GLZ) was used to determine exposure group differences on NNNS outcomes. Exposure group (CON, MDD or MDD+SRI) was the primary independent variable; NNNS summary variables were the dependent measures. The majority of the NNNS variables were normally distributed and a normal distribution model with identity link was used for 9 of the summary variables. Non-optimal reflexes, asymmetry, hypertonia and hypotonia had skewed distributions; therefore Poisson models with a log link were used for these variables. Infant age at the time of NNNS assessment varied and was therefore used as a continuous predictor variable to examine possible contributions to neurobehavioral outcomes. This variable was highly skewed toward younger ages; therefore two approaches were tested to address this statistically. First, age was divided into three groups based on distribution and clinical significance (age in days 1–2, 3–9, 10–21) and tested in the models as a fixed factor. Second, age was log transformed (log10) and used as a continuous covariate in the models. The log transformed covariate model produced lower log likelihood and Pearson Chi square values in the goodness of fit tests and was therefore used. The interaction term of exposure group by infant age at NNNS was included to examine the potential contribution of infant age on main outcomes. Pairwise comparisons of the parameter estimates for any significant exposure group main effects were examined with sequential Bonferonni corrections.
Model testing was conducted for the best fit for all NNNS analyses with the inclusion of additional potential covariates, including infant GA, Apgar score, marital status, SES, and race. Only GA at birth contributed significantly to outcomes and was retained in the final models as a continuous predictor variable with infant age at assessment and exposure status as the factorial predictor. Within group analyses of mean HRSD score, duration and timing of SRI use and mean total standard dose equivalent of SRI were conducted for all significant outcome variables in the GLZ models.
Maternal demographics and infant characteristics were compared between exposure groups using one-way ANOVA for continuous measures and χ2 tests of independence for categorical measures using exact methods. Analyses were conducted using SPSS 19.0.
RESULTS
Demographic Characteristics
There were no significant exposure group differences in the percentage of participants that completed the study through delivery (N(%) =76 (85%) CON, 27(77%) MDD, 46(79%) MDD+SRI; p=.47) or in the percentage of infants who completed NNNS assessments (N(%)=56(74%) CON, 20(74%) MDD, and 36(78%) MDD+SRI; p=.20). However, across all exposure groups, those completing the infant assessment had a higher GA at birth (39.3 vs 37.1 weeks, p=<.0001) and lower maternal depression scores at 36 weeks GA (8.3 vs 11.5, p<.03) than those not completing the exam.
Demographics of participants completing NNNS assessments are listed in Table 1. The three study groups differed significantly on two maternal demographic variables. MDD+SRI group women had a mean of 0.8 more pregnancies than CON group women (p<.04); 54.0% more women in the MDD group and 25.7% more women in the MDD+SRI group were not married, compared to those in the CON group (p<.001). No demographic characteristics were related to birth outcomes or infant neurobehavioral outcomes.
Table 1.
Maternal and Newborn Characteristics by Clinical Group
| Total Sample | NonMDD | MDD | MDD+SRI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=112 | n=56 | n=20 | n=36 | |||||||
| f(%) or M (SD) | f(%) or M (SD) n miss | f(%) or M (SD) n miss | f(%) or M (SD) | F or χ2 | p< | |||||
| Maternal Characteristics | ||||||||||
| Maternal Age (Years) | 29.3 | (5.5) | 29.6 | (4.4) | 26.8 | (6.4) | 30.1 | (6.1) | 2.7 | 0.07 |
| Gravida | 2.7 | (1.9) | 2.3 | (1.2) | 3.2 | (2.5) | 3.1 | (2.1) | 3.4 | 0.04 |
| Parity | 1.1 | (1.2) | 0.8 | (1.0) | 1.2 | (1.5) | 1.3 | (1.4) | 2.1 | 0.15 |
| 1st Prenatal Visit (weeks) | 8.0 | (3.1) | 8.2 | (2.9) | 7.7 | (3.5) | 7.9 | (3.3) | 0.1 | 0.88 |
| Pre-pregnancy Weight (lbs) | 157.1 | (43.2) | 155.4 | (42.2) | 151.6 | (28.2) | 162.9 | (51.2) | 0.5 | 0.62 |
| Depression Severity | 8.0 | (6.0) | 3.8 | (2.3) | 13.5 | 4.0 | 12.0 | (6.1) | 65.7 | <.001 |
| % Drank alcohol in pregnancy | 51 | (46.79%) | 23 | (42.59%) 2 | 13 | (68.42%) 1 | 15 | (41.67%) | 4.3 | 0.12 |
| % Smoked in pregnancy | 20 | (18.35%) | 8 | (14.81%) 2 | 7 | (36.84%) 1 | 5 | (13.89%) | 5.3 | 0.07 |
| % Low SES | 11 | (9.82%) | 4 | (7.14%) | 2 | (10.00%) | 5 | (13.89%) | 1.3 | 0.57 |
| % Not Married | 38 | (34.23%) | 9 | (16.36%) 1 | 14 | (73.68%) | 15 | (41.67%) | 20.1 | <.001 |
| % Hispanic | 14 | (12.72%) | 7 | (12.73%) 1 | 5 | (26.32%) 1 | 2 | (5.56%) | 4.8 | 0.09 |
| % NonWhite | 16 | (14.55%) | 6 | (10.91%) 1 | 6 | (31.58%) 1 | 4 | (11.11%) | 5.2 | 0.07 |
| Newborn Characteristics | ||||||||||
| GA at Birth (Weeks) | 39.35 | (1.06) | 39.48 | (1.01) | 39.66 | (1.17) | 38.99 | (1.00) | 3.6 | 0.03 |
| Birth Weight (g) | 3453.25 | (473.12) | 3553.82 | (463.07) | 3466.95 | (478.59) | 3320.69 | (469.23) | 2.3 | 0.11 |
| 1 Min Apgar %<8 | 19 | (17.27%) | 6 | (10.71%) | 3 | (16.67%) 2 | 11 | (30.56%) | 5.6 | 0.03 |
| 5 Min Apgar %<9 | 9 | (8.18%) | 3 | (5.36%) | 1 | (5.56%) 2 | 6 | (16.67%) | 3.2 | 0.23 |
| % Males | 56 | (50.00%) | 28 | (50.00%) | 9 | (45.00%) | 19 | (52.78%) | 0.3 | 0.86 |
| % C-Section | 32 | (29.36%) | 13 | (23.64%) 1 | 4 | (22.22%) 2 | 15 | (41.67%) | 3.9 | 0.14 |
| % Breastfeeding at birth | 88 | (80.73%) | 47 | (87.04%) 2 | 15 | (78.95%) 1 | 26 | (72.22%) | 3.1 | 0.21 |
Continuous measures tested with one-way ANOVA, F values presented; categorical variables tested with exact tests, χ2 values presented; n indicated for variables with missing data.
Depression Severity and SRI Use
As expected, women in the MDD+SRI and MDD groups had significantly higher depression severity scores than CON group women (mean difference, CON:MDD=9.7; CON:MDD+SRI=8.3; p<.00, see Table 1). However, the MDD and MDD+SRI groups were not significantly different (p<.38).
The majority of MDD+SRI group women were taking sertraline (n=19; 52.8%), with an additional 9 (25.0%) taking fluoxetine, 4 (11.1 %) paroxetine, 3 (8.3%) escitalopram, and 1 (2.8%) venlafaxine. Mean duration of treatment during pregnancy in the MDD+SRI group was 24.3 weeks, with a range of 4–40 weeks. The average standard dose equivalent prescribed was 0.91 with a range of 0.11 to 3.33.
Birth Outcomes
Table 1 also presents newborn characteristics. Despite exclusion of participants who delivered preterm (before 37 weeks GA), MDD+SRI-exposed infants were born significantly earlier than MDD group infants (mean difference (SE) = −.68 (.17) weeks, p<.03), but only had a marginal difference from the CON group (mean difference (SE) = −.49 (.06) weeks, p<.07) groups. Significantly more infants in the MDD+SRI group had 1 minute Apgar scores <8 (30.6% compared to 10.9% in the CON group, p<.05); there was no difference in number of infants scoring <9 on the 5 minute Apgar (p=.23).
Neurobehavioral Assessment (NNNS)
There were no significant group differences in infant age in days at the time of NNNS assessment (M(SD), CON: 4.08(5.13), MDD:5.32(5.14), MDD+SRI:3.91(4.37); F=0.60, df=2,109, p<.56).
Main Effects of Exposure Group
Table 2 presents the NNNS estimated marginal means for the three exposure groups with parameter estimates (95% confidence intervals) and tests of model effects. Significant main effects for exposure group were found for attention, quality of movement, arousal, CNS stress signs, and hypertonia. Pairwise comparisons revealed that MDD group infants had lower attention scores compared to CON group infants (mean diff (SE)= −1.49 (.33), p<.001) and MDD+SRI group infants (mean diff (SE) = −1.60(.39), p<.001). MDD+SRI group infants had lower quality of movement scores and more CNS stress signs than infants in the CON (mean diff (SE) =−0.36(.13), p<.05; mean diff (SE) = .08(.02), p<.001) and MDD (mean diff (SE) = −.55 (.17), p<.005; mean diff (SE)= .07(.02), p<.001) groups. Although hypertonicity and arousal were significant in the overall model, conservative pair-wise comparisons were not significant for either summary variable (p’s >.10).
Influence of Infant Age at Assessment on NNNS outcomes
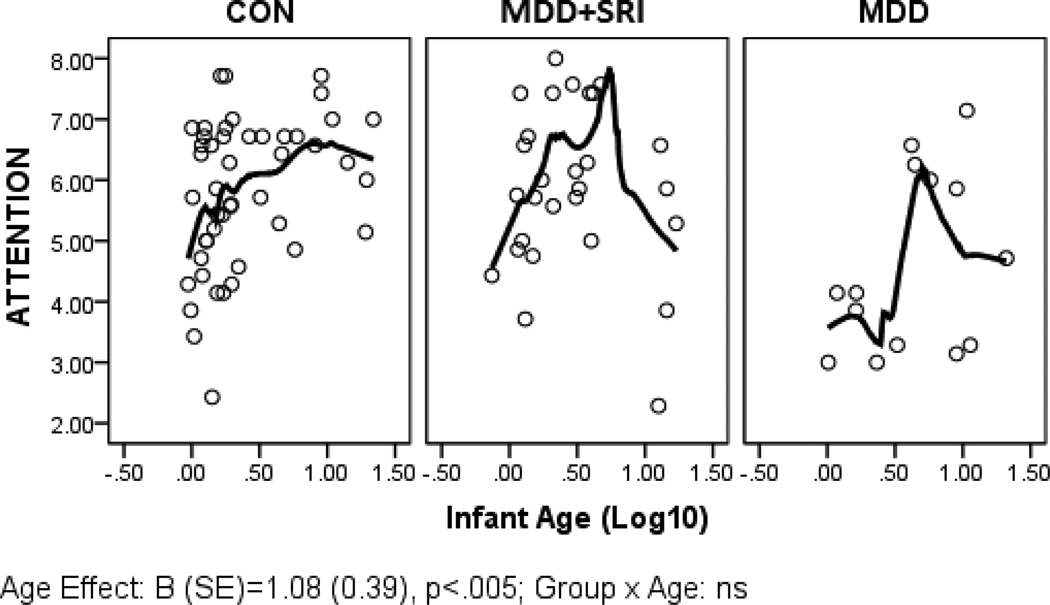
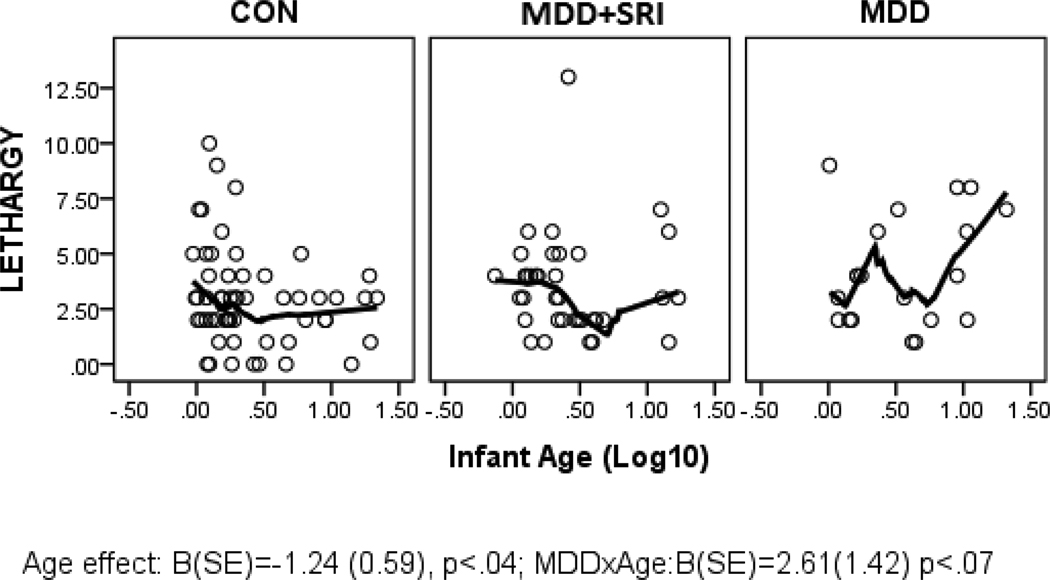
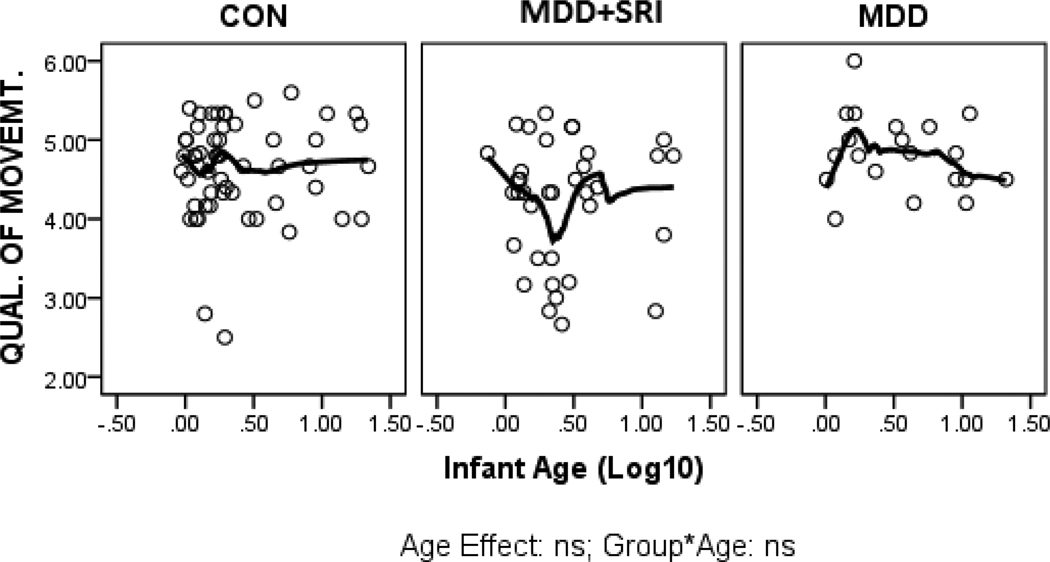
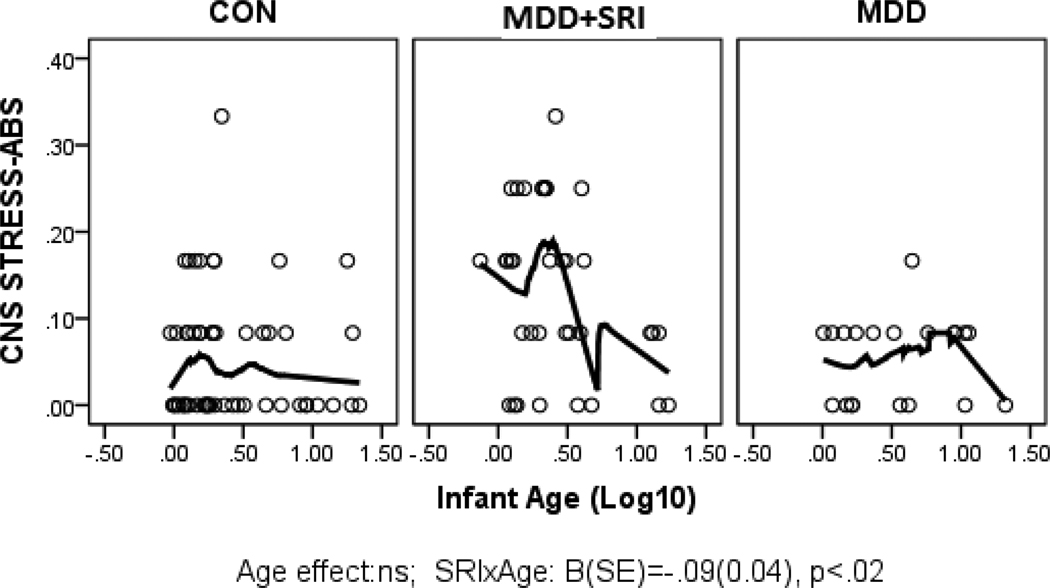
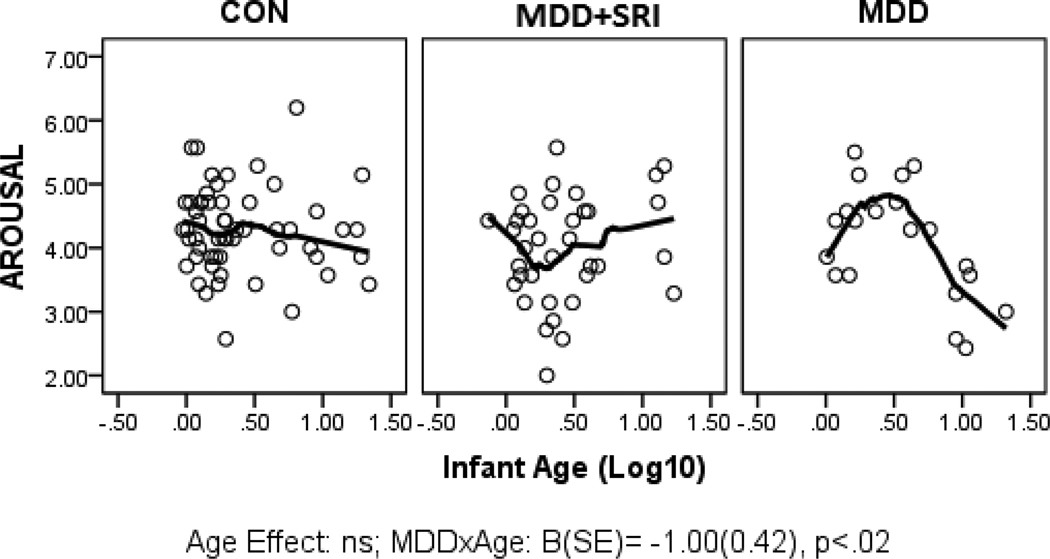
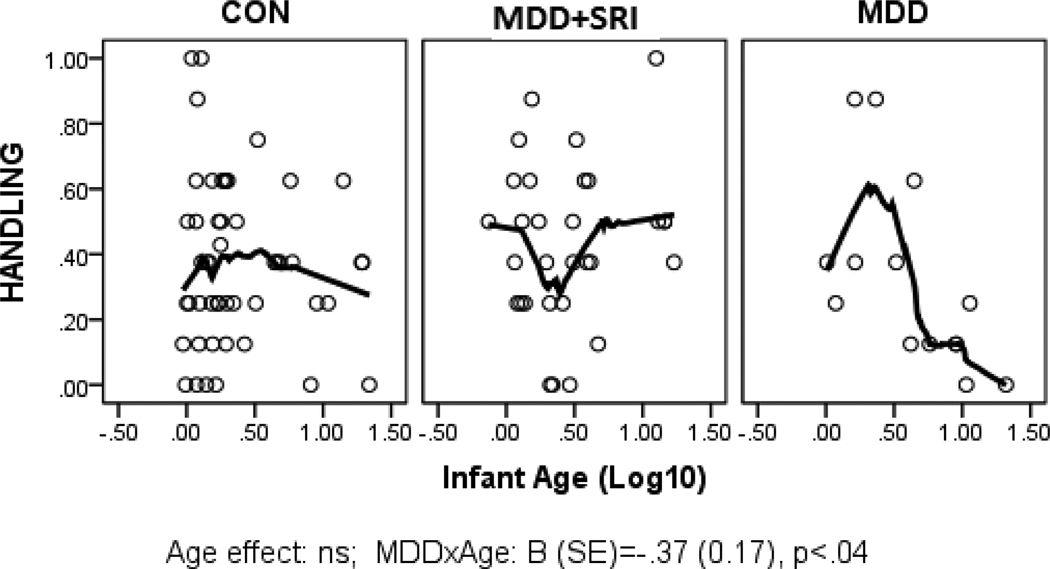
Parameter estimates from the GLZ models were examined for relationships between infant age at assessment and NNNS summary variables by exposure groups (CON*age as referent). Figures 1 through 6 present scatter plots of the relevant NNNS variables by the log-transformed infant age at assessment for each exposure group. Attention scores were higher with increasing age at assessment over all groups (Figure 1). For quality of movement, there was only a significant main effect for exposure group and no relationships with infant age (Figure 2). The number of CNS stress signs in the MDD+SRI group was lower with increasing age at assessment compared to the CON group (Figure 3). MDD group infants had lower arousal (Figure 4) and handling scores (Figure 5) with increasing age. However, while there was a decrease over all groups in lethargy scores with increasing age, the MDD group infants showed a marginally significant increase with age at assessment (Figure 6).
Figure 1.
Attention scores. Although the plots show varying relationships by group, the interactions were not significant. In addition to the exposure group main effect, the main effect of age was significant; overall attention scores increased with age at assessment.
Figures 1–6 represent the scatterplots of estimated marginal means of significant NNNS summary variables plotted against the log-transformed age at infant assessment for each medication exposure group. A Loess curve was fit to the data to examine possible nonlinear relationships.
Figure 6.
Lethargy scores; Overall, infants had lower lethargy scores with increasing age at assessment. However, there was marginal but non-significant MDD × AGE interaction, suggesting higher lethargy scores with increasing age at assessment compared to the CON group.
Figure 2.
Quality of Movement scores. Although there were significant group differences, there were no significant relationships with infant age at assessment.
Figure 3.
Number of CNS Stress Signs. Infants in the MDD+SRI group had fewer CNS stress signs with increasing age at assessment compared to the CON group.
Figure 4.
Arousal scores. Infants in the MDD group had lower arousal scores with increasing age at assessment compared to the CON group.
Figure 5.
Number of Handling maneuvers required to maintain a quiet alert state in the infant. Infants in the MDD group required less handling with increasing age at assessment compared to the CON group.
Secondary analyses of depression severity and SRI use
Separate GLZ models examined relationships between NNNS variables and mean HRSD scores during pregnancy for all exposure groups (CON as referent with GA and age covariates). Only the handling summary score was significant; overall handling scores increased as HRSD scores increased (b=0.03, CI=0.003, 0.06, p=.03), however, within the MDD group, the relationship was negative (b=−.03, CI=0.10, −.01, p=.01).
Within the MDD+SRI group, relationships were examined for NNNS variables and duration of SRI use and mean standard dose of SRI. No significant relationships resulted (p’s>.05).
DISCUSSION
These findings show a different neurobehavioral profile for infants exposed to maternal MDD treated with SRIs from those exposed to MDD without SRI treatment. As expected, MDD+SRI-exposed infants had lower quality of movement scores, more hypertonia, and a higher number of CNS stress signs on the NNNS examination than infants in the MDD and CON groups. This profile of results suggests that MDD+SRI-exposed newborns have more startles, tremors, back-arching, and hypertonic reflexes than non-exposed newborns. Confidence intervals were similarly small across groups and suggest a small to moderate effect of MDD+SRI on quality of movement and CNS-stress. In contrast, MDD-exposed infants were not different from infants in the CON group for quality of movement or stress-abstinence signs, but had significantly lower attention scores than infants in the CON and MDD+SRI groups. Previous studies using the Brazelton Neurobehavior Scale, from which the NNNS attention score was original derived, to examine effects of maternal depression on neonates have consistently reported approximately a 1 point lower attention score for MDD-exposed infants compared to controls.[67; 68] In this study, infants in the MDD group had at least a 1.5 lower score than those in the CON and MDD+SRI groups. According to published reference values for the NNNS summary scales for infants at 3 and 30 days after birth, mean attention scores for infants in the current sample were lower than 75% of previously studied infants in normative groups. [62; 69] These findings contribute new information suggesting that while these findings agree with previous studies of neurobehavior in SRI-exposed newborns,[70; 71] prenatal MDD exposure without SRI treatment may have a different risk profile that requires further study.
The varying ages at the time of the assessment provided an opportunity to examine potential relationships between infant age (days since birth) and NNNS variables between groups. There were fewer CNS stress signs with increasing age of assessment in the MDD+SRI-exposed infants, and although limited by cross sectional analyses, the data are consistent with previous database and cohort studies that reported more startles and tremors only in the first two weeks post-birth in MDD+SRI-exposed infants than those not exposed.[34; 72; 73] There were no age related relationships for quality of movement, suggesting that while CNS stress related behaviors in SRI-exposed newborns may be transient, other indicators of less optimal motor development in the first postpartum month may be different with increasing time from birth. Data from previous long term studies suggest that subtle motor effects may persist beyond the first year of life, including less optimal fine motor skills [74] and slower gross-motor development [75] in prenatal SRI exposed compared to non-SRI exposed children.
MDD-exposed infants had lower arousal and handling scores and marginally higher lethargy scores with increasing age at assessment. This pattern suggests that although irritability and the need for external soothing may be lower with increasing age from birth, this may not reflect a more optimal progression as higher lethargy scores may be indicative of more depressed and under-aroused infants. Although the effect size of this finding is small, previous studies also reported depressed neurobehavior in newborns of mothers with prenatal MDD.[29; 67; 68] Longitudinal studies with systematic repeated measures will be critical to further examine these neurobehavioral profiles and their trajectory over age as well as their ability to predict long term outcomes.
The sample size was not large enough to adequately compare rate of pre-term birth across groups. Therefore, we limited this cohort to infants who were born after 37 weeks GA. Even within the full-term infants, MDD+SRI-exposed infants were born at significantly younger GA. Further, more MDD+SRI-exposed infants had 1 minute Apgar scores below 8 than infants in both CON and MDD groups.
Strengths and Limitations
A major strength of this study was the separation of exposure definitions beyond SRI vs. CON to include assessment of the underlying maternal disease state (MDD) assessed by structured interview. The majority of previous studies have not included a group with pharmacologically untreated maternal depression when examining effects of prenatal SRI exposure. This study included pregnant women taking SRIs who had MDD in various degrees of remission. It is not possible to completely separate out the effects of MDD vs SRI exposure, and we emphasize that the “SRI effects” reported in this paper are indeed effects of SRI in addition to MDD. Larger samples will be required to adequately examine potential effects on infant outcomes based on pharmacological and non-pharmacological treatment response.
Another major strength is use of a well-validated, standardized infant assessment to differentiate a broad range of neurobehavioral outcomes related to MDD from SRI exposure; results of which can be mapped onto standardized prenatal assessments of fetuses exposed to MDD and SRIs.[76] The same type of assessment can be used to characterize infant neurobehavioral profiles over time and has been shown to predict medical and behavioral outcomes through age four.[65] A standardized tool such as the NNNS provides a systematic and comprehensive assessment of newborn neurobehavior that may help tease apart a possible withdrawal syndrome versus signs analogous to side effects with repeated assessment throughout and beyond the first month post-birth. The effects sizes in this study were small to moderate; the clinical significance of these findings to later development is unknown.
Demographic differences are likely to occur in a study of depression and medication use and this sample was also characterized by such differences. However, importantly, these variables were examined and did not contribute to the NNNS neurobehavioral outcomes. This study did not obtain objective confirmation of prenatal medication levels and no objective confirmation was obtained regarding lack of illicit drug use. Medication use was obtained prospectively from the second trimester of pregnancy in a detailed, time line format.
Implications of the findings
Our findings provide evidence that both maternal MDD and SRI treatment during pregnancy result in risks for infants in the first month of life, with neurobehavioral profiles that vary depending upon the type of exposure. While MDD+SRI effects on the variables of CNS-stress and quality of movement appear to be small to moderate, the effect of MDD on attention scores appears to be moderate, with those infants comparable to only 25% of other samples tested at similar ages. Infants in the MDD+SRI group were similar to CON infants, suggesting that SRI treatment of MDD may contribute to more optimal infant attention in the first month of life. Further study will be needed to replicate these findings and explore other factors that might influence these outcomes. Our data also suggest that MDD+SRI exposure risks are not different based on depression severity or on timing or length of SRI exposure, although the study may have lacked sufficient power to adequately address these relationships. The long term significance of these findings is yet to be examined.
Long term outcomes from SRI exposure are difficult to determine due to the potential cumulative and additive effects of MDD and SRI exposure. Indeed, a confound exists in that prenatal depression, stress, and anxiety have all been associated with preterm birth and lower birth weight as well as acute and long term neurobehavioral sequelae. Findings from the few available long term studies were limited to less optimal motor development before 2 years of age. [74; 75; 77] Data from the current study also suggest less optimal motor quality in SRI-exposed infants that may not be limited to the first few weeks of life. It is important to note that these differences were reported to be within normal limits and were no longer significant after 19 months of age.
While long term effects of SRI exposure are just beginning to be explored, many studies have investigated effects of maternal MDD on child development. The long-term effects of prenatal MDD are highly confounded by postpartum and concurrent depression, however, when controlling for these factors, prenatal depression remains related to later developmental delays[78] and depression in adolescence.[79] Conversely, a subsequent lack of maternal MDD in the postpartum or childhood years is associated with fewer problems in adolescence,[80; 81] and at least one study provided evidence that psychotropic treatment of MDD during pregnancy compared to no medication treatment was associated with more attenuated cortisol increases in infants following mild stress.[82] The current study is the first step toward a better understanding of MDD treatment effects on infant and child outcomes.
Acknowledgments
Katherine Halloran, M.A., infant assessments and manuscript review; Mary Roberts, M.S., Beth Hott, B.A., Matthew Hinckley, B.A., and Marion Young, Ph.D. for data processing assistance.
Disclaimers:
This work was supported by The National Institutes of Health, grant K23MH65479 and R01 MH078033 (Salisbury), K23MH066402 (Battle), and K23MH065443 (Stroud) Bethesda, MD.; Dr. Wisner’s time was supported by National Institutes of Health R01MH60335. Drs. Stroud and Salisbury received grant support from the Flight Attendant Medical Research Institute, Clinical Innovator Award. Dr. Wisner served on the Advisory Committee "Neuroscience - Comorbidities across conditions" for Eli Lilly Corp., and received a donation of placebo estradiol patches from Novogyne for an NIMH funded randomized clinical trial. Dr. Pearlstein received grant support in the form of clinical supplies from Pfizer.
Abbreviations
- MDD
major depressive disorder
- SRI
Serotonin Reuptake Inhibitors
- CON
Control
- SSRI
Selective Serotonin Reuptake Inhibitors
- SNRI
Selective Norepinephrine Reuptake Inhibitors
- GA
Gestational Age
- NICU
Neonatal Intensive Care Unit
- NNNS
NICU Network Neurobehavioral Scale
- HRSD
Hamilton Rating Scale for Depression
- GLM
General Linear Model
- DSM-IV-R
Diagnostic and Statistical Manual of Psychiatric Disorders, Version IV-R
- SCID
Structured Clinical Interview for the DSM
- TLFB
Timeline Follow-Back
- CNS
Central Nervous System
REFERENCES
- 1.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 2.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 3.Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54(Suppl 1)(5):24–27. doi: 10.1016/j.metabol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Shaffery J, Hoffmann R, Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9(1):82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- 5.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, Kuhn C, Gonzalez-Garcia A. Prenatal maternal biochemistry predicts neonatal biochemistry. Int J Neurosci. 2004;114(8):933–945. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- 7.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, Schanberg S, Kuhn C. Prenatal dopamine and neonatal behavior and biochemistry. Infant Behav Dev. 2008;31(4):590–593. doi: 10.1016/j.infbeh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandman CA, Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Belman J, Porto M, Murata Y, Garite TJ, Crinella FM. Psychobiological influences of stress and HPA regulation on the human fetus and infant birth outcomes. Annuals of the New York Academy of Science. 1994;739:198–210. doi: 10.1111/j.1749-6632.1994.tb19822.x. [DOI] [PubMed] [Google Scholar]
- 9.Wadhwa PD, et al. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86(1):104–109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. British Medical Journal. 1999;318(7177):153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF. Dev Psychobiol. 2011. Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: Implications for fetal programming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer LT, Garber R, Kliegman R. Neurobehavioral sequelae of fetal cocaine exposure. Journal of Pediatrics. 1991;119(4):667–672. doi: 10.1016/s0022-3476(05)82426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum Dev. 2004;80(1):31–42. doi: 10.1016/j.earlhumdev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Grassi-Zucconi G. Effect of paradoxical sleep deprivation on DNA synthesis in fetal rat brain. International Journal of Developmental Neuroscience. 1984;2(6):585–590. doi: 10.1016/0736-5748(84)90036-4. [DOI] [PubMed] [Google Scholar]
- 16.Altman J, Das GD, Sudarshan K. The influence of nutrition on neural and behavioral development. I. Critical review of some data on the growth of the body and the brain following dietary deprivation during gestation and lactation. Dev Psychobiol. 1970;3:281–301. doi: 10.1002/dev.420030408. [DOI] [PubMed] [Google Scholar]
- 17.Welder AA, Grant R, Kutschke RL, Anthony M, Bradlaw J, Acosta D. Effects of maternal calorie-restricted diet on development of the foetal heart, as evaluated in primary cultures of rat myocardial cells. Food Chem Toxicol. 1991;29(7):445–452. doi: 10.1016/0278-6915(91)90089-p. [DOI] [PubMed] [Google Scholar]
- 18.Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093–1099. doi: 10.1016/0895-4356(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 19.Grella CE. Services for perinatal women with substance abuse and mental health disorders: the unmet need. J Psychoactive Drugs. 1997;29(1):67–78. doi: 10.1080/02791072.1997.10400171. [DOI] [PubMed] [Google Scholar]
- 20.Zaid A, Fullerton JT, Moore T. Factors affecting access to prenatal care for U.S./Mexico border-dwelling Hispanic women. J Nurse Midwifery. 1996;41(4):277–284. doi: 10.1016/0091-2182(96)00030-4. [DOI] [PubMed] [Google Scholar]
- 21.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 22.Van Dijk AE, Van Eijsden M, Stronks K, Gemke RJ, Vrijkotte TG. Maternal depressive symptoms, serum folate status, and pregnancy outcome: results of the Amsterdam Born Children and their Development study. Am J Obstet Gynecol. 2010;203(6):563, e1–e7. doi: 10.1016/j.ajog.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT. Major Depression and Antidepressant Treatment: Impact on Pregnancy and Neonatal Outcomes. Am J Psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Thurin M, Trinchieri G, Wang E, Wigginton J, Chaussabel D, et al. A systematic approach to biomarker discovery; preamble to "the iSBTc-FDA taskforce on immunotherapy biomarkers". J Transl Med. 2008;6:81. doi: 10.1186/1479-5876-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20(3):189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 27.Salisbury AL, Lester BM, Seifer R, Lagasse L, Bauer CR, Shankaran S, Bada H, Wright L, Liu J, Poole K. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29(3):331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. Journal of Developmental and Behavioral Pediatrics. 1990;11(4):190–194. [PubMed] [Google Scholar]
- 29.Jones NA, Field T, Fox N, Davalos M, Lundy B, GHart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior & Development. 1998;21(3):537–541. [Google Scholar]
- 30.Armstrong KL, O'Donnell H, McCallum R, Dadds M. Childhood sleep problems: association with prenatal factors and maternal distress/depression. Journal of Paediatrics and Child Health. 1998;34(3):263–266. doi: 10.1046/j.1440-1754.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 31.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194, e1–e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Battle CL, Zlotnick C, Miller IW, Pearlstein T, Howard M. Clinical characteristics of perinatal psychiatric patients: a chart review study. J Nerv Ment Dis. 2006;194(5):369–377. doi: 10.1097/01.nmd.0000217833.49686.c0. [DOI] [PubMed] [Google Scholar]
- 33.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60(7):720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 35.Anderson GM. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanisms of action. Int J Dev Neurosci. 2004;22(5–6):397–404. doi: 10.1016/j.ijdevneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 37.Boucher N, Bairam A, Beaulac-Baillargeon L. A new look at the neonate's clinical presentation after in utero exposure to antidepressants in late pregnancy. J Clin Psychopharmacol. 2008;28(3):334–339. doi: 10.1097/JCP.0b013e318173aa2e. [DOI] [PubMed] [Google Scholar]
- 38.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 39.Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365(9458):482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 40.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160(2):173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- 41.Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479. doi: 10.1111/j.1600-0447.2009.01490.x. [DOI] [PubMed] [Google Scholar]
- 42.Oberlander TF, Eckstein Grunau R, Fitzgerald C, Ellwood AL, Misri S, Rurak D, Riggs KW. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002;51(4):443–453. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115(2):411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- 44.Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008;84(10):689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einarson A, Choi J, Einarson TR, Koren G. Adverse effects of antidepressant use in pregnancy: an evaluation of fetal growth and preterm birth. Depress Anxiety. 2010;27(1):35–38. doi: 10.1002/da.20598. [DOI] [PubMed] [Google Scholar]
- 46.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry. 2008;192(5):338–343. doi: 10.1192/bjp.bp.107.037101. [DOI] [PubMed] [Google Scholar]
- 47.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055–2061. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 48.Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman AK, Cook L, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study.[see comment] Journal of the American Medical Association. 1998;279(8):609–610. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 49.Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Women Ment Health. 2004;7(3):193–200. doi: 10.1007/s00737-004-0057-5. [DOI] [PubMed] [Google Scholar]
- 50.Cohen LS, Heller VL, Bailey JW, Grush L, Ablon JS, Bouffard SM. Birth outcomes following prenatal exposure to fluoxetine. Biol Psychiatry. 2000;48(10):996–1000. doi: 10.1016/s0006-3223(00)00877-5. [DOI] [PubMed] [Google Scholar]
- 51.McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461–475. doi: 10.1016/0890-6238(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 52.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 53.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 54.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 55.Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, et al. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse. 2001;36(3):313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- 56.Dum M, Sobell LC, Sobell MB, Heinecke N, Voluse A, Johnson K. A Quick Drinking Screen for identifying women at risk for an alcohol-exposed pregnancy. Addictive behaviors. 2009;34(9):714–716. doi: 10.1016/j.addbeh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS care. 2004;16(2):199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- 58.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, Marzani-Nissen G. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug and alcohol dependence. 2011;116(1–3):177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottfried AW. Measures of socioeconomic status in child development research: Data and recommendations. Merrill-Palmer-Quarterly. 1985;31(1):85–92. [Google Scholar]
- 60.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):634–640. [PubMed] [Google Scholar]
- 61.Maza PL, Wright LL, Bauer CR, Shankaran S, Bada HS, Lester B, Krause-Steinrauf H, Smeriglio VL, Bowler A, Katsikiotis V. Maternal Lifestyles Study (MLS). Caretaking environment and stability of substance-exposed infants at one month corrected age. Ann N Y Acad Sci. 1998;846:358–361. [PubMed] [Google Scholar]
- 62.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. 2004;113(3 Pt 2):668–675. [PubMed] [Google Scholar]
- 63.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 64.Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lagasse LL, Lester B. Maternal smoking during pregnancy and newborn neurobehavior: effects at 10 to 27 days. J Pediatr. 2009;154(1):10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, Bauer C, Shankaran S, Bada H. Neonatal Neurobehavior Predicts Medical and Behavioral Outcome. Pediatrics. 2010;125(1):e90–e98. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salisbury A, Lester B, Seifer R, Lagasse L, Bauer C, Shankaran S, Bada H, Wright L, Liu J, Poole K. Prenatal cocaine use and maternal depression: Effects on infant neurobehavior. Neurotoxicology and Teratology. 2007;29(3):331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Field T. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27(2):216–229. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Lundy B, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, Schanberg S, Kuhn C. Prenatal Depression Effects on Neonates. Infant Behav Dev. 1999;22(1):119–129. [Google Scholar]
- 69.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):676–678. [PubMed] [Google Scholar]
- 70.Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Forman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42(3):95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- 71.Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113(2):368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- 72.Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293(19):2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 73.Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237. doi: 10.4088/jcp.v65n0214. [DOI] [PubMed] [Google Scholar]
- 74.Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 75.Pedersen LH, Henriksen TB, Olsen J. Fetal Exposure to Antidepressants and Normal Milestone Development at 6 and 19 Months of Age. Pediatrics. 2010;125(3):e600, e608. doi: 10.1542/peds.2008-3655. [DOI] [PubMed] [Google Scholar]
- 76.Salisbury AL, Fallone MD, Lester B. Neurobehavioral assessment from fetus to infant: The NICU network neurobehavioral scale and the fetal neurobehavior coding scale. Ment Retard Dev Disabil Res Rev. 2005;11(1):14–20. doi: 10.1002/mrdd.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010;27(7):675–686. doi: 10.1002/da.20706. [DOI] [PubMed] [Google Scholar]
- 78.Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043–1051. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 79.Pawlby S, Hay DF, Sharp D, Waters CS, O'Keane V. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J Affect Disord. 2009;113(3):236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 80.Hay DF, Pawlby S, Waters CS, Sharp D. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J Child Psychol Psychiatry. 2008;49(10):1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 81.Pilowsky DJ, Wickramaratne P, Talati A, Tang M, Hughes CW, Garber J, Malloy E, King C, Cerda G, Sood AB, et al. Children of depressed mothers 1 year after the initiation of maternal treatment: findings from the STAR*D-Child Study. Am J Psychiatry. 2008;165(9):1136–1147. doi: 10.1176/appi.ajp.2008.07081286. [DOI] [PubMed] [Google Scholar]
- 82.Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 2008;49(10):1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]