Abstract
Patterning the Drosophila retina for color vision relies on post-mitotic specification of photoreceptor subtypes. R8 photoreceptors express one of two light-sensing Rhodopsins, Rh5 or Rh6. This fate decision involves a bistable feedback loop between Melted, a PH-domain protein, and Warts, a kinase in the Hippo growth pathway. Here, we show a subset of the Hippo pathway—Merlin(Mer), Kibra(Kib), and Lethal(2)giant larvae(Lgl), but not Expanded or Fat--is required for Warts expression and activity in R8 to specify Rh6 fate. Melted represses warts transcription to disrupt Hippo pathway activity and specify Rh5 fate. R8 Hippo signaling therefore exhibits ON-or-OFF regulation, promoting mutually exclusive fates. Furthermore, Mer and Lgl are continuously required to maintain R8 neuronal subtypes. These results reveal a role for Mer, Kib, and Lgl in neuronal specification and maintenance, and show that the Hippo pathway is re-implemented for sensory neuron fate by combining canonical and non-canonical regulatory steps.
Keywords: binary cell fate, color vision, photoreceptor, rhodopsin, Hippo pathway, neural development, neural maintenance, Merlin, Warts, Kibra, Lgl
Introduction
In neural development, neurons progressively differentiate towards terminal fates, culminating in the post-mitotic specialization of subtypes with subtle, yet critical differences. For example, sensory neurons of the same class often express one sensory receptor from among several options to endow each neuron with a precise functional identity. While neural progenitor specification (Jessell, 2000) and the role of transcription factors in generating neural diversity (Hobert, 2008) are well studied, the mechanisms by which signaling pathways control terminal neuronal subtype specification events, like mutually exclusive sensory receptor expression, are less well understood. Here, we use terminal differentiation of R8 photoreceptor subtypes in the Drosophila eye as a model to study how an otherwise equipotent precursor cell uses a signaling pathway to instruct one of two alternate fates.
The Drosophila compound eye comprises ~800 ommatidia, or unit eyes, that each contains 8 photoreceptor neurons (Hardie, 1985) which express light-sensitive Rh proteins and transmit visual information to the brain. Six outer photoreceptors (R1–R6) express Rh1 and, like vertebrate rods, mediate motion detection and dim light vision (Yamaguchi et al., 2008). Two inner photoreceptors, R7 and R8, are involved in color vision, analogous to vertebrate cones, and detect light by expressing one of four Rhs of different wavelength sensitivity that define ommatidial subtypes (reviewed in Rister and Desplan, 2011). In ‘yellow’ (y) ommatidia, yR7 expresses long-UV-sensitive Rh4 and yR8 expresses green-Rh6, while in ‘pale’ (p) ommatidia, pR7 expresses short-UV-Rh3 and pR8 expresses blue-Rh5. ‘y’ and ‘p’ subtypes are distributed stochastically in a roughly 70% ‘y’ to 30% ‘p’ ratio (Figure 1A). R7 and R8 coordinate their subtype identities in two sequential post-mitotic fate decisions. First, stochastic expression of the transcription factor gene spineless (ss) in ~70% of R7 cells induces yR7 fate and rh4 (Wernet et al., 2006). pR7s without ss express rh3 and instruct the underlying R8 to become pR8 and express rh5. The remaining yR8s express rh6 by default (Chou et al., 1999).
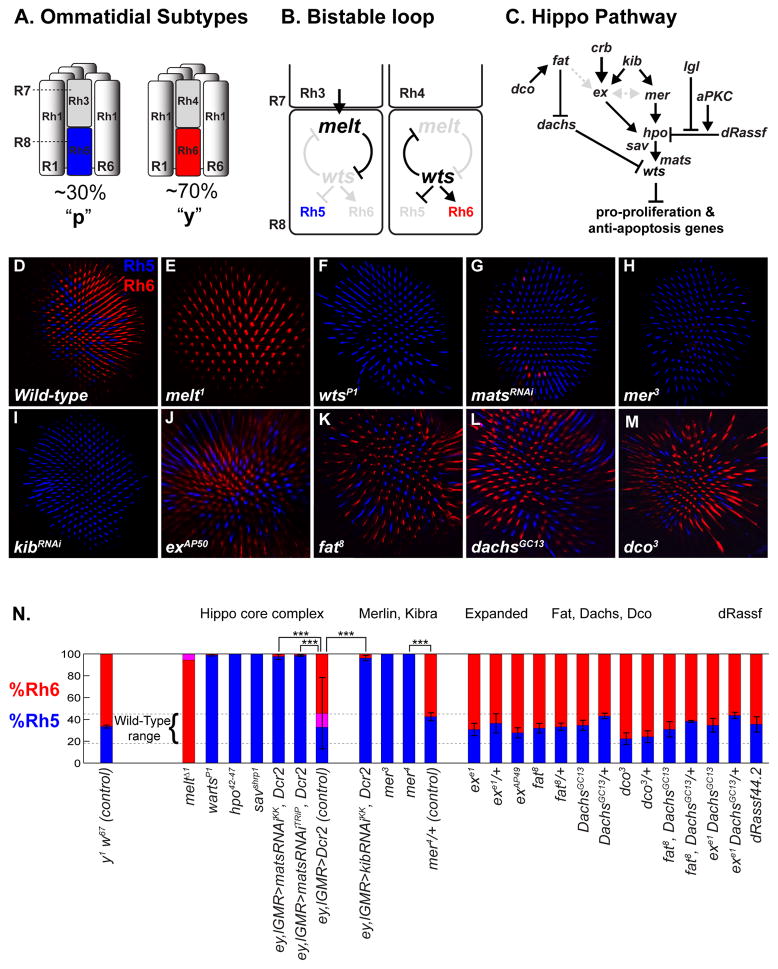
Figure 1. A Merlin/Kibra branch of the Hippo pathway regulates R8 subtypes specification.
(A) R7/R8 Rhodopsin pairing defines ommatidial subtypes. Outer photoreceptors (R1–R6) express rh1, whereas R7/R8 primarily express either rh3/rh5 or rh4/rh6.
(B) A bistable feedback loop regulates mutually exclusive Rh5 and Rh6 expression in R8: warts and melted are expressed in y- and p-R8, respectively, and repress each other’s transcription.
(C) The Hippo signaling network regulates proliferation and cell death. Upstream inputs converge onto the Hpo/Sav/Mats/Wts “core complex” to regulate Wts activity.
(D–M): Confocal images of adult retinas stained with Rh5 (blue) and Rh6 (red) antibodies that label R8 subtypes. (E–F, H, J–M) are whole mutant retinas. Genotypes in Supp Methods.
(D) wild-type. Note the roughly 70:30 ratio of Rh6:Rh5.
(E) melt1
(F) wartsP1
(G) retina expressing mats-RNAi
(H) mer3
(I) retina expressing kib-RNAi. All R8s in kibdel null mutants also express Rh5, but the kibdel chromosome contains a mutation in rh6 preventing analysis of Rh6 expression (data not shown).
(J) exAP50
(K) fat8
(L) dachsGC13
(M) dco3
(N) Quantification of R8 subtypes in Hippo pathway mutants. Graph presents proportion R8s (y-axis) that contain Rh5 (blue), Rh6 (red), or co-expression (pink). Dashed lines indicate the wild-type range of 20%–45% Rh5 based on counts in wild-type or balancer strains of various genetic backgrounds (data not shown). Heterozygote controls contained either a wild-type chromosome (chr), or, for mutants on chr X, FM7c; chr II, the CyO balancer; chr III, TM2 or TM6B. Mean %Rh5 were compared with a two-tailed, unpaired t-test. (***) p<0.001. wild-type: n=9 retinas, N=3055 ommatidia, mats-RNAi: n=8, N=1561; kib-RNAi: n=11, N=3214; exe1: n=8, N=1869; all others genotypes: n≥4, N≥800.
Two proteins previously known as growth regulators--the NDR Ser/Thr kinase Warts (Wts), and a PH-domain containing protein, Melted (Melt)--control post-mitotic specification of R8 subtypes (Figure 1B) (Mikeladze-Dvali et al., 2005). melt is necessary and sufficient for pR8 fate and rh5 expression (Figure 1E) while wts is necessary and sufficient for yR8 and rh6 (Figure 1F). wts and melt repress each other’s transcription in a double negative, bistable feedback loop that directs robust expression of either rh5 or rh6 in R8. The bistable loop ensures an unambiguous R8 fate decision in response to an instructive signal from the R7 cell.
Wts is a tumor suppressor and nexus of the Hippo pathway, which coordinates the balance between proliferation and apoptosis (Figure 1C) (reviewed in Pan, 2010; Halder and Johnson, 2011). Wts inhibits growth by phosphorylating the transcriptional co-activator Yorkie (Yki) in the cytoplasm, preventing it from entering the nucleus (Huang et al., 2005; Dong et al., 2007; Oh and Irvine, 2008) where Yki would otherwise interact with transcription factors such as Scalloped (Sd) or Homothorax (Hth) (Goulev et al., 2008; Wu et al., 2008; Zhang et al., 2008, Peng et al., 2009) to upregulate pro-proliferation and anti-apoptosis target genes.
Wts is activated upstream by another Ser/Thr kinase, Hippo (Hpo), bound by the scaffolding protein Salvador (Sav) and co-factor Mob-as-tumor suppressor (Mats) (Harvey and Tapon, 2007). Further upstream are several independent or semi-redundant input branches (Figure 1C): FERM domain proteins Expanded (Ex) and Merlin (Mer) (Hamaratoglu et al., 2006; Pellock et al., 2006; Tyler and Baker, 2007) appear to act with the WW-domain protein Kibra (Kib) to activate Hpo (Baumgartner et al., 2010, Genevet et al., 2010, Yu et al., 2010). The membrane protein Crumbs (Crb) mediates upstream activation of Ex (Chen et al., 2010; Ling et al., 2010; Robinson et al., 2010), and Ex can also directly inhibit Yki (Badouel et al., 2009, Oh et al., 2009). Another input consists of the atypical cadherin, Fat, and the kinase Discs overgrown (Dco), which act through the unconventional myosin Dachs to control Wts protein levels (Bennett and Harvey, 2006; Cho et al., 2006; Silva et al., 2006; Willecke et al., 2006; Tyler and Baker, 2007). Finally, apical-basal polarity regulators Lethal giant larvae (Lgl) and atypical protein kinase C (aPKC) act antagonistically to influence the Hippo pathway by unknown mechanisms, possibly by affecting the inhibition of Hpo by dRassf (Polesello et al., 2006; Grzeschik et al., 2010).
Mutations in fat, lgl, crb, ex, mer, hpo, sav, mats, and wts, or over-expression of yki, cause overgrowth in developing tissues, while pathway activation or yki depletion blocks proliferation and induces cell death (Pan, 2010). Many Hippo pathway members have orthologs implicated in human cancers, in particular, the mer ortholog, NF2, whose mutations cause Neurofibromatosis type 2 (MacCollin et al., 1993).
The Hippo pathway also regulates non-growth processes in Drosophila, implying that pathway regulation can be heterogeneous and context-dependent. The Hippo pathway functions in autophagic cell death in salivary glands (Dutta and Baehrecke, 2008), apico-basal polarity (Hamaratoglu et al., 2009; Genevet et al., 2009), establishment and maintenance of dendritic arborizations (Emoto et al., 2006), and differentiation of the optic lobe neuroepithelia (Reddy et al., 2010). In these processes, wts is believed to be ubiquitously expressed and controlled post-transcriptionally to mediate its activation.
R8 subtype specification is the only known cellular process in which wts is controlled transcriptionally through a genetic interaction with Melt. Melt modulates the TOR/Insulin signaling pathway in fat metabolism (Teleman et al., 2005), yet mutants in other TOR/Ins pathway genes do not exhibit R8 phenotypes (Mikeladze-Dvali et al., 2005), suggesting that Melt acts independently to regulate R8 subtypes. Whether Wts acts with its canonical signaling partners besides Hpo and Sav for cell fate determination, and how diverse biological processes such as tissue growth and sensory receptor expression can re-use the same signaling modules, is not known.
Here, we report that Wts is regulated by the integration of canonical (tumor suppressor pathway) and non-canonical mechanisms for R8 subtype specification. mer, kib, lgl, and the hpo/sav/mats/wts core complex specify yR8 subtypes while Ex and Fat upstream inputs are not involved. mer and kib are required for wts expression and to repress melt such that the state of the wts-melt transcriptional feedback loop is coupled to upstream Hippo pathway signaling. Conditional inactivation of mer or lgl in adult flies reveals that continuous Hippo pathway activity also maintains mutually exclusive Rh5 and Rh6 expression, and melt can disrupt Hippo signaling long after R8 subtype specification has occurred. Therefore, constitutive and continuous activation by upstream Hippo inputs keep Wts “ON” to specify yR8, while repression of wts transcription by Melt acts as a circuit breaker to switch pathway activity “OFF” to specify pR8. Such regulation allows Hippo pathway activity to depend on the presence or absence of Wts, which ensures binary output of Rh5 and Rh6.
Results
To test whether the upstream Hippo growth network regulates Wts in R8 photoreceptor subtype specification, we genetically manipulated Mats, Hpo, Sav, Ex, Kib, Mer, Fat, Fj, Dco, Dachs, dRassf, aPKC, and Lgl and assayed for changes in the ratio of R8 subtypes as defined by the expression of Rh5 and Rh6.
The Hippo “core complex” module is functionally conserved in R8 subtype specification
Wts, Hpo, Sav, and Mats physically interact to form the “core complex” of Hippo growth signaling (reviewed in Zhao et al., 2010). Yet core complex members can behave differently in distinct contexts, as Hpo activates another NDR-kinase, Tricornered (Trc), instead of Wts, to establish dendritic fields of larval body wall neurons (Emoto et al., 2006). In R8, mutations in hpo and sav phenocopy wts mutants (Mikeladze-Dvali et al., 2005). To confirm that the core complex functions canonically during R8 photoreceptor subtype specification, we analyzed mats and performed epistasis among mats, wts, and hpo.
We generated wts, hpo, or sav whole mutant eyes with the FLP/FRT system using eyeless-FLP (ey-FLP) and a chromosome containing a cell lethal mutation and GMR-hid that eliminates all non-homozygous mutant cells in the eye (Stowers and Schwartz; 1999; Newsome et al., 2000). In these mutants, all R8s adopted the pR8 subtype fate and expressed Rh5 (Figures 1F and 1N; and Figure S1D-E) (Mikeladze-Dvali et al., 2005). As homozygous mats mutant eyes do not fully differentiate photoreceptors (Lai et al., 2005), we examined late mats function in the retina by expressing mats-RNAi with either the lGMR-Gal4 driver, in cells posterior to the morphogenetic furrow (Wernet et al., 2003), or a combination of lGMR-Gal4 and ey-Gal4 in the entire eye. Three different mats-RNAi transgenes resulted in loss of Rh6 and expansion of Rh5 (Figures 1G and 1N; Figure S1A-B and S1F). Thus, all four members of the Hippo core complex are required to specify yR8.
Expression of hpo in all photoreceptors (GMR-hpo) induced Rh6 expression in all R8s (Figure S1G) (Mikeladze-Dvali et al., 2005). wtsP1 hypomorphic mutant eyes suppressed the hpo mis-expression phenotype, as about half of R8s expressed Rh5 (Figure S1H). mats-RNAi also suppressed the “all Rh6” hpo gain-of-function phenotype, as ey, lGMR>matsRNAi; GMR-hpo retinas contained both Rh5 and Rh6 (~80% of R8s expressed Rh5; 60% Rh5+Rh6, and ~20% only Rh6) (Figure S1I). Similarly, removing hpo from lGMR>wts retinas reduced the proportion of R8s that expressed Rh6 exclusively (data not shown). Thus, wts, hpo, and mats are required for each other’s ability to promote yR8 fate and Rh6, consistent with functional conservation of the Hippo core complex in R8 subtype specification.
mer and kib are required to specify R8 photoreceptor subtypes independently of upstream Hippo pathway regulators Ex and Fat
To test whether upstream regulators of wts act in R8, we first generated whole mer mutant eyes. Hypomorphic mer3 led to Rh5 expression in almost all (>99%) R8 cells, with concomitant loss of Rh6 (Figure 1H). Similar results were obtained with null alleles (mer1, mer2, mer 4), in mer4 mutant clones, or after eye-specific expression of mer RNAi (Figure 1N). Wild-type UAS-mer expressed in all photoreceptors using lGMR-Gal4 rescued the mer4 mutant phenotype. We next analyzed mutants for Kib, which acts in a complex with Mer and Ex to promote Hippo pathway activity. kibdel null mutant retinas contained mostly Rh5-expressing R8s, indicating that kib is also required for yR8 fate. Eye-specific kib RNAi expression (ey+lGMR-Gal4; UAS-kib-RNAi, UAS-Dcr2) led to similar results (Figure 1I). Thus, mer and kib are required to specify yR8 fate and induce Rh6 expression.
We also generated eyes mutant for ex, which acts upstream of wts for growth regulation, in part redundantly with mer (McCartney et al., 2000; Hamaratoglu et al., 2006). Three different alleles of ex (exe1, exAP49, exAP50) did not significantly affect the R8 subtype ratio (Figures 1J and 1N; Figures S2A-B). To test for redundancy with mer, we removed one copy of mer in homozygous ex mutant clones and again observed no detectable difference in the Rh5:Rh6 ratio (Figures S2C-C″), consistent with mer functioning non-redundantly in the Hippo pathway to specify R8 subtypes. Several other studies have suggested that mer has functions separate from ex in the Hippo pathway (Pellock et al., 2006; Bennett and Harvey, 2006; Cho et al., 2006; Silva et al., 2006; Willecke et al., 2006), but to our knowledge this is the first example where mer mutant phenotypes perfectly match hpo, sav, and wts mutant phenotypes with ex completely dispensable (Figure 1N). Null or strong alleles of fat (fat8, fatGrv, fatfd), dco (dco3), dachs (dGC13), and dRASSF (dRASSFX16, dRASSF44.2), or exe1, fatGr-v double mutants, also all had normal pR8:yR8 ratios (Figures 1K-N; Figures S2D-D′). Therefore, the Mer/Kib upstream branch of the Hippo pathway is required for R8 subtype specification, while the Fat/Fj/Dco/Dachs pathway, Ex, and dRASSF are not involved.
Mer requires its BB-domain and C-terminus for activity in R8 subtype specification
For viability, Mer requires the N-terminal FERM domain, as well as portions of the protein C-terminal half (LaJeunesse et al., 1998). We tested whether these domains are also required for Mer in R8. Mer constructs missing either the C-terminal half (UAS-merΔC) or the 7-aa “Blue Box” sequence (170YQMTPEM177) in the FERM domain (UAS-merΔBB) were ectopically expressed in photoreceptors with lGMR-GAL4. Almost all R8s expressed Rh5 and not Rh6 (Figures 2C and 2G), suggesting that these proteins act as dominant negatives. Furthermore, merΔBB and merΔC constructs expressed in mer whole-mutant eyes failed to rescue the loss of yR8s (>99% of R8s expressed Rh5) (Figure 2D–G). Thus, the 7-aa Blue-Box domain, which is perfectly conserved in human NF2, and the C-terminal half of the protein are essential for Mer function in R8 subtype specification.
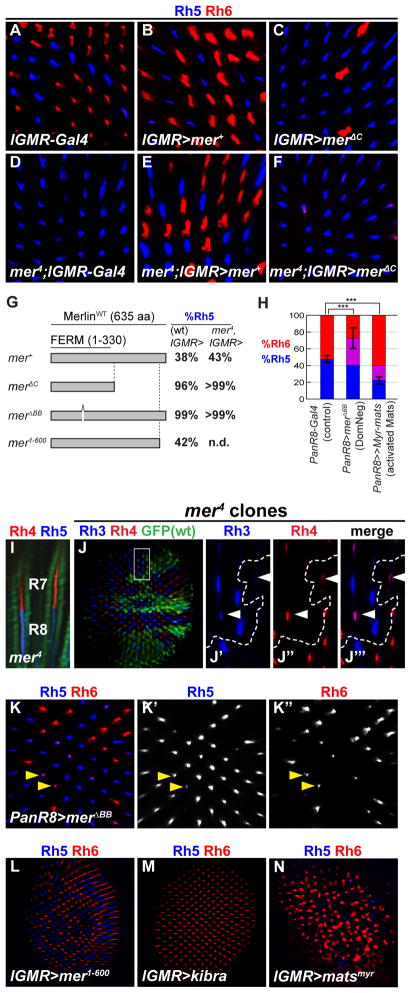
Figure 2. Merlin acts specifically in R8 and requires the FERM domain and C-terminus to regulate yR8 photoreceptor subtypes.
(A–F, J–N) adult retinas stained for Rh5 (blue) and Rh6 (red). Full genotypes listed in Supplemental Methods.
(A) lGMR-Gal4 control
(B) lGMR>mer+
(C) lGMR>merΔC
(D) mer4; lGMR-Gal4 (negative control for rescue experiments)
(E) mer4; lGMR>mer+ (rescue of R8 subtype specification)
(F) mer4; lGMR> merΔC
(G) Merlin deletion constructs (LaJeunesse et al., 1998) mis-expressed in wild-type and mer4 mutant retinas. Columns indicate percent of R8s that express Rh5. n.d.= not determined.
(H) Proportion of R8s that express Rh5 (blue), Rh6 (red), or both (purple) in PanR8>merΔBB (comparing %Rh5; n=8 retinas, N= 2329 ommatidia) PanR8>Myr-mats(comparing %Rh6; n=7, N=2355) and PanR8-Gal4 controls (n=7, N=2131). Two-tailed, unpaired t-test; *** denotes p<0.001.
(I) Side view of mer4 mutant ommatidia showing Rh4 (red) and Rh5 (blue) mis-coupling in the same ommatidium. Alexa-488 conjugated Phalloidin (green) stains actin and labels rhabdomeres (outer photoreceptors in image).
(J-J″) mer4 mutant clones (absence of GFP) stained with antibodies for R7 Rhs, Rh3 (blue) and Rh4 (red). (J′-J″) “Dorsal third” yR7 ommatidia, where Rh3 and Rh4 are co-expressed (white arrowheads) (Mazzoni et al. 2008). White box in panel (J) indicates area shown in adjacent panels. Dashed line indicates clone boundary.
(K-K″) PanR8>merΔBB (K′) Rh5 (K″) Rh6. Arrows show co-expression of Rh5 and Rh6. Note the decrease in Rh6 intensity in co-expressing cells (K″).
(L) lGMR>mer1-600
(M) lGMR>kib
(N) lGMR>Myr-mats-GFP (activated mats containing a Myristoylation sequence) The disorganized ommatidia and altered rhabdomere structure in some R8s is likely due to earlier growth functions of mats (Ho et al., 2010).
Mer acts autonomously in R8 for subtype specification
The R8 mer mutant phenotype is not due to general photoreceptor fate defects as mer retinas did not aberrantly express the cell fate markers Spalt (R7 & R8) and Rh1 (R1–R6) (Figures S3D-D′ and S3G-G′). As pR8 fate depends on signaling from pR7, mer could affect R7 subtype specification, and only indirectly affect R8 rh expression. However, Rh3 and Rh4 protein expression was normal in mer or kib mutant retinas (Figures 2J-J ‴; Figures S3A-B′), leading to extensive Rh4/Rh5 (yR7/pR8) mis-coupling (Figure 2I) never observed in wild-type.
To assess whether mer acts cell-autonomously in R8, we mis-expressed the dominant negative UAS-merΔBB using PanR8-Gal4, which is expressed exclusively in all R8s starting at ~70% pupation, after subtypes are specified (Mikeladze-Dvali et al., 2005). PanR8>MerΔBB retinas exhibited a dramatic gain of Rh5 (Figure 2K-K′), and partial loss of Rh6 (Figure 2K″), leading many R8s to co-express Rh5 and Rh6 (Figures 2H and 2K). This is likely because the PanR8 driver is expressed late, after Rh6 has been expressed. Rh6 protein levels appeared lower in R8 cells that co-expressed Rh5 (Figure 2K ″), confirming that yR8 subtype fate had been re-specified to pR8. Thus, mer is specifically required in R8 to regulate R8 subtype fate.
Kib and the core complex are necessary and sufficient to specify yR8 fate
We next tested if Mer was sufficient to induce yR8 fate in wild-type animals by using lGMR-Gal4 to mis-express either a full length (UAS-mer+) or a 35-amino acid truncated protein (UAS -mer1-600) proposed to be an activated form (LaJeunesse et al., 1998). Neither Mer construct affected R8 subtypes (Figures 2B and 2L). In contrast, lGMR>kib retinas exhibited a complete fate transformation and all R8s expressed Rh6 (Figure 2M), similar to mis-expression of wts, sav, or hpo (Mikeladze-Dvali et al., 2005). Thus, kib is necessary and sufficient to induce yR8 fate.
An activated, membrane-tethered Mats containing a myristoylation sequence (UAS-Myr-matsGFP) (Ho et al., 2010) driven with lGMR-GAL4 also induced Rh6, with a corresponding loss of Rh5 (Figure 2N). Late expression of Myr-matsGFP with PanR8-Gal4 was also sufficient to reprogram pR8 subtypes to express Rh6 (Figure 2H). In contrast, expression of a dominant negative construct with a mutated myristoylation sequence (lGMR-Gal4, UAS-MyrG2A-matsGFP) induced Rh5 (Figure S1C). Therefore, kib and all four core complex members are sufficient to induce yR8 subtypes. These results also support the idea that membrane localization of core complex proteins is important for their activation (Ho et al., 2010). mer might be permissively required for Hippo pathway activation in R8, as mer is necessary, but not sufficient, to induce the yR8 fate when ectopically expressed at high levels.
Wts and Sav require Mer to induce the yR8 subtype
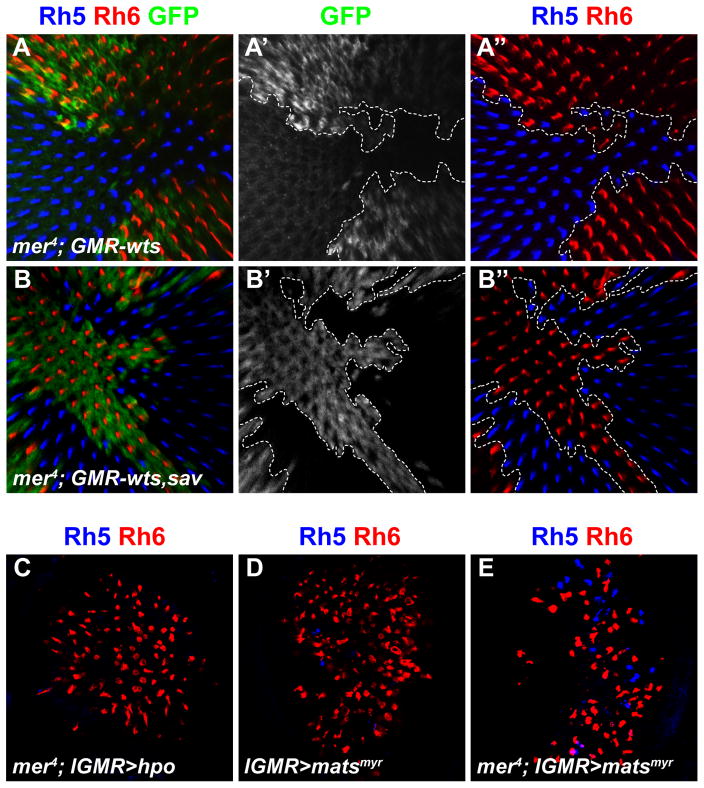
In the tumor suppressor pathway, Mer acts upstream of Wts to promote Wts activity (Hamaratoglu et al., 2006). However, removing mer in clonal patches of GMR-wts retinas completely suppressed the ability of wts to activate Rh6 and led to a mer mutant phenotype (>99% of R8s expressed Rh5) (Figures 3A-A ″). All R8s in neighboring GMR-wts only tissue expressed Rh6 exclusively (Figure 3A ″). Similar results were obtained in mer mutant clones when sav (mer; GMR-sav) or sav and wts (mer; GMR-sav, wts) were mis-expressed in all photoreceptors (Figures 3B-B″ and data not shown). Therefore, mer might act downstream of the pathway, or it might act upstream but be required for the ability of sav and wts to regulate R8 subtype fate.
Figure 3. Warts requires Merlin to induce yR8 subtype.
(A–B″) all depict adult retina stained with antibodies for Rh5 (blue), Rh6 (red), and GFP (green). Dashed lines indicate clonal boundaries.
(A) mer4; GMR-wts (mer4 mutant clones (marked by absence of GFP) in retina that mis-expresses wts in all photoreceptors.) Note the presence of only Rh6 in GMR-wts tissue when mer is wild-type (GFP+), and absence of Rh6 when mer is removed (non-GFP).
(A′) GFP only.
(A″) Rh6 and Rh5 only.
(B) mer4; GMR-sav, wts (mer4 mutant clones marked by absence of GFP in retinas that mis-expresses sav and wts in all photoreceptors).
(B′) GFP only.
(B″) Rh6 and Rh5 only.
(C–E) Adult retinas stained with antibodies for Rh5 (blue) and Rh6 (red).
(C) mer4; lGMR>hpo. Compare to Figure 2D (mer4 mutants) and Figure S1H (GMR-hpo). Experiment was performed at 18 °C as lGMR>hpo flies do not eclose at 25 C. (D) lGMR>Myr-mats
(E) mer4; lGMR>Myr-mats. Compare to mer4 mutants in Figure 2D.
To address these possibilities, we used Hpo to activate the pathway independently of mer. If mer acts upstream of hpo and wts, as in growth signaling, activated Hpo should suppress the mer phenotype. Hpo can auto-activate when expressed at high levels (Praskova et al., 2004). Indeed, lGMR-hpo led all R8s to express Rh6 (data not shown, see Figure S1G) and suppressed the mer phenotype, inducing Rh6 in most R8s (Figure 3C). Activated Mats (lGMR>Myr-mats) also suppressed the mer mutant phenotype (Figures 3D–E). These results suggest that mer acts upstream of the hpo/sav/mats/wts core complex in R8, as in growth control. Moreover, because mer is required for wts and sav activity in R8, even when wts and sav are expressed at high levels, the Mer upstream branch is a critical activator of Wts for R8 subtype specification.
ex and dRassf are not required for R8 subtype specification
Intriguingly, two upstream genes whose functions are not required for R8 subtype determination, ex and dRassf, were sufficient to alter R8 subtypes when mis-expressed. lGMR>dRassf induced Rh5 which led to partial co-expression of Rh5 and Rh6 (Figure S4B), while lGMR>ex mimicked Hippo pathway activation and induced gain of Rh6 and loss of Rh5 (Figure S4C). ex and dRassf are likely sufficient to artificially perturb the Hippo pathway by performing their canonical activities—Ex to activate Hpo or sequester Yki, dRassf to inhibit Hpo—when ectopically expressed at high levels. This suggests that normal Ex and dRassf protein function is not prohibited in R8 subtypes; rather, they might be sequestered from the Wts/Hpo complex, or not highly expressed in post-mitotic R8s. Indeed, Ex levels are lower posterior to the morphogenetic furrow and in pupation relative to the larval eye disc (Milton et al., 2010). Supporting this hypothesis, we were unable to detect a difference in Ex immunoreactivity between wild-type and ex mutant clones in adult photoreceptors (data not shown), and temporal RNA-seq data from Drosophila modENCODE indicate that dRassf reaches its lowest expression level during late pupation (Graveley et al., 2010).
A role for Lgl and aPKC in R8 subtype specification
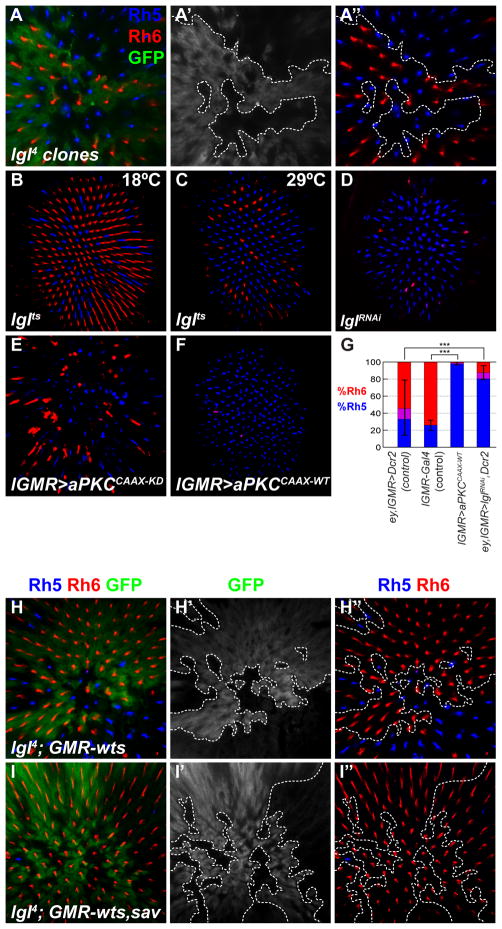
We next tested lgl, a neoplastic tumor suppressor and apico-basal cell polarity regulator that genetically interacts with the Hippo pathway in the developing eye disc (Grzeschik et al., 2010). lgl4 mutant clones contained R8s that expressed Rh5 almost exclusively, with little or no Rh6 (Figures 4A-A″). lgl-RNAi also led to induction of Rh5 and loss of Rh6 (ey, lGMR>lglRNAi: 80.6% Rh5, 11.7% Rh6, 7. 7% co-expression), as did a conditional allele (lglts3) at the non-permissive temperature (Figures 4B–D and 4G). The lgl mutant defect is specific to R8, as R7 and outer photoreceptor Rh expression was unaffected (Figures S3C-C′ and S3F-F′). Loss of lgl, or RNAi targeting another apico-basal polarity regulator, bazooka, severely disrupted photoreceptor polarity and rhabdomere morphology, as measured by F-actin localization (Figures S5A-A ″). But only lgl affected the Rh5:Rh6 ratio, suggesting that the lgl mutant yR8 specification defect is not due to general cell polarity aberrations.
Figure 4. Lgl is required and aPKC is sufficient to specify yR8 subtype.
(A–F, H–I″) Adult retinas stained for Rh5 (blue), Rh6 (red), GFP (green). Dashed lines indicate clonal boundaries.
(A-A″) lgl4 clones (non-GFP)
(A′) GFP only
(A″) Rh6 and Rh5 only. Dashed line indicates clone boundary.
(B) lglts3 raised at 18°C
(C) lglts3 shifted to 29°C during early pupation
(D) retina expressing lgl-RNAi
(E) lGMR>aPKCCAAX-KD(F) lGMR>aPKCCAAX-WT (active)
(G) Proportion of R8 that express Rh5 (blue), Rh6 (red), or both (purple) in listed genotypes. For all genotypes: n≥5, N≥1100; %Rh5 means compared with two-tailed, unpaired t-test; ***p<0.001;*p<0.05.
(H) lgl4 clones in GMR-wts retina background.
(H′) GFP only
(H″) Rh5 and Rh6 only
(I) lgl4 clones in GMR-wts, sav background.
(I′) GFP only
(I″) Rh5 and Rh6 only
We investigated another polarity regulator, aPKC, which acts antagonistically to Lgl (Lee et al., 2006), and is genetically linked to the Hippo growth pathway (Grzeschik et al., 2010). Over-expression of an “activated” aPKC targeted to the plasma membrane with a CAAX prenylation motif (lGMR>aPKCCAAX-WT) (Sotillos et al., 2004) disrupted photoreceptor polarity, and converted all R8s to pR8 (Figure 4F) without affecting R7 Rhs (Figure S5B). aPKC kinase function is required for this phenotype, as over-expression of a membrane tethered kinase-dead version (lGMR>aPKCCAAX-KD) did not strongly increase Rh5 (Figure 4E).
Wts requires Lgl to specify yR8 in parallel to Mer
Because lgl, mer, and wts have identical Rh phenotypes, we examined genetic interactions between lgl and core complex members, wts and sav. lgl mutant clones suppressed the ability of ectopic Wts to induce yR8 as most R8s expressed Rh5 in lgl; GMR-wts tissue (Figures 3H-H″) indicating that lgl, like mer, is required for Wts activity. But when sav was co-overexpressed with wts in lgl mutants, all R8s expressed Rh6 (Figures 4I-I″). Thus, the ability of Wts to become activated in lgl mutant R8s is sensitive to levels of sav. Furthermore, as mer can fully suppress the GMR-sav, wts overexpression phenotype while lgl cannot (compare Figures 3B-B ″ to 4I-I ″), Lgl might promote Wts activity in parallel to the Mer/Kib branch.
lgl mutants and aPKC gain-of-function have identical R8 phenotypes, suggesting that aPKC also influences the Hippo pathway to induce pR8 fate. Wts mis-expression significantly suppressed Rh5 induction but not the polarity phenotype of activated aPKC (Figure S5C), consistent with aPKC acting upstream or in parallel to Wts. These data are consistent with Lgl and aPKC acting upstream of Wts, likely in parallel to Mer/Kib, to regulate the core complex, although other possibilities cannot be excluded.
Upstream Hippo pathway regulators control wts and melt expression
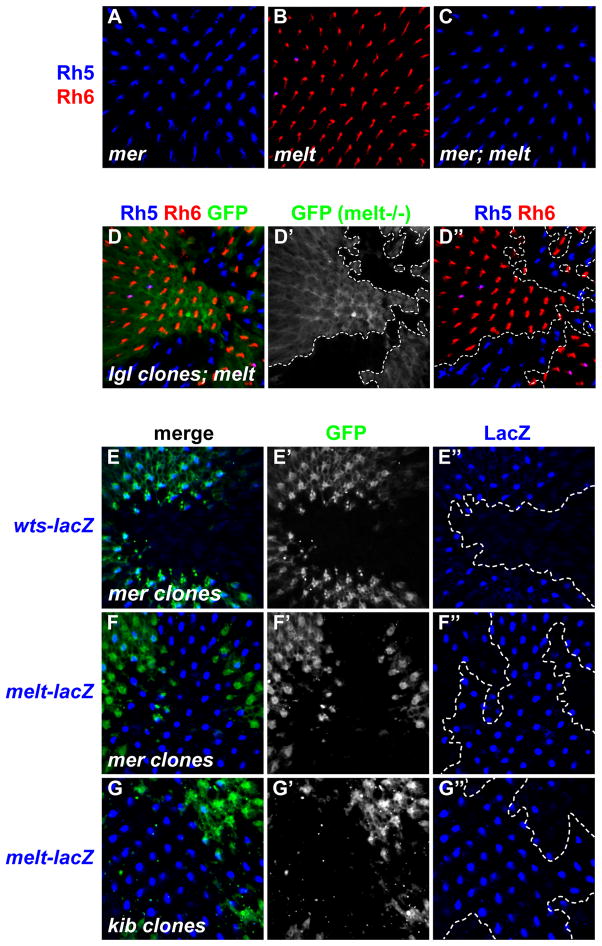
In R8, melt represses wts while wts represses melt transcription and wts is the ‘output’ of the bistable feedback loop for Rh expression (Mikeladze-Dvali et al., 2005). To test the epistatic relationship between mer and melt, we generated retinas double mutant for mer and melt (Figures 5A–C) which phenocopied mer single mutants: most R8 cells expressed Rh5 and less than 1% expressed Rh6 (Figure 5C). Similarly, loss of lgl suppressed the melt phenotype, as lgl mutant clones in melt mutant retinas expressed only Rh5 (Figures 5D-D″). Thus, like wts, mer and lgl are required for the melt phenotype, consistent with a role in R8 to activate Wts genetically downstream of melt.
Figure 5. Merlin and Kibra regulate the expression of Warts and Melted in R8.
(A–C) Adult retinas stained for Rh5 (blue) and Rh6 (red).
(A) hemizygous merts/Y mutants kept at the non-permissive temperature from early pupation.
(B) melt1.
(C) merts/Y; melt1 double mutants.
(D-D″) lgl4 clones in melt1 mutant background. Rh5 (blue), Rh6 (red), GFP (green). melt1 tissue is GFP-positive; lgl4; melt1 double mutant tissue is GFP-negative. Dashed lines indicate clonal boundaries.
(E–G″) Adult retinas showing either mer or kib mutant clones (GFP-negative) stained with β-gal antibody that labels nuclearly localized warts- or melt-lacZ. Confocal image taken in the R8 nucleus layer focal plane. In (E), (F), and (G), lacZ-positive R8 cells that overlap GFP represent wild-type R8 nuclei. Dashed lines indicate clonal boundaries.
(E) mer4 clones (GFP-negative) and warts-LacZ. Note the presence of warts-LacZ expression in wild-type tissue (GFP+).
(E′) grayscale of GFP in (E)
(E″) wts-lacZ of (E).
(F) mer4 mutant clones (GFP-negative) and melt-lacZ. Note that melt-lacZ expression is present in roughly a third of wild-type R8 cells, but in almost all mer4 mutant R8s.
(F′) grayscale of GFP in (F)
(F″) melt-lacZ of (F)
(G) kibdel mutant clones (GFP-negative) and melt-lacZ.
(G′) grayscale of GFP in (G)
(G″) melt-lacZ of (G).
To determine if upstream Hippo signaling influences the transcriptional feedback loop between wts and melt, we asked whether loss of mer affected wts or melt transcription. wts-lacZ expression was lost in mer mutant clones, and instead all R8s expressed melt-lacZ (Figures 5E–F″). Similar results were obtained for kib mutants, with slightly less expressivity (Figures 5G-G″ and data not shown). These data are consistent with a model wherein the upstream Mer/Kib branch represses melt and promotes wts transcription by keeping Wts protein active. In the absence of mer or kib, Wts protein kinase is inactive and unable to promote its own expression through the bistable feedback loop. Therefore, transcriptional feedback between wts and melt is coupled to the “ON/OFF” state of the entire upstream Mer/Kib activation branch of the Hippo pathway.
Mer and Hippo pathway are first required in R8 at ~40% pupation
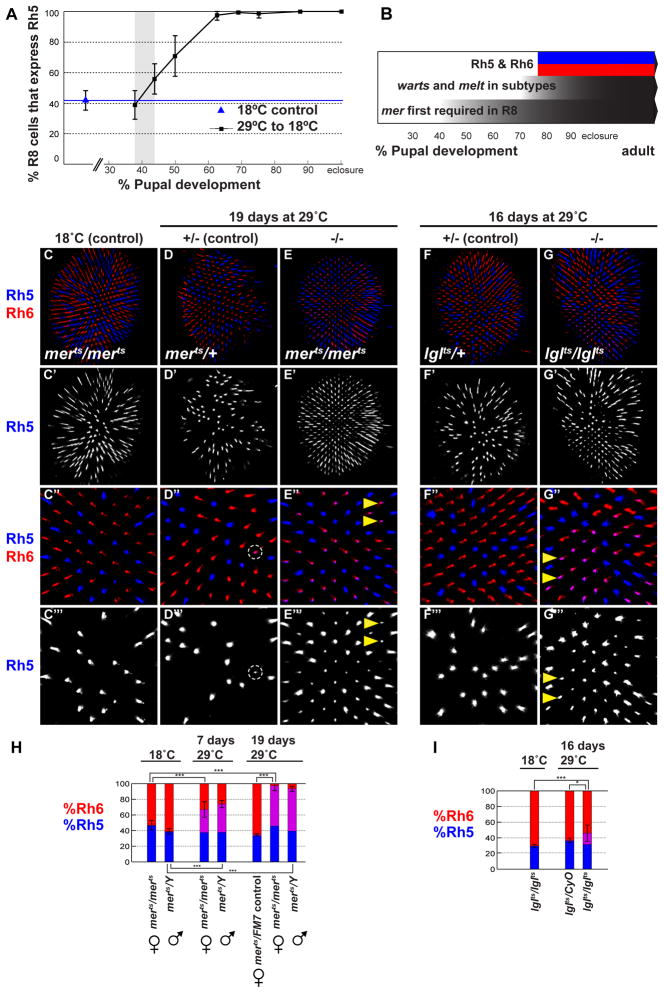
The genetic program that defines p- and y- ommatidial subtypes begins in early pupation with stochastic expression of ss in R7 cells (Wernet et al., 2006) followed by an R7-to-R8 signal that induces R8 subtypes, and then Rh5 and Rh6 expression around 70–80% pupation (Earl and Britt, 2006). To examine temporal dynamics of the Hippo pathway in R8 subtype specification, we took advantage of a temperature sensitive merts allele, which at the non-permissive temperature behaves genetically as to a null allele (MacDougall et al., 2001). To define the earliest requirement for mer, we moved flies to the non-permissive temperature at 10% pupation to remove mer function, and then shifted them permanently to the permissive temperature (18°C) to restore mer function at successively later time points. When mer was inactivated until up to ~38% pupation, we observed a wild-type Rh5:Rh6 ratio in R8 (Figure 6A). Yet when inactivation persisted until 44% pupation, the proportion of R8 cells expressing Rh5 increased, and continued to increase as mer function was removed for longer periods (Figure 6A). Complete transformation into pR8 was achieved when mer function was restored later than ~70% pupation, when R8 subtypes are fully established. Therefore, between 38 and 44% pupation (or even later as it might take time for wild-type mer function to recover), mer and upstream Hippo signaling become required in presumptive yR8 photoreceptors. Subtypes appear fully specified after 70–80% pupation, when melt/Rh5 and wts/Rh6 are expressed in p- or y- R8 subtypes, respectively (Figure 6B).
Figure 6. Continuous Merlin and Lgl signaling is required to maintain the yR8 subtype fate.
(A) mer is first required for yR8 specification at ~38–44% pupation (shaded gray bar). Graph shows proportion of R8s expressing Rh5 in adult retinas of merts flies where mer function was restored after shift from the restrictive to permissive temperature at various time points during pupation (black squares). (B) R8 subtype specification timeline during development. mer is required as early as ~40% pupation, followed by R8 subtype-specific expression of wts and melt, which define y- and p-R8, respectively, and induce Rh6 and Rh5 expression around 70–80% pupation. R8 subtypes and mutually exclusive Rh expression are maintained in adults.
All images in (C-G‴) are adult retinas stained for Rh5 (blue) and Rh6 (red).
(C-C‴) homozygous merts flies reared at the permissive temperature.
(C′) Rh5 of (C).
(C″) close up of (C).
(C‴) Rh5 of (C′)
(D) heterozygous merts/+ control flies shifted to the restrictive temperature (29°C) for 19 days during adulthood.
(D′) Rh5 of (D)
(D″) close up of (D). Rh5 and Rh6 are co-expressed in fewer than 2% of R8 cells (Dashed circle).
(D‴) Rh5 of (D″). Dashed circle indicates the rare yR8 that has weakly de-repressed Rh5.
(E) homozygous merts flies shifted to the restrictive temperature (29°C) for 19 days during adulthood.
(E′) Rh5 of (E). (E″) close-up of (E′). Arrowheads indicate examples of Rh5 de-repressed in the former yR8 subtype.
(E‴) Rh5 of (E″).
(F) heterozygous lglts/+ flies shifted to the restrictive temperature for 16 days during adulthood.
(F′) Rh5 of (F).
(F″) close-up of (F′).
(F‴) Rh5 of (F″).
(G) homozygous lglts flies shifted to the restricted temperature for 16 days during adulthood.
(G′) Rh5 of (G).
(G″) close-up of (G′). Many Rh6-expressing cells co-express Rh5 (pink); examples indicated by arrowheads.
(G‴) Rh5 of (G″).
(H) Proportion of R8s that express Rh5 when mer function is removed in adult flies, as compared to controls. y-axis shows percent exclusively Rh5 (blue), exclusively Rh6 (red), and Rh5/Rh6 co-expression (purple). Two-tailed, unpaired t-test; (***) denotes p<0.0001.
(I) Proportion of R8s that express Rh5 when lgl function is removed in adult flies, as compared to controls. y-axis shows percent exclusively Rh5 (blue), exclusively Rh6 (red), and Rh5/Rh6 co-expression (purple). Two-tailed, unpaired t-test; (***) denotes p<0.001. (*) denotes p<0.05.
Mer, Lgl, and Hippo pathway signaling are continuously required to maintain yR8 photoreceptor neuron subtypes
We next attempted to identify the latest point at which mer is required by using merts to remove mer function at successively later time points. Even when we inactivated mer as late as 90% pupation—after Rh5 and Rh6 are expressed—retinas always exhibited some de-repression of Rh5 into yR8s. Retinas from merts adult flies reared at 18°C and shifted to 29°C for 7 days (when they were 5–10 days old) also showed an increase in Rh5 expressing R8s, which often co-expressed Rh6, revealing that Rh5 is de-repressed into yR8 cells as R8s shift to pR8 fate upon loss of mer (Figure 6H). Strikingly, when merts flies were kept at 29°C for 19 days, almost all R8s expressed Rh5, and often co-expressed Rh6 (Figures 6E-E‴ and 6H; compare to 6C-D‴). Thus, in addition to its role in yR8 establishment, the mer upstream branch of the Hippo pathway is also required to maintain yR8 fate in adult flies.
To determine whether yR8 fate maintenance was specific to the Mer branch, or if it involved the entire Hippo pathway, we performed a similar experiment using lglts. Homozygous lglts flies reared at 18°C until several days post-eclosure and shifted to 29°C for 16 days de-repressed Rh5 into the yR8 subtype, which co-expressed Rh5 and Rh6 in 14.4% of R8s (Figures 6G-G‴). Control lglts flies reared continuously at 18°C or lglts/+ flies shifted to 29°C as adults exhibited a wild-type Rh5:Rh6 ratio (Figures 6F-F‴ and 6I). Thus, two different upstream regulators, mer and lgl, are required to specify as well as maintain yR8 subtypes, suggesting that the entire R8 Hippo pathway is required to keep Wts protein active for the life of the neuron.
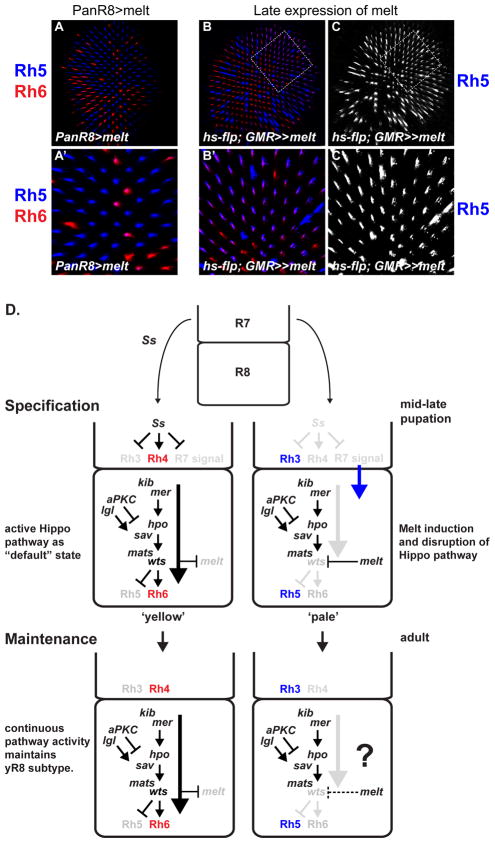
Melt is sufficient to re-specify adult yR8 into pR8
As wts expression and Rh6 (i.e., yR8) are the default state for R8 in the absence of an R7 signal, the role of melt in specifying pR8 appears to be as an “OFF” switch that can disrupt Hippo pathway activity (Figures 7A-A ′). This model predicts that ectopic Melt should still be able to turn off Hippo pathway activity in adult photoreceptors. To test this hypothesis, we mis-expressed Melt long after R8 subtype specification, starting in 7 day old flies using an inducible “flp-out” cassette (hs-FLP; GMR-FRT-w+, STOP-FRT-Gal4). This led almost all R8s in the dorsal half of the retina to express Rh5 while ~70% still contained Rh6 (Figures 7B–C ′). This phenotype is similar to late removal of mer or lgl function and demonstrates that melt is sufficient to abrogate Hippo signaling in yR8, to allow reactivation of Rh5 even in adult flies.
Figure 7. Model for regulation of Hippo pathway signaling in a post-mitotic fate decision.
(A–C′) All panels show adult retinas stained for Rh5 (blue) and Rh6 (red).
(A) PanR8>melt. melt expressed immediately after subtype-specific Rh expression can reset the bistable feedback loop and induce the pR8 fate.
(A′) close up of (A). Note increase in Rh5-only expressing pR8s and decrease in Rh6-expressing yR8 cells.
(B) ectopic melt expressed in adult flies (see methods).
(B′) close-up of (B). Note that Rh6 staining appears weaker in cells that co-express Rh5 and Rh6.
(C) Rh5 of (B).
(C′) Rh5 of (B′).
(D) Model of Hippo pathway regulation for binary fate specification of R8. Constitutive and continuous upstream pathway signaling by Mer, Kib, and Lgl promotes yR8 fate, while repression of warts by Melt disrupts Hippo signaling to specify pR8 fate. Genes in black or colored font are expressed and active in that particular cell; genes in gray font are not expressed or not active.
Discussion
This work describes a mechanism for a post-mitotic neuronal subtype decision wherein a complex signaling pathway is regulated both canonically and non-canonically to instruct either of two cell states. The control point for Hippo pathway signaling in R8 is the expression or repression of Wts, whose activity is promoted by constitutive upstream Hippo pathway activity (Figure 7D). Several lines of evidence support this model. First, two upstream inputs, mer/kib and lgl, and the core complex of hpo/sav/mats/wts are required for R8 subtype specification and all have identical Rh phenotypes. Second, mer is required for Wts activity and expression, indicating the entire Hippo pathway is coupled to the wts-melt transcriptional feedback loop and binary fate mechanism. Third, mer and lgl temperature-sensitive experiments showed that upstream Hippo pathway signaling is continuously required to maintain yR8 fate. Finally, Hippo signaling can be abrogated long after the fate decision by late induction of melt, which re-specifies pR8 fate, demonstrating that melt can disrupt active Hippo signaling.
Given that wts acts genetically downstream of melt in R8 subtype specification, why engage transcriptional regulation of wts instead of simply inactivating Wts kinase? Regulating signal transduction members by transcription might be an efficient way to arrest signaling permanently, especially if the regulation is mediated by a bistable loop, a common genetic motif used by cells to transition from one cell state to another, often irreversibly (reviewed in Ferrell, 2002). It is thus well suited to promote mutually exclusive gene expression and switch-like fate decisions (reviewed in Jukam and Desplan, 2010). Regulation of wts by melt acts as an “OFF” switch for Hippo pathway activity, and the bistable feedback loop reinforces this cell state transition, despite the activity of multiple upstream Hippo regulators.
Hippo pathway and R8 subtype specification dynamics
These results deepen our understanding of key temporal steps in R8 subtype specification. Ectopic kib, mats, and hpo can induce the yR8 fate in all R8s, but require Wts for their function, indicating that some basal level of Wts must be present initially in all R8s. Such Wts is likely dispensable for yR8 specification until at least 40% pupation, when mer is first required.
wts is de-repressed in melt mutants, and melt is de-repressed in wts mutants. Thus, general factor(s) must be competent to activate transcription of wts and melt in all R8s, independent from the R7 signal that coordinates R7/R8 subtypes. This activation would be restricted to mutually exclusive R8s by the bistable feedback loop. In presumptive yR8s, this general activator might boost Wts levels to allow sustained activation by constitutive upstream Hippo signaling. In presumptive pR8, the R7 signal could induce a further increase in melt expression or protein activity that allows Melt to inactivate the Hippo pathway and repress wts. Alternatively, the R7 signal might inhibit upstream activators of the Hippo pathway like Mer or Lgl, which likely act near the membrane and are candidates for substrates that mediate reception of the R7 signal. Melt could then be activated by loss of Wts, and reinforce the loss in Wts activity by transcriptionally repressing wts.
Mer, Lgl, and the Hippo pathway in neuronal maintenance
Neural subtypes, once specified, appear to require active genetic programs to maintain their fate (Lesch and Bargmann, 2010). A second role for the Hippo pathway in R8, in addition to specifying yR8, is to maintain exclusion of the opposite fate in old R8 neurons, by continuously repressing rh5 in adult yR8 subtypes. When mer or lgl was removed late, the yR8 character was partially lost, as many R8 cells contained both Rh5 and Rh6. Photoreceptors must coordinate sensory receptor expression with hard-wired axon projections to the visual processing centers (reviewed in Sanes and Zipursky, 2010), so the functional identity of the R8 neuron should be stable or the fly risks sensory confusion.
Context-Specificity and Mer, Kib, and Lgl protein function in the Hippo signaling network
Upstream inputs and downstream transcriptional targets of Wts signaling in development are context-dependent (Pellock et al., 2006; Cho et al., 2006; Peng et al., 2009; Milton et al., 2010, Grusche et al., 2010). Evidence that Kib can bind separately to either Ex or Mer, as well as synergistically in a complex to both, implies that Ex, Kib, and Mer act semi-redundantly in growth control (Genevet et al., 2010; Grusche et al., 2010). Our results demonstrate that Mer/Kib can operate as a branch completely independent from Ex upstream of Wts for R8 specification.
mer and lgl mutants phenocopy wts mutants with respect to R8 subtype specification. If Mer and Lgl do not act in a linear pathway or complex, why would Hippo signaling require two non-redundant upstream inputs to keep Wts protein active? Mer/Kib and Lgl might simply provide complementary means to promote Wts function in R8. The protein-protein interactions between Mer, Kib and Sav (Yu et al., 2010) and Kib and Wts (Genevet et al., 2010) support a localization or scaffolding role for Mer and Kib in activation of Hpo and Wts, perhaps by recruiting Hpo, Sav, and Mats near the membrane where Hpo can phosphorylate Wts. Membrane tethered Mats promotes Wts activation in our system and in growth (Ho et al., 2010). Kib, like Mats, Hpo, Sav, or Wts, is sufficient to induce the yR8 subtype. Dramatically increasing the expression level of kib, hpo, mats, sav, or wts might promote synergistic localization and binding of all six proteins near the membrane, resulting in phosphorylated and active Wts.
Lgl could function to inhibit a negative pathway regulator, such as the dSTRIPAK PP2A phosphatase complex that acts on Hpo (Ribeiro et al., 2010). Lgl would then promote activation of Wts by preventing inactivation of Wts or Hpo. Such regulation would be useful where a continuously active Hippo pathway is necessary, as in the maintenance of yR8 subtype fate. Alternatively, the intracellular polarity roles of Lgl and aPKC might generically affect localization of Hippo pathway components. Perhaps the molecular signal from R7 that promotes Melt and represses Wts activity shares features with non-autonomous signals that affect polarity regulators of Hippo signaling in dividing cells.
R8 subtypes as a model for studying Mer/NF2 function
Several reports suggest that Hippo pathway signaling might underlie some pathology of neurofibromatosis type 2 (NF-2). Mer/NF2 can regulate contact-dependent growth inhibition and nuclear localization of the Yki ortholog and oncogene, YAP, in human meningioma cell lines (Striedinger et al., 2008), as well as genetically interact with YAP during tumorigenesis in the mouse liver (Zhang et al., 2010). Other studies implicated mis-regulated EGFR signaling in NF-2 mutant tumors (Benhamouche et al., 2010) or suggested that Hippo pathway-like overgrowth phenotypes in mer; ex double mutants are a secondary consequence of EGFR mis-regulation in Drosophila (Maitra et al., 2006). Our results support a role for Mer upstream of the Hippo pathway in the context of R8 subtype specification. The binary assay of Rh5 or Rh6 in R8 photoreceptors can be used to dissect Mer protein function in Hippo signaling without complications from redundant upstream regulators of Wts.
Re-use of signaling pathways in development
The modular re-use of signaling pathway components for different purposes in development is widespread, and is likely a key molecular conduit for the evolution of cellular and morphological diversity (Wilkins, 2002). The uncoupling of mer, kib, lgl and the core Hippo signaling cassette from growth control allows the genes to contribute to a broader array of developmental decisions. It will be interesting to see if the Lgl/Mer/Kib/Hpo/Sav/Mats/Wts module is regulated by Melt in other post-mitotic fate specification events during neural development.
Experimental Procedures
Drosophila Stocks
For Drosophila genotypes and fly strains, see Supplemental Experimental Procedures.
Drosophila Genetics and Induction of Mutant Tissue
Flies were raised on corn meal-molasses-agar medium under standard laboratory conditions. y1, w67;+;+ flies were considered “wild-type” and used as a control for rh expression. For details on Gal4 drivers, generating whole-mutant retinas, the mer and lgl temperature sensitive experiments, and late induction of melt, please see Supplemental Experimental Procedures.
Immunostaining and Imaging
Adult retina dissections were performed as described (Mazzoni et al., 2008). Briefly, retinas were dissected in PBS over ice and fixed in PBS + 4% paraformaldehyde for 20 min at room temperature. After three washes in PBT (PBS + 0.2% Triton-X), the lamina was removed and retinas incubated overnight at room temperature with primary antibodies diluted in PBT. After three rinses and one 30min wash in PBT, retinas were incubated in secondary antibody diluted in PBT for 4–6 hours at room temperature, followed by four washes in PBT. Retinas were mounted using SlowFade (Molecular Probes, Invitrogen, Eugene, OR) on glass slides with coverslip.
Antibodies and dilutions were as follows: mouse anti-Rh3 (1:100, gift form S. Britt, University of Colorado), rabbit anti-Rh4 (1:100, gift from C. Zuker, Columbia University), mouse anti-Rh5 (1:200, S Britt), rabbit anti-Rh6 (1:2000), rabbit anti-Sal (1:100, gift from B. Mollereau, Ecole Normale Superieure-Lyon), goat anti-β-gal (1:5000, Biogenesis), rabbit anti-β-gal (1:5000, Cappel), sheep anti-GFP (1:1000, AbD Serotec), rabbit anti-GFP (1:1000, Invitrogen), mouse anti-Elav (1:40, DSHB). All secondary antibodies were Alexa Flour (488, 555, or 647)-conjugated made in donkey (1:800, Molecular Probes). Alexa Fluor 488 coupled Phalloidin (1:150, Invitrogen) was used to visualize actin to outline photoreceptor rhabdomeres.
All fluorescent images were taken with a Leica TCS SP2 or SP5 confocal laser scanning microscope.
Quantification and Statistics
Confocal images were taken and the number of R8 cells that expressed Rh5, Rh6, both, or neither were counted. The percentage of R8s expressing Rh5 (%Rh5) was calculated for each retina, and mean %Rh5 of all retinas within a genotype was used to compare across genotypes. A two-tailed, unpaired t-test was used to calculate statistical significance when appropriate. Retinas were scored if there were 75 or more ommatidia present in a single focal plane Most retinas contained ~200–300 ommatidia in a single image. For all genotypes, more retinas were observed than quantified to confirm a particular phenotype. All error bars in figures are ± one standard deviation around the mean.
Supplementary Material
Highlights.
mer, kib, and lgl specify yR8 photoreceptor neurons and induce Rh6 expression.
mer and kib are required for warts expression and melted repression in R8.
mer and lgl activity are continuously required to maintain the yR8 fate in adults.
melted is sufficient to disrupt active Hippo pathway signaling in R8.
Acknowledgments
We are grateful to S. Blair, S. Britt, S. Cohen, I. Davis, B. Dickson, R. Fehon, G. Halder, I. Hariharan, K. Irvine, J. Jiang, ZC. Lai, A. Laughton, D. Pan, N. Tapon, J. Treisman, T. Xu, C. Zuker, and the Bloomington Stock Center for generously providing fly stocks and antibodies. We thank N. Baker, G. Halder, P. McKenney, P. Sood, and members of the Desplan lab for helpful discussions and comments on the manuscript. D. J. was supported by a New York University Dean’s Dissertation Fellowship. C.
D. was supported by NIH grant RO1 EY13012.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, McNeill H. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev Cell. 2009;16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–616. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Baehrecke EH. Warts is required for PI3K-regulated growth arrest, autophagy, and autophagic cell death in Drosophila. Curr Biol. 2008;18:1466–1475. doi: 10.1016/j.cub.2008.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl JB, Britt SG. Expression of Drosophila rhodopsins during photoreceptor cell differentiation: insights into R7 and R8 cell subtype commitment. Gene Expr Patterns. 2006;6:687–694. doi: 10.1016/j.modgep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Emoto K, Parrish JZ, Jan LY, Jan YN. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rister J, Desplan C. The retinal mosaics of opsin expression in invertebrates and vertebrates. Dev Neurobiol. 2011 doi: 10.1002/dneu.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci. 2009;122:2351–2359. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hardie RC. Functional organization of the fly retina. In: Ottoson D, editor. Progress in Sens Physiol. 5. Springer; Berlin, Heidelberg, New York, Toronto: 1985. pp. 1–79. [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour- suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Ho LL, Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated at the cell membrane to control tissue growth and organ size in Drosophila. Dev Biol. 2010;337:274–283. doi: 10.1016/j.ydbio.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008;105:20067–71. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000 Oct;1(1):20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jukam D, Desplan C. Binary fate decisions in differentiating neurons. Curr Opin Neurobiol. 2010;20:6–13. doi: 10.1016/j.conb.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol. 1998;141:1589–1599. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Lesch BJ, Bargmann CI. The homeodomain protein hmbx-1 maintains asymmetric gene expression in adult C. elegans olfactory neurons. Genes Dev. 2010;24:1802–1815. doi: 10.1101/gad.1932610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCollin M, Mohney T, Trofatter J, Wertelecki W, Ramesh V, Gusella J. DNA diagnosis of neurofibromatosis 2. Altered coding sequence of the merlin tumor suppressor in an extended pedigree. JAMA. 1993;270:2316–2320. doi: 10.1001/jama.270.19.2316. [DOI] [PubMed] [Google Scholar]
- MacDougall N, Lad Y, Wilkie GS, Francis-Lang H, Sullivan W, Davis I. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development. 2001;128:665–673. doi: 10.1242/dev.128.5.665. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Celik A, Wernet MF, Vasiliauskas D, Johnston RJ, Cook TA, Pichaud F, Desplan C. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 2008;6:e97. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Milton CC, Zhang X, Albanese NO, Harvey KF. Differential requirement of Salvador-Warts-Hippo pathway members for organ size control in Drosophila melanogaster. Development. 2010;137:735–743. doi: 10.1242/dev.042309. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo analysis of Yorkie phosphorylation sites. Oncogene. 2009;28:1916–1927. doi: 10.1038/onc.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;304:102–115. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009;23:2307–2319. doi: 10.1101/gad.1820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesello C, Huelsmann S, Brown NH, Tapon N. The Drosophila RASSF homolog antagonizes the hippo pathway. Curr Biol. 2006;16:2459–2465. doi: 10.1016/j.cub.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137:2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PS, Josue F, Wepf A, Wehr MC, Rinner O, Kelly G, Tapon N, Gstaiger M. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell. 2010;39:521–534. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Rister J, Desplan C. The retinal mosaics of opsin expression in invertebrates and vertebrates. Dev Neurobiol. 2011 May 9; doi: 10.1002/dneu.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sotillos S, Diaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Chen YW, Cohen SM. Drosophila Melted modulates FOXO and TOR activity. Dev Cell. 2005;9:271–281. doi: 10.1016/j.devcel.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Tyler DM, Baker NE. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev Biol. 2007;305:187–201. doi: 10.1016/j.ydbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AS. The Evolution of Developmental Pathways. Sinauer; Sunderland, MA: 2002. [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.