Abstract
Background
Twin studies demonstrate that measures of alcohol consumption (AC) show evidence of genetic influence, suggesting they may be useful in gene identification efforts. The extent to which these phenotypes will be informative in identifying susceptibility genes involved in alcohol dependence depends on the extent to which genetic influences are shared across measures of AC and alcohol problems. Previous studies have demonstrated that AC reported for the period of heaviest lifetime drinking shows a large degree of genetic overlap with alcohol dependence; however, many studies with genetic material assess current AC. Further, there are many different aspects of AC that can be assessed (e.g., frequency of use, quantity of use, and frequency of intoxication).
Methods
Here, we use data from 2 large, independent, population-based twin samples, Finn-Twin 16 and The Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, to examine the extent to which genetic influences are shared across many different measures of AC and alcohol problems.
Results
Genetic correlations across current AC measures and alcohol problems were high across both samples. However, both samples suggest a complex genetic architecture with many different genetic factors influencing various aspects of current AC and problems.
Conclusions
These results suggest that careful attention must be paid to the phenotype in efforts to “replicate” genetic effects across samples or combine samples for meta-analyses of genetic effects influencing susceptibility to alcohol-related outcomes.
Keywords: Alcohol Consumption, Alcohol Dependence, Gene Finding, Genetic Influence, Twin Studies
Alcohol dependence is under substantial genetic influence (Dick et al., 2009), and twin studies demonstrate that measures of alcohol consumption (AC) are under significant genetic influence as well (Dick and Bierut, 2006; Goldman, 1993; Prescott and Kendler, 1999; Rose, 1998). That evidence has fostered studies investigating the extent to which the same genetic factors underlie patterns of consumption and the development of problems. Data from the Australian twin registry indicated moderate correlations (r = 0.42 for women and r = 0.45 for men) between genetic influences on weekly AC and lifetime alcohol problems and between heavy drinking and alcohol dependence (r = 0.63) (Heath and Martin, 1994). More recently, Grant and colleagues (2009) found a genetic correlation of 0.97 between a composite AC factor score, comprised of drinking measures from the period of heaviest use, and alcohol dependence symptoms. Similarly, Kendler and colleagues (2010), using data from the Virginia Twin Study of Adult Psychiatric and Substance Use Disorders, found complete overlap between the genetic risk for alcohol dependence and 4 measures of AC at the time of heaviest intake in women; in men, the consumption measures captured 85% of the genetic risk for dependence. Both studies concluded that the high genetic overlap between consumption and alcohol dependence suggests that continuous consumption measures may be useful in the discovery of genes contributing to dependence risk.
The extent to which genetic influences on alcohol dependence are shared with genetic influences on the measures of AC has important implications for gene identification efforts. It is more practical to collect information on AC from large samples of individuals than to recruit alcohol-dependent probands and appropriate controls and assess psychiatric diagnoses. Measures of AC also have attractive statistical properties because analyzing quantitative traits can improve power in association analyses (Agrawal et al., 2009). While a small number of studies are under way with the express purpose of identifying genes involved in alcohol dependence (Edenberg et al., 2005; Prescott et al., 2005), many projects with genetic material have collected data on AC, making it possible to use existing data sets for gene identification, replication, and/or meta-analyses. However, the relevance of these findings for understanding predispositions to develop alcohol-related problems hinges on the extent to which genes associated with measures of AC also relate to alcohol problems.
One critical aspect that has not been widely addressed in this burgeoning literature is the fact that there are many different ways to assess “AC,” reflecting the many different aspects and facets of drinking patterns. For example, in the studies reviewed above, measures of AC included frequency (weekly and annually), quantity by frequency, maximum drinks in a 24-hour period, frequency of heavy drinking (5+ drinks), and frequency of intoxication. The most recent studies (Grant et al., 2009; Kendler et al., 2010) addressing genetic overlap have used measures of AC at the heaviest point of drinking. However, many studies assess current AC, rather than lifetime consumption patterns. Here, we use data from 2 twin studies to conduct an exploratory set of analyses examining the extent to which different measures of past-year AC share genetic overlap with various indices of alcohol problems. We test the extent to which genetic influences are shared across different measures of consumption and between these different consumption measures and measures of alcohol-related problems.
MATERIALS AND METHODS
FinnTwin16
FinnTwin16 (FT16) is a population-based study consisting of 5 consecutive birth cohorts of Finnish twins. All twins were identified through Finland’s Population Register Center, permitting exhaustive and unbiased ascertainment. Zygosity was determined using a well-validated questionnaire completed by both co-twins at the baseline, as described elsewhere (Kaprio et al., 1991). FT16 consists of twins born 1975 to 1979 (Kaprio et al., 2002). The 5 birth cohorts contained 3,065 families of twins in which both twins were living and residing in Finland at the age of 16. Details about data collection have previously been published (Kaprio, 2006; Kaprio et al., 2002). Briefly, 4 waves of postal questionnaires were completed at ages 16, 17, 18.5, and as young adults. Here, we analyze data from the most recent questionnaire and focus on AC and alcohol problems in adulthood. The average age for the respondent twins at this assessment was 24.4 years (SD = 1.50, range 22.8 to 27.2), with a response rate of 88.1%. For ease of presentation, this assessment is referred to as age 25 throughout this paper. Parallel to current practice in gene identification efforts for alcohol dependence, only individuals who had evidence of alcohol exposure were included in twin analyses, so that genetic and environmental influences on the decision to initiate alcohol are not confounded with genetic and environmental influences on AC or problems. After exclusion of individuals who had not been exposed to alcohol, data were available for 685 complete pairs of twin brothers (287 monozygotic [MZ] and 398 dizygotic [DZ]) and 693 complete pairs of twin sisters (378 MZ and 315 DZ).
Measures
Frequency was assessed with the following question: “At the present, how often do you drink alcohol?” Response options included: (1) I don’t use alcohol; (2) Once or year or less frequently; (3) 3 to 4 times a year; (4) About once in 2 months; (5) About once a month; (6) A couple times a month; (7) About once a week; (8) About twice a week; (9) Daily. Note that responses were reverse-coded from the actual order asked so that higher numbers reflected more drinking across all items used in analyses.
Frequency × quantity was a composite of 2 items; the frequency of reported alcohol use in the past 28 days multiplied by the quantity of drinks (drinks defined as 1 beer, 1 glass of wine, or 1 mixed drink containing hard liquor) consumed per drinking day during the past 28 days. Because this measure was highly skewed, with over representation of those who drank on less than 1 occasion in the past 28 days, we log-transformed this variable.
Frequency of heavy drinking was assessed with the following question: “At the present, how often do you within 1 occasion use more than 5 bottles of beer, or more than a bottle of wine, or more than half a bottle of hard liquor?” Response options included: (1) I don’t use alcohol; (2) Never; (3) Once or year or less frequently; (4) 3 to 4 times a year; (5) About once in 2 months; (6) About once a month; (7) A couple times a month; (8) About once a week; (9) About twice a week; (10) Daily.
Frequency of intoxication was assessed with the following question: “At the present, how often do you use alcohol to get drunk?” Response options included: (1) I don’t use alcohol/Never; (2) Once or year or less frequently; (3) 3 to 4 times a year; (4) About once in 2 months; (5) About once a month; (6) A couple times a month; (7) About once a week; (8) About twice a week; (9) Daily.
Maximum drinks (Max Drinks) was the maximum number of drinks twins reported ever consuming in a 24-hour period, with 1 drink defined as 1 beer, 1 glass of wine, or 1 mixed drink containing hard liquor. Responses ranged from 1 to 100 (mean = 16.49, SD = 9.46).
The Malmo-modified Michigan Alcoholism Screening Test (Mm-MAST; Kristenson and Trell, 1982) is a 9-item self-report scale of current drinking patterns and problems designed for application in Nordic cultures (Seppa et al., 1999). Representative items include taking a drink before going to a party, increased tolerance over time, and having difficulty not drinking more than one’s friends. Our scale added 2 items more directly overlapping DSM diagnostic criteria: finding it hard to stop after having had a drink and feeling that someone close to you thinks you should drink less. Each of these questions was asked of “current and past drinking habits” and had a “Yes” or “No” response option. For those twins who answered at least 9 of the 11 items, we calculated a Mm-MAST score by taking the average response (yes/no) across the number of items answered. This scoring method permitted us to retain participants who completed the majority of the items but who may have neglected to answer a few of them.
Rutgers Alcohol Problem Index (RAPI) is a reliable 22-item scale designed to assess problematic drinking (White and Labouvie, 1989). The RAPI contains items assessing dependence, withdrawal, blackouts, neglect of responsibilities in several domains, shame and/or embarrassment to self or others, and inappropriate behaviors such as fighting. Individuals indicated how often each consequence of alcohol use had happened in the past 12 months using the following 5 response options: (1) Never/I don’t use alcohol, (2) Rarely, (3) Sometimes, or (4) Quite often. For subjects who answered at least 18 of the 22 items, we calculated a RAPI severity score by taking the average response (1 to 4) across the number of items answered.
Because of the limitations of the genetic statistical analysis program, we were unable to simultaneously analyze both continuous and ordinal variables; thus, we collapsed the drinking measures into 4 categories (once individuals who had indicated that they do not use alcohol were removed). An alcoholic drink was defined as “1 bottle of beer, 1 glass of wine, or 1 shot of liquor” across all questions. For drinking frequency, frequency of heavy drinking, and frequency of intoxication, these categories were (1) About 1 to 4 times a year, (2) About once in 2 months, (3) About 1 to 2 times a month, (4) About 1 to 2 times a week. Max Drinks, the Mm-MAST, and RAPI scores were each collapsed into 5 levels using the SAS System’s univariate quintiles procedure, where the first level contains those individuals lowest on problem drinking and the fifth level contains those highest on problem drinking (SAS, 2000–2004).
Virginia Adult Twin Study of Psychiatric and Substance Use Disorders
Participants in this study derive from 2 interrelated studies of Caucasian same-sex twin pairs who participated in Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (Kendler, 2006). All subjects for the VATSPSUD were ascertained from the population-based Virginia Twin Registry formed from a systematic review of birth certificates in the Commonwealth of Virginia. Female–female twin pairs (FF), from birth years 1934 to 1974, became eligible if both members previously responded to a mailed questionnaire in 1987 to 1988, the response rate to which was approximately 64%. Zygosity was determined by discriminate function analyses using standard twin questions validated against DNA genotyping in 496 pairs (Kendler and Prescott, 1999). All female–female data on AC and alcohol dependence used in this report were collected at the fourth wave of interviews (FF4), conducted in 1995 to 1997. For this wave, we succeeded in interviewing 85% of the sample who had responded to the previous questionnaire. Data on the male–male (MM) pairs, birth years 1940 to 1974, came from a sample initially ascertained directly from registry records, which contained all twin births. The first interview (MM1) was completed largely by phone in 1993 to 1996 and obtained a 72% response rate. This was followed by a second wave of interviews (MM2), conducted in 1994 to 1998 with a follow-up response rate of 83%. Data on AC and alcohol dependence were collected at both of these waves. We used the measures of drink frequency, regular quantity, maximum quantity, and alcohol dependence from MM1 because of the larger sample size, but frequency of intoxication was only assessed at MM2 and so those data were used. The mean (SD) age of the twins was 36.3 (8.2) at the FF4 interview and 35.5 (9.1) at the MM1 interview. Note that the FT16 sample is age standardized (~age 25) and differs in this sense from the wide age range covered in the VATSPSUD sample. The VATSPSUD alcohol section began by asking about any lifetime alcohol use. In our FF4, MM1, and MM2 interviews, 8.0, 5.0, and 4.3% of participants, respectively, denied any lifetime alcohol use and were excluded from all subsequent analyses. After excluding abstainers, the total sample size on which we had data for AC and alcohol dependence was 5,073 and consisted of 1,766 complete pairs and 893 twins whose co-twins did not participate. By zygosity, the numbers of complete pairs were MZ male twins 613, DZ male 435, MZ female 440, and DZ female 278.
Measures
Frequency was assessed by the following question: “In a typical month over the last year, how often do you drink alcohol?” Response options included: (1) 1 to 3, (2) 4 to 9, (3) 10 to 15, (4) 16 to 27, and (5) 28 to 30 days per month.
Regular quantity was assessed with the following question on drinking habits in the past year: “On those days when you drank, how many drinks did you usually have in a day?” Response options included: (1) 1 to 2, (2) 3, (3) 4 to 5, (4) 6 to 9, and (5) ≥9 drinks/day.
Frequency of intoxication was assessed with the following question: “During the past year, how often did you use alcohol to get drunk?” Response options were: (1) 1 to 2, (2) 3 to 5, (3) 6 to 7, (4) 8, and (5) 9 to 11 times/year.
Maximum drinks was assessed with the following question: “What is the largest number of drinks you had on any single day during the past year?” Response options were: (1) 1 to 5, (2) 6 to 9, (3) 10 to 12, (4)13 to 20, and (5) ≥21 drinks/day.
DSM-IV alcohol dependence symptoms were assessed for lifetime in the interviews based on 7 DSM-IV criteria (American Psychological Association, 1994), and was the only VATSPSUD measure that did not reflect current alcohol problems.
Multivariate Cholesky
A multivariate Cholesky model was used to estimate genetic and environmental influences across the measures of consumption/problem drinking (Neale and Cardon, 1992). Analyses were conducted separately using the measures available in each sample. The Cholesky model allows us to evaluate (1) the magnitude of genetic and environmental influences on each phenotype and (2) the extent to which these influences contribute to the covariation between the phenotypes. Phenotypic variance was decomposed into 3 components: variance because of additive genetic factors (a2); variance because of shared environmental factors (c2); and variance because of nonshared environmental, or individual-specific, factors (e2). Calculation of variance accounted for by each of these factors is performed by comparing MZ twin correlations to DZ twin correlations. Genetic influences correlate 1.0 between MZ twins, who share all of their genetic variation identical-by-descent, and 0.5 between DZ twins, who share, on average, 50% of their segregating genes, as do ordinary siblings. Common/shared environmental effects, as defined in biometrical twin modeling, refer to all environmental influences that make siblings more similar to one another. By definition, these influences correlate 1.0 between both MZ and DZ twins. Unique/nonshared environmental influences are uncorrelated between co-twins and have the effect of decreasing the covariance between siblings. When data on multiple phenotypes are available, these models can be extended to evaluate the extent to which genetic and environmental contributions to the disorders are shared. This is calculated by comparing cross-twin, cross-trait correlations, with the logic extended from the basic twin model that comparison of the cross-twin, cross-trait correlations between MZs and DZs provides information about the extent to which a2, c2, and e2 contribute to the phenotypic correlations between traits.
The full model (depicted in Fig. 1 for FT16 and Fig. 2 for the VATSPSUD) calculated variance components separately by sex. Thresholds for each variable were adjusted by age to account for the variability in age in the samples. Additional models were tested to evaluate goodness-of-fit in which estimates of the variance components were constrained to be equal across sex. Estimates were obtained from observed twin data using maximum likelihood estimation in the software program Mx (Neale et al., 1999). Model fit was evaluated by Akaike’s Information Criterion (AIC), and the probability (p) value associated with the chi-squared statistic. Lower AIC values indicate an optimal balance between explanatory power and parsimony. Additionally, nonsignificant chisquared values (p > 0.05) indicate a good fit. We compared nested alternative models by the change in chi-square between models, which is used to evaluate the significance of dropping parameters. A significant change in chi-squared (p < 0.05) for the difference in degrees of freedom of the models indicates that the model with fewer degrees of freedom should be adopted, because the gain in degrees of freedom of the alternate model caused a significant decrease in fit. Missing data were handled by reading raw data into Mx and fitting to the observed and unobserved data vectors using full-information maximum-likelihood estimation.
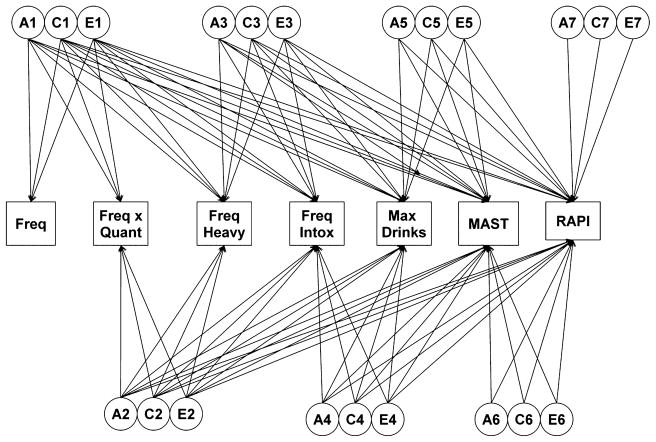
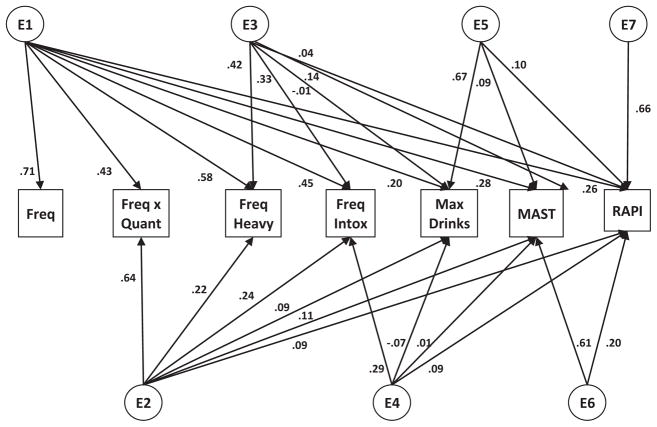
Fig. 1.
Full Cholesky model in the FinnTwin16 sample containing 7 latent genetic (A), shared environmental (C), and unique environmental (E) factors each loading onto the 7 measured alcohol variables (frequency, frequency × quantity, frequency of heavy drinking, frequency of intoxication, maximum drinks in a 24-hour period, the MAST, and the RAPI). MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
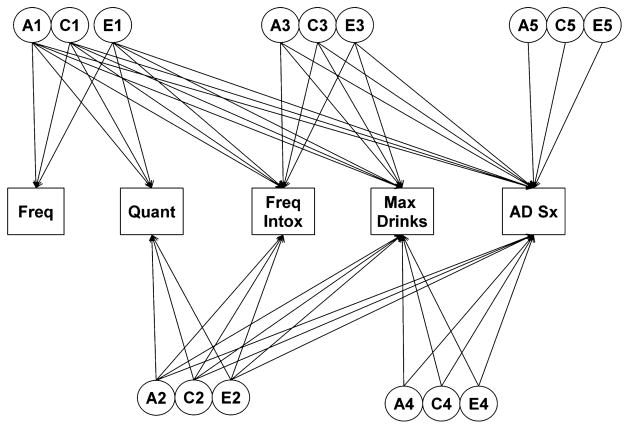
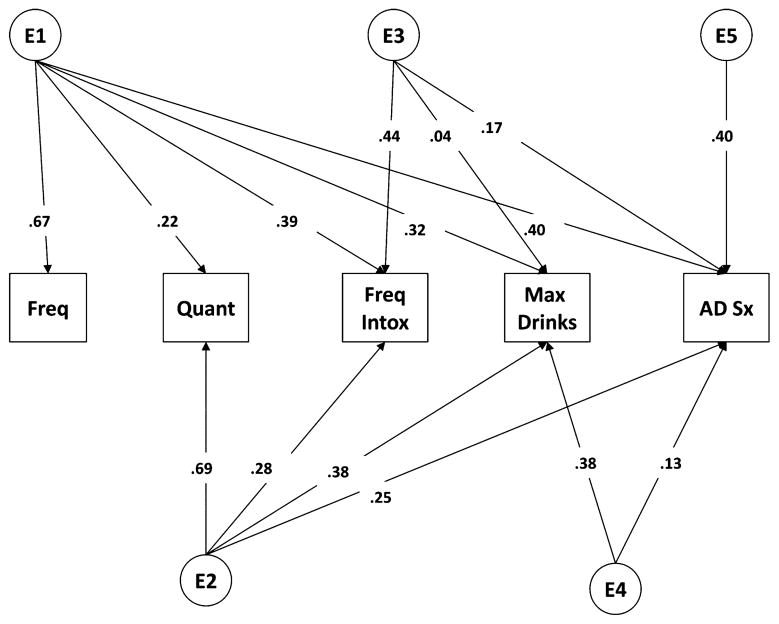
Fig. 2.
Full Cholesky model in the VATSPSUD sample containing 5 latent genetic (A), shared environmental (C), and unique environmental (E) factors each loading onto the 5 measured alcohol variables (frequency, quantity, frequency of intoxication, maximum drinks in a 24-hour period, DSM-IV alcohol dependence symptoms [AD Sx]). VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.
RESULTS
FinnTwin16
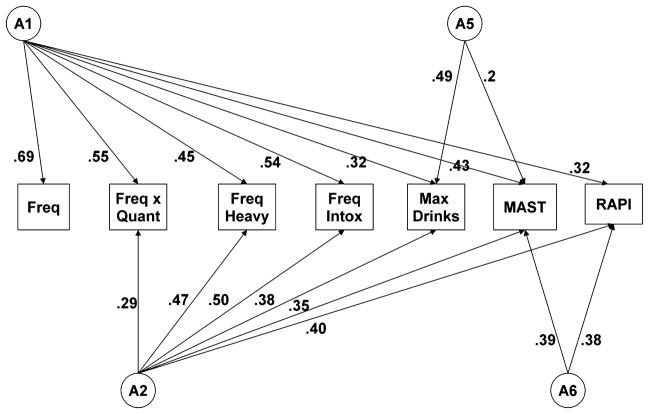
Table 1 details the phenotypic correlations across the different measures of AC and problem drinking. Polychoric correlations were computed on only 1 twin from each pair, chosen randomly. Table 2 shows the MZ and DZ twin correlations for each of the measures. The results of the series of models fit are shown in Table 3. We initially fit a full Cholesky model including full A, C, and E matrices separately for each sex (AIC = 5967.906, df = 16618; Model I in Table 3). Next, we tested a model in which we constrained all parameters to be equal in men and women (Model II). The AIC decreased and the chi-squared change was nonsignificant for the change in degrees of freedom, indicating that the more parsimonious model constraining men and women to be equal provided a better fit. We next tested a model including full A and E matrices and dropping the full C matrix representative of all shared environmental influences (Model III). The AIC decreased and the chi-squared change was nonsignificant for the change in degrees of freedom, indicating that the more parsimonious model dropping all shared environmental influences on the measures provided a better fit. Models IV to VI are submodels that test for a reduced number of genetic factors. We systematically tested the significance of each genetic factor and each pathway in the following sequence: (1) tested the significance of the entire A matrix; (2) tested the significance of each latent genetic factor; and (3) tested the significance of each individual genetic pathway. Each of the pathways retained in the best-fitting model is by definition significant. Model IV allows for only 1 latent genetic factor (A1 in Fig. 1), Model V allows for 2 latent genetic factors (A1 and A2), and Model VI allows for 3 latent genetic factors (A1, A2, and A3). For each of these submodels, the AIC increased and the chi-squared change was significant for the change in degrees of freedom, indicating that these models provided a worse fit to the data. The best-fitting model (Model VII; shown in Fig. 3), obtained by systematically dropping parameters based on the order of magnitude until no further pathways could be dropped without causing a significant decrease in fit, allowed for 4 latent genetic factors. Additionally, this model dropped the individual pathway from the third latent genetic factor (A5 in Fig. 1) loading onto the RAPI. This model indicates that genetic variance across the measures of AC and problems is accounted for by multiple latent genetic factors. The genetic correlations, computed for each pair of variables as the covariance of the 2 measures divided by the square root of the product of the variances of each of the measures, are shown in Table 4. They range from 0.45 (frequency of alcohol use with max drinks) to 0.99 (frequency of heavy drinking and frequency of intoxication).
Table 1.
FinnTwin16 Phenotypic Correlations
| Measure | Freq | Freq × quant | Freq of Heavy | Freq of Intox | Max Drinks | MAST | RAPI |
|---|---|---|---|---|---|---|---|
| Frequency | 1 | ||||||
| Freq × Quant | 0.77 | 1 | |||||
| Freq Heavy | 0.73 | 0.79 | 1 | ||||
| Freq Intox | 0.73 | 0.80 | 0.91 | 1 | |||
| Max Drinks | 0.46 | 0.53 | 0.56 | 0.53 | 1 | ||
| MAST | 0.33 | 0.41 | 0.44 | 0.45 | 0.39 | 1 | |
| RAPI | 0.23 | 0.31 | 0.34 | 0.35 | 0.26 | 0.47 | 1 |
All correlations significant at p < 0.001.
MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
Table 2.
FinnTwin16 Heritability Estimates, MZ and DZ Correlations
| Measure | Heritability | Females
|
Males
|
||
|---|---|---|---|---|---|
| MZr | DZr | MZr | DZr | ||
| Frequency | 0.48 | 0.59 | 0.43 | 0.75 | 0.47 |
| Freq × Quant | 0.39 | 0.45 | 0.30 | 0.61 | 0.37 |
| Freq Heavy | 0.43 | 0.54 | 0.34 | 0.64 | 0.42 |
| Freq Intox | 0.54 | 0.64 | 0.38 | 0.65 | 0.45 |
| Max Drinks | 0.49 | 0.55 | 0.35 | 0.65 | 0.29 |
| MAST | 0.50 | 0.55 | 0.34 | 0.63 | 0.52 |
| RAPI | 0.42 | 0.43 | 0.23 | 0.52 | 0.25 |
All correlations significant at p < 0.001.
MZ, monozygotic; DZ, dizygotic; MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
Table 3.
FinnTwin16 Model Fitting Results
| Model | Compared to Model | Δ Fit
|
||||
|---|---|---|---|---|---|---|
| Δ χ2 | Probability | Δ df | Δ AIC | |||
| Ia | Full Model | – | – | – | – | – |
| II | Sexes equated | I | 16.60 | 0.96 | 84 | 39.39 |
| III | C Matrix dropped | II | 60.05 | 0.98 | 28 | 107.95 |
| IV | A1 | III | 337.39 | 0.00 | 21 | +127.39 |
| V | A1 + A2 | III | 216.36 | 0.00 | 15 | +18.36 |
| VI | A1 + A2 + A3 | III | 145.48 | 0.00 | 10 | +145.48 |
| VIIb | A1 + A2 + A3 + A4 | III | 111.60 | 0.12 | 6 | 78.36 |
AIC, Akaike’s Information Criterion.
Fit of Model I: 2LL = 39203.91, df = 16618, AIC = 5967.91.
Best fitting model.
Fig. 3.
FinnTwin16 best-fitting model: additive genetic pathways. MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
Table 4.
FinnTwin16 Genetic Correlations
| Measure | Freq | Freq × Quant | Freq of Heavy | Freq of Intox | Max Drinks | MAST | RAPI |
|---|---|---|---|---|---|---|---|
| Frequency | 1 | ||||||
| Freq × Quant | 0.88 | 1 | |||||
| Freq Heavy | 0.69 | 0.95 | 1 | ||||
| Freq Intox | 0.74 | 0.97 | 0.99 | 1 | |||
| Max Drinks | 0.45 | 0.65 | 0.70 | 0.70 | 1 | ||
| MAST | 0.60 | 0.76 | 0.77 | 0.78 | 0.73 | 1 | |
| RAPI | 0.50 | 0.73 | 0.80 | 0.80 | 0.57 | 0.95 | 1 |
MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
Virginia Adult Twin Study of Psychiatric and Substance Use Disorders
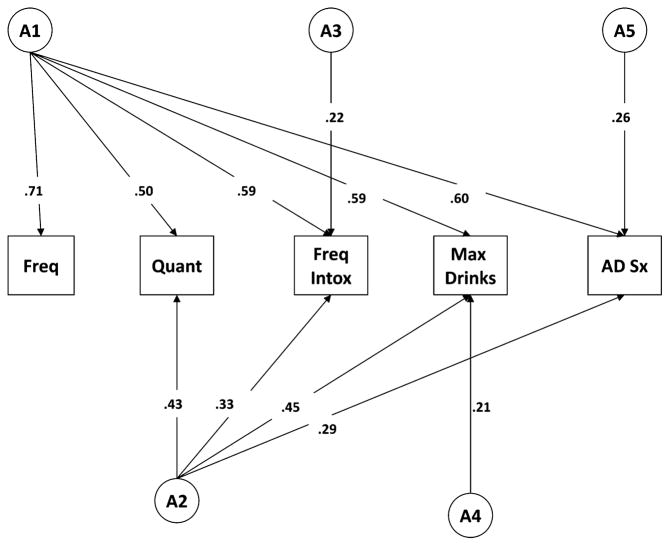
Table 5 details the phenotypic correlations across the different measures of current AC and lifetime symptoms of problem drinking. Polychoric correlations were computed on only 1 twin from each pair, chosen randomly. Note that while FT16 phenotypic correlations ranged from 0.25 to 0.75, VATSPSUD phenotypic correlations were somewhat higher ranging from 0.53 to 0.84. Table 6 shows the MZ and DZ twin correlations for each of the measures. We fit a series of models paralleling those fit in the FT16 data, as described above. The results of those models are shown in Table 7. Constraining all parameters to be equal in men and women (Model II), dropping the full C matrix (representing all shared environmental influences; Model III) provided better fits to the data, as indicated by decreases in the AIC and a nonsignificant chi-squared change. A systematic series of fitting submodels to test the significance of the individual genetic factors/pathways resulted in the best-fitting model (Model VII, shown in Fig. 4). Parallel to the results from the Finn- Twin16 data, this model contained multiple latent genetic factors across the measures of AC and alcohol problems. Genetic correlations for this sample are shown in Table 8 and range from 0.76 (drinking frequency and quantity) to 0.96 (drinking quantity and max drinks).
Table 5.
VATSPSUD Phenotypic Correlations
| Measure | Drinking Frequency | Drinking Quantity | Frequency of Intoxication | Max Drinks | DSM-IV AD Sx |
|---|---|---|---|---|---|
| Frequency | 1 | ||||
| Quantity | 0.53 | 1 | |||
| Freq of Intoxication | 0.73 | 0.76 | 1 | ||
| Max Drinks | 0.68 | 0.84 | 0.79 | 1 | |
| DSM AD Symptoms | 0.73 | 0.70 | 0.80 | 0.79 | 1 |
All correlations significant at p < 0.001.
VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders; AD Sx, alcohol dependence symptoms.
Table 6.
VATSPSUD Heritability Estimates, Monozygotic and Dizygotic Correlations
| Measure | Heritability | Females
|
Males
|
||
|---|---|---|---|---|---|
| MZr | DZr | MZr | DZr | ||
| Frequency | 0.50 | 0.56 | 0.34 | 0.46 | 0.29 |
| Quantity | 0.42 | 0.39 | 0.24 | 0.42 | 0.24 |
| Freq of Intoxication | 0.51 | 0.48 | 0.29 | 0.46 | 0.29 |
| Max Drinks | 0.56 | 0.48 | 0.30 | 0.53 | 0.34 |
| DSM AD Symptoms | 0.52 | 0.47 | 0.27 | 0.48 | 0.24 |
All correlations significant at p < 0.001. MZ, monozygotic; DZ, dizygotic; VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders; AD, alcohol dependence.
Table 7.
VATSPSUD Model Fitting Results
| Model | Compared to Model | Δ Fit
|
||||
|---|---|---|---|---|---|---|
| Δ χ2 units | Probability | Δ DF | Δ AIC | |||
| Ia | Full Model | – | – | – | – | – |
| II | Sexes equated | I | 9.42 | 0.86 | 45 | 20.58 |
| III | C Matrix dropped | II | 34.42 | 0.87 | 15 | 55.57 |
| IV | A1 | III | 220.71 | 0.00 | 10 | 23.72 |
| V | A1 + A2 | III | 199.32 | 0.00 | 6 | 47.36 |
| VI | A1 + A2 + A3 | III | 185.44 | 0.00 | 3 | 56.44 |
| VI | A1 + A2 + A3 + A4 | III | 74.08 | 0.05 | 1 | 58.09 |
| VIIb | A1 + A2 + A3 + A4 + A5 | III | 35.96 | 0.90 | 3 | 60.04 |
AIC, Akaike’s Information Criterion; VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.
Fit of Model I: 2LL = 43147.81, df = 17540, AIC = 8067.81; All subsequent models are compared to Model I.
Best fit model.
Fig. 4.
VATSPSUD best-fitting model: additive genetic pathways. VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders; AD Sx, alcohol dependence symptoms.
Table 8.
VATSPSUD Genetic Correlations
| Measure | Drinking Frequency | Drinking Quantity | Frequency of Intoxication | Max Drinks | DSM-IV AD Sx |
|---|---|---|---|---|---|
| Frequency | 1 | ||||
| Quantity | 0.76 | 1 | |||
| Freq of Intoxication | 0.82 | 0.94 | 1 | ||
| Max Drinks | 0.79 | 0.96 | 0.92 | 1 | |
| DSM AD Symptoms | 0.83 | 0.91 | 0.89 | 0.89 | 1 |
VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders; AD Sx, alcohol dependence symptoms.
In summary, the best-fitting model across both samples indicated that a single latent genetic factor cannot explain the genetic influences on all consumption and problem measures. Rather, several latent genetic factors are needed (Figs. 3 and 4). The first (A1) loads most heavily on the frequency items, but retains considerable influence across the other items. A second latent genetic factor (A2) loads more heavily on the heavier drinking items but again retains considerable influence on all items. Additional latent genetic factors are more specific to other consumption measures, with both samples showing some latent genetic influences specific to measures of alcohol problems (unshared with any of the measures of consumption).
The goal of these analyses was to examine the underlying genetic architecture across measures of consumption and alcohol problems; accordingly, we did not test any models in which we dropped any component of the E matrix for either sample. Path estimates for the E parameters from the best-fitting models for the FT16 and VATSPSUD samples are shown in Figs. 5 and 6, respectively.
Fig. 5.
FinnTwin16 best-fitting model: unique environmental pathways. MAST, Michigan Alcoholism Screening Test; RAPI, Rutgers Alcohol Problem Index.
Fig. 6.
VATSPSUD best-fitting model: unique environmental pathways. VATSPSUD, Virginia Adult Twin Study of Psychiatric and Substance Use Disorders; AD Sx, alcohol dependence symptoms.
DISCUSSION
The initial genome-wide association studies have taught us that very large sample sizes will be necessary to identify genes of small effect (Wellcome Trust Case Control Consortium, 2007), as are assumed involved in psychiatric and substance- use disorders. Failure to identify robust genetic effects reaching genome-wide significance has led to large-scale meta-analytic efforts (McMahon et al., 2010). But often, the increase in sample size comes with a reduction in phenotypic specificity, because different assessment measures or outcomes have been used across different samples. Rather than assuming that different measures are influenced by the same genetic factors, twin studies provide a method to explicitly evaluate these relationships. In this study, we examined the genetic architecture across different measures of current AC and problems in 2 independent twin samples from 2 different cultures: FT16 and the VATSPSUD. Previous analyses found a large proportion of overlap in the genetic factors that influence alcohol dependence and measures of AC during the heaviest period of drinking. Our analyses also suggest considerable overlap of genetic influences across different indices of current drinking and different measures of alcohol problems, across both samples, as evidenced by genetic correlations ranging from 0.45 to 0.99. Across both samples, frequency of intoxication and quantity of alcohol use were more strongly genetically correlated with alcohol problems than frequency of use. The Kendler and colleagues’ (2010) study of lifetime indices of consumption also found that drinking frequency had the lowest shared genetic overlap with alcohol problems. The Grant and colleagues’ (2009) study only evaluated a composite consumption factor score, making it impossible to evaluate differential informativeness of various drinking indices. However, the available data from this study and the Kendler study suggest that quantity of AC and frequency of heavy drinking or intoxication have greater shared genetic overlap with alcohol problem measures than measures of the frequency of alcohol use, which likely reflects a number social factors as well. Overall, genetic correlations were higher in the VATSPSUD sample, which may reflect the somewhat older mean age of the sample (36 vs. 24 years of age) and more stabilized drinking patterns as individuals move further into adulthood. This suggests that meta-analytic studies may want to test for heterogeneity across samples according to age when using studies assessing consumption to replicate genetic findings originally identified with alcohol dependence, as drinking indices among slightly older adults may be more genetically correlated with alcohol problems than among younger adults, for whom drinking patterns are still more transitional.
Despite high genetic correlations, across both samples the genetic architecture is complex. A single latent genetic factor influencing all the consumption measures did not provide a good fit to the data in either sample. Rather, there are several different genetic factors that influence different measures of AC. This indicates that there is not complete overlap across measures of AC and alcohol problems, and there are different genetic influences impacting different indices of drinking. This has implications for gene identification studies in the area of alcohol dependence. It suggests that there are valid reasons why genetic findings may not “replicate” across studies that have assessed different aspects of alcohol use and dependence. In practice, this has already been seen in candidate gene studies, where genes have been associated with aspects of alcohol use, but not with alcohol dependence diagnoses (Dick et al., 2005; Foroud et al., 2007). Meta-analytic efforts that combine different indices of alcohol use and alcohol problems may enhance power to detect genetic influences that are shared across these measures, but they may miss some genetic influences specific to different aspects of alcohol use.
These findings should be interpreted in the context of several limitations. Although we believe that the demonstration of similar effects across 2 independent samples is a strength of the study, we note that the exact measures of alcohol use and alcohol problems collected in the 2 projects differed. Even when the construct was the same (e.g., drinking frequency), the exact wording of the item and response options varied across the samples. Differential reliabilities and distributional properties of the items could have influenced the emergent genetic factor structures. Differences in psychometric properties across the samples likely contributed to some of the observed sample variability. We believe that the convergence of results across these studies is notable, given that the samples contained slightly different measures of current consumption and different indices of problem drinking, covered different age ranges (the FT16 sample was limited to young adults while the VATSPSUD sample covered a much broader age range of adults), and come from different drinking cultures.
In summary, our analyses are consistent across 2 independent twin samples in finding fairly high genetic correlations across current AC measures and alcohol problems. This is true across several different indices of consumption (frequency of drinking, quantity of alcohol use, frequency of heavy drinking/drunkenness) and using different measures of alcohol-related problems (Mm-MAST, RAPI, DSM-IV symptom counts). Frequency of drinking appears to be the least genetically correlated with other measures of alcohol (less so than quantity of alcohol use/frequency of heavy drinking or drunkenness), suggesting there is more unique environmental variance on this aspect of alcohol use. This suggests that this measure may be least likely to “replicate” genetic effects identified with alcohol dependence. Both samples indicate that there is not a single genetic factor responsible for the phenotypic overlap between different measures of consumption and problem use. Accordingly, combining studies using different indices of alcohol use and problems may help increase power to identify shared genetic influences, but may introduce noise if the gene under study is more specific to a particular aspect of AC. Creating multivariate genetic factor scores that take into account the extent to which different indices of alcohol use are reflective of the underlying genetic predisposition allows researchers to capitalize on all available information, while taking into account the differential informativeness of various indices of use. This illustrates 1 of the ways in which twin studies remain informative in the evolving era of gene identification.
Acknowledgments
The Finnish Twin studies have been supported by the National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to RJR), the Academy of Finland (grants 100499 and 118555), and the Academy of Finland Centre of Excellence in Complex Disease Genetics (to JK). The present study has been supported by grants from the National Institute of Alcohol Abuse and Alcoholism (AA-15416 and K02 AA018755 to DMD) and also supported in part by NIH grants R37AA011408, P20AA017828. Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, now directed by Judy Silberg, PhD, has received support from the NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations. Carol Prescott, PhD, contributed to the design and implementation of this study.
References
- Agrawal A, Grant JD, Littlefield A, Waldron M, Pergadia ML, Lynskey MT, Madden PA, Todorov A, Trull T, Bucholz KK, Todd RD, Sher K, Heath AC. Developing a quantitative measure of alcohol consumption for genomic studies on prospective cohorts. J Stud Alcohol Drugs. 2009;70:157–168. doi: 10.15288/jsad.2009.70.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Dick DM, Bierut L. The genetics of alcohol dependence. Curr Psychiatry Rep. 2006;8:151–157. doi: 10.1007/s11920-006-0015-1. [DOI] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Hesselbrock V, Schuckit M, Crowe R, Foroud T. No association of the GABA-A receptor genes on chromosome 5 with alcoholism in the Collaborative Study on the Genetics of Alcoholism sample. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:24–28. doi: 10.1002/ajmg.b.30058. [DOI] [PubMed] [Google Scholar]
- Dick DM, Prescott C, McGue M. The genetics of substance use and substance use disorders. In: Kim Y-K, editor. Handbook of Behavior Genetics. Springer; New York: 2009. pp. 433–453. [Google Scholar]
- Edenberg H, Bierut L, Boyce P, Cao M, Cawley S, Chiles R, Doheny KF, Hansen M, Hinrichs AL, Jones K, Kelleher M, Kennedy GC, Liu G, Marcus G, McBride C, Murray SS, Oliphant A, Pettengill J, Porjesz B, Pugh EW, Rice JP, Rubano T, Shannon S, Steeke R, Tischfield JA, Tsai YY, Zhang C, Begleiter H. Description of the data from the Collaborative Study on the Genetics of Alcoholism (COGA) and single-nucleotide polymorphism genotyping for Genetic Analysis Workshop 14. BMC Genetics. 2005;6(Suppl 1):S2. doi: 10.1186/1471-2156-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Wetherill LF, Liang T, Dick DM, Hesselbrock V, Kramer J, Nurnberger J, Schuckit M, Carr L, Porjesz B, Xuei X, Edenberg HJ. Association of alcohol craving with alpha-synuclein (SNCA) Alcohol Clin Exp Res. 2007;31:537–545. doi: 10.1111/j.1530-0277.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Goldman D. Recent developments in alcoholism: genetic transmission. Recent Dev Alcohol. 1993;11:231–248. [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PA, Pergadia ML, Nelson EC, Lynskey MT, Todd RD, Todorov AA, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biol Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Genetic influences on alcohol consumption patterns and problem drinking: results from the Australian NH & MRC twin panel follow-up survey. Ann NY Acad Sci. 1994;708:72–85. doi: 10.1111/j.1749-6632.1994.tb24699.x. [DOI] [PubMed] [Google Scholar]
- Kaprio J. Twin studies in Finland 2006. Twin Res Hum Genet. 2006;9:772– 777. doi: 10.1375/183242706779462778. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Rose RJ, Romanov K, Koskenvuo M. Genetic and environmental determinants of use and abuse of alcohol: the Finnish Twin Cohort studies. Alcohol Alcohol Suppl. 1991;1:131–136. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcohol Clin Exp Res. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kristenson H, Trell E. Indicators of alcohol consumption: comparisons between a questionnaire (Mm-MAST), interviews and serum y-glutamyl transferase (GGT) in a health survey of middle-aged males. Br J Addict. 1982;77:297–304. doi: 10.1111/j.1360-0443.1982.tb02459.x. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Akula N, Schulze TG, Muglia P, Tozzi F, Detera-Wadleigh SD, Steele CJ, Breuer R, Strohmaier J, Wendland JR, Mattheisen M, Mühleisen TW, Maier W, Nöthen MM, Cichon S, Farmer A, Vincent JB, Holsboer F, Preisig M, Rietschel M Bipolar Disorder Genome Study (BiGS) Consortium . Meta-analysis of genome-wide association data identifies a risk locus for major mood disorders on 3p21.1. Nat Genet. 2010;42:128–131. doi: 10.1038/ng.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 5. Department of Psychiatry, Medical College of Virginia; Richmond, VA: 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Prescott C, Sullivan PF, Myers J, Patterson D, Devitt M, Halberstadt LJ, Walsh D, Kendler KS. The Irish Affected Sib Pair Study of Alcohol Dependence: study methodology and validation of diagnosis by interview and family history. Alcohol Clin Exp Res. 2005;29:417–429. doi: 10.1097/01.alc.0000156085.50418.07. [DOI] [PubMed] [Google Scholar]
- Rose RJ. A developmental behavior-genetic perspective on alcoholism risk. Alcohol Health Res World. 1998;22:131–143. [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS 9.1.3 Help and Documentation. SAS Institute Inc; Cary, NC: 2000–2004. [Google Scholar]
- Seppa K, Pitkajarvi T, Sillanaukee P. Alcohol consumption profile by time in middle-aged men: a longitudinal study based on three different diagnostic instruments. Alcohol Alcohol. 1999;34:65–70. doi: 10.1093/alcalc/34.1.65. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium . Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Toward the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]