Abstract
Capacity of certain Mycobacterium tuberculosis isolates to grow more rapidly in human macrophages may be indicative of increased virulence. Significant differences were observed in intracellular growth of two isolates from sites of tuberculosis transmission, with an outbreak-associated strain growing faster than a strain causing disease in only one person. Activated THP-1 cells are a suitable alternative to peripheral blood monocyte models.
Virulence of Mycobacterium tuberculosis strains has been traditionally assessed in terms of the ability of organisms to replicate within specific organs of mice and guinea pigs following aerosol infection. Human monocyte models have been used to examine intracellular growth rates of M. tuberculosis reference strains and clinical isolates to determine if these correlate with virulence as previously defined in animal models. In these models, a low ratio of M. tuberculosis bacteria to human macrophages was used to mimic in vivo conditions, and isolates were assessed for their initial uptake, intracellular growth rates, ability to resist peripheral blood lymphocyte-mediated limitation of growth, and induction of cytokines. M. tuberculosis H37Rv and its avirulent counterpart, H37Ra, have been shown to grow at similar rates in human monocyte-derived macrophages (5), whereas in another study intracellular growth and induction of tumor necrosis factor alpha (TNF-α) correlated with previous assessments of virulence in animal models, i.e., H37Rv grew more rapidly and induced less TNF-α than H37Ra (11). Results with the clinical isolate CDC 1551, which demonstrated an unusually high rate of transmission as evidenced by a high rate of tuberculin skin test conversion, have varied. In mice, CDC 1551 grew at a rate similar to that of H37Rv (9) and slightly faster than Erdman (4). Manca et al. (3) observed the intracellular growth rate of CDC 1551 in mice to be slightly higher than H37Rv, whereas Li et al. (2) found CDC 1551 to grow slightly more slowly than Erdman and H37Rv in human monocytes. In addition, the human macrophage model has also been used to determine whether M. tuberculosis strain 210 associated with outbreaks correlated with the capacity to grow (10). Isolates representing strain 210 grew significantly more rapidly than small-cluster and unique isolates but elicited production of similar amounts of cytokines. These variable observations indicate that further studies are needed to evaluate the human monocyte model for assessing virulence of clinical M. tuberculosis isolates.
Studies of mycobacteria and macrophage interactions have employed many systems (primary and in vitro-differentiated cells) to mimic what is thought to be the in situ situation, i.e., the association of M. tuberculosis with alveolar macrophages. Macrophage-like cell lines of human origin have been shown to be a good model for in vitro-differentiated monocyte-derived macrophages (7) and have the advantages of no donor variability of macrophage function and that large numbers of cells can be grown reproducibly, cells can be studied at different stages (resting versus activated), and the cells closely model alveolar macrophages for M. tuberculosis-induced apoptosis (6). The human acute monocytic leukemia cell line THP-1 develops macrophage functions following the addition of stimulators. Recently we examined the THP-1 cell line and its derivative, activated THP-1 (A-THP-1), which demonstrates characteristics of activated macrophages in the absence of stimulators, under different conditions to refine a model for differentiating clinical M. tuberculosis isolates on the basis of their growth rates. Herein, we describe a comparison of the two cell lines using two M. tuberculosis strains thought to differ in their abilities to spread in a community.
The A-THP-1 cells (graciously provided by Toshio Kudo, Tohoku University School of Medicine, Aoba, Sendai, Japan) were selected as adherent cells from a 9-year continuous culture of the original THP-1 cells (8). These adherent THP-1 cells showed remarkable phenotypic changes demonstrating their activated state, e.g., increased phagocytic activity and HLA-DR expression, and had the tendency to form multinucleated giant cells. In addition, these cells have an enhanced constitutive production of interleukin 1β. However, TNF-α was not detected in the culture supernatant by L929 bioassay (8). The A-THP-1 cells demonstrated enhanced stimulator activity in the mixed lymphocyte reaction test and increased complement receptor and FcγR expression. Our interest in examining A-THP-1 cells was based on their feature of being continuously activated by these criteria without the addition of any exogenous stimulators.
The clinical isolates TB282 and TB284 were selected from an epidemiologic study in central Los Angeles (1). TB282 represents strain 210, which is characterized by a 21-band IS6110 DNA fingerprint pattern and the Beijing spoligotype. TB282 was associated with a tuberculosis outbreak (cluster of 43 patients) and has since been found widely distributed in the United States. TB284 is characterized by a four-band IS6110 DNA fingerprint pattern, was isolated from a single patient (unique isolate) who resided in the same homeless shelter while infectious, and was assumed to be less successful in causing disease than strain 210. Strain 210 grows more rapidly in blood monocyte-derived macrophages than strains that demonstrate poor transmissibility, such as TB284 (10). M. tuberculosis H37Rv was included for comparison. Strain designation was blinded in all experiments.
THP-1 cells and A-THP-1 cells were grown and maintained as previously described (7). THP-1 cells were subcultured every third day, and A-THP-1 cells were subcultured every fifth day, for an initial density of 2 × 105 cells/ml. Prior to plating of the cell lines, viability was assessed by trypan blue exclusion staining. THP-1 cells were differentiated into adherent, well-spread macrophages by the addition of 100 nM phorbol myristate acetate (PMA) and, in some experiments, 150 U of recombinant human gamma interferon (IFN-γ) and maintained for 3 days at 37°C, 5% CO2. A-THP-1 cells were allowed to adhere overnight prior to utilization in experiments. The adherent cells were washed twice in binding medium (138 mM NaCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl, 0.6 mM CaCl2, 1 mM MgCl2, 5.5 mM d-glucose) prior to the addition of M. tuberculosis at a ratio of either 50 bacteria to 1 macrophage or 10 bacteria to 1 macrophage. M. tuberculosis bacteria and the cells were then gently rocked for 1 h at 37°C, 5% CO2 followed by 2 h of stationary incubation at 37°C, 5% CO2. After 3 h, M. tuberculosis bacteria not associated with the cells were removed by repeated washing of the monolayers with binding medium. Infected monolayers that were not immediately processed to assess uptake (day zero) were supplemented with fresh RPMI and reincubated at 37°C, 5% CO2. Binding of the M. tuberculosis bacteria to the macrophages was carried out in eight-well Nunc Lab-Tek chamber slides according to a method previously described (7). The binding of the M. tuberculosis bacteria with the cell lines was estimated by determining the percentage of cells with at least one mycobacterium associated. No attempt was made to differentiate between attachment and ingestion. Viability of the cell lines was assessed and estimation of cell numbers of the monolayers was done at 3 h and 1 day following infection by using 0.25% trypsin for 30 min at 37°C to detach the cells, followed by trypan blue exclusion staining. The level of M. tuberculosis infection was determined by quantitative culture of cell lysates at 3 h and 1, 2, 3, 5, 7, 9, and 14 days after infection. Cell lysates and supernatants were ultrasonically dispersed, and then serial dilutions were plated on Middlebrook 7H10 agar plates, with the number of CFU being determined after 3 to 4 weeks of incubation. Cultures of supernatants showed no growth at each time point, indicating that extracellular bacteria and intracellular bacteria in nonadherent macrophages were not present in significant numbers. Values represent at least three independent experiments.
Figure 1A illustrates the association of M. tuberculosis with PMA-activated THP-1 cells, PMA- and IFN-γ-activated THP-1 cells, and A-THP-1 cells at a 50:1 ratio. There was no significant difference between TB282 and TB284 for binding to the three macrophage cell types. However, binding of H37Rv was less to the fully activated (PMA and IFN-γ) THP-1 cells and more to A-THP-1 cells than was the case with the clinical isolates (P ≤ 0.05). Interestingly, THP-1 cells activated by PMA alone showed significantly less binding with all three strains than was the case with either of the fully activated cell lines (P ≤ 0.05). At a ratio of 10:1, binding was about 11% less than with the higher ratio (data not shown). These findings suggest that fully activated THP-1 macrophages bind more effectively and that binding is not strain dependent.
FIG. 1.
Binding of M. tuberculosis bacteria to PMA-activated THP-1 cells (THP-1/PMA), PMA- and IFN-γ-activated THP-1 cells (THP-1/PMA + IFN), and A-THP-1 cells (ATHP-1) at a ratio of 50 bacteria to 1 macrophage. Two clinical M. tuberculosis isolates and the virulent strain M. tuberculosis H37Rv were tested. The percentage of the macrophage population binding one or more mycobacteria was assessed microscopically. Each bar represents the mean ± standard error of the mean from three separate experiments. (A) THP-1 monolayers were established and incubated with M. tuberculosis bacteria in the absence of human serum. There was no significant difference in binding to the three cell types between TB282, TB284, and H37Rv, except for the binding of H37Rv to the fully activated (by PMA and IFN-γ) THP-1 cells and A-THP-1 cells (P ≤ 0.05). THP-1 cells activated by PMA alone showed significantly less binding (P ≤ 0.05) with all three strains than did either of the fully activated cell lines. (B) Binding was carried out in the presence of 1% normal human serum. THP-1 cells treated with PMA or PMA and IFN-γ and A-THP-1 cells showed increased association compared to binding in the absence of serum. No differences in binding among the three strains were observed.
Since activated THP-1 and A-THP-1 cells were able to bind to similar amounts of M. tuberculosis in the absence of serum, we decided to assess macrophage binding in the presence of 1% normal human serum (not heat inactivated) (Fig. 1B). It has previously been shown that normal human serum greatly enhances the binding of M. tuberculosis to THP-1 cells, and we decided to test this with activated THP-1 and A-THP-1 cells. At a ratio of 50:1, THP-1 cells treated with PMA or PMA and IFN-γ and A-THP-1 cells showed increased association (by 8 to 12%) with all three strains compared to binding in the absence of serum. No differences in binding were observed among the three strains. At 10:1, the percentage of macrophages associated with M. tuberculosis was essentially the same as that with the higher ratio (data not shown). As expected, normal serum enhances binding; however, there is no difference between THP-1 or A-THP-1 cells in their ability to associate with M. tuberculosis in the presence of serum.
Viabilities of PMA-activated THP-1, PMA- and IFN-γ-activated THP-1, and A-THP-1 cells infected with TB282, TB284, and H37Rv were similar regardless of the ratio of M. tuberculosis bacteria to macrophages. After the initial binding period and through day 3, viabilities ranged from 96 to 100%. Seven days following infection, cell viabilities decreased to 82 to 92%. By 14 days postinfection, 69 to 85% of cells were viable. The addition of serum did not alter these values. These results demonstrate that the cell lines are not immediately killed following infection with any of the M. tuberculosis strains and can survive infection at the ratios tested.
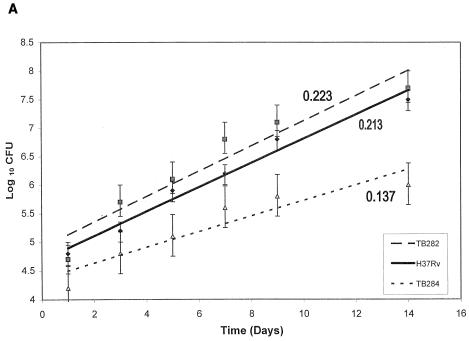
The capacity for intracellular growth of each of the strains was measured in the two cell lines under fully activated conditions at both ratios. Figure 2A shows growth rates for a 14-day infection with TB282, TB284, and H37Rv at a ratio of 50:1 in PMA- and IFN-γ-activated THP-1 cells. Numbers of CFU in the macrophage lysates were comparable after the 3-h incubation period for all the strains, indicating that equivalent numbers of bacteria were ingested and the extent of phagocytosis was independent of strain type. The mean intracellular CFU for each strain in both cells lines was log 4.65 ± 0.3 at 1 day postinfection. Differences in the intracellular growth rates between strains were observed as soon as day 5. After 14 days, the mean log CFU for TB282 and H37Rv were essentially the same and significantly higher than for TB284 (P ≤ 0.05), and the growth rates over this period of time were significantly higher than for TB284 (P ≤ 0.01). After 7 days, growth rates were even higher; however, TB284 showed a significantly decreased rate compared to the other strains. Similar intracellular growth rates were observed in the infected A-THP-1 cells (Fig. 2B). By day 7, there was an obvious difference in the growth rates for the three strains. Again, strains TB282 and H37Rv showed significantly increased growth rates compared to TB284 (P ≤ 0.01). At the 10:1 ratio, the two activated cell lines ingested fewer bacilli and the growth rates were overall lower; however, the relationship of TB284 to TB282 and H37Rv remained the same, i.e., TB284 grew significantly more slowly than the other two strains (data not shown). Likewise, the number of bacteria ingested with serum binding was higher than with non-serum binding and comparable with all strains, and the growth rate of TB284 was lower (data not shown).
FIG. 2.
Intracellular growth of TB282, TB284, or H37Rv was measured in THP-1 cells activated with PMA and IFN-γ (A) and A-THP-1 cells (B), and extracellular growth was measured in 7H9 broth (C). Fourteen days of incubation in both fully activated THP-1 cell lines showed a significantly decreased growth rate for TB284 in contrast to that for TB282 and H37Rv (P ≤ 0.01). A significant difference in growth rates between TB284 and TB282 or H37Rv was observed as early as 7 days following infection. Although the growth rates were higher, TB284 showed a significantly decreased rate compared to the other strains (for PMA-activated THP-1 cells: TB282, 0.333; H37Rv, 0.274; TB284, 0.217; for A-THP-1 cells: H37Rv, 0.328; TB282, 0.283; TB284, 0.165) (P ≤ 0.01). To investigate if TB284 was incapable of growing at a rate similar to those for TB282 and H37Rv, growth rates were compared in broth (C). There were no significant differences in the growth rates between strains. Error bars indicate standard deviation for at least three separate experiments.
To investigate whether strain TB284 was simply incapable of growing at a rate similar to those of TB282 and H37Rv, growth rates in broth culture were determined following culture in Middlebrook 7H9 medium for 7 days (Fig. 2C). Growth rates for the three strains were the same, indicating that the decreased intracellular growth rate of TB284 was not due to some intrinsic growth characteristic.
In summary, despite the similar innate growth characteristics (in broth culture), differences among these strains are consistently maintained in an epidemiological context in blood monocyte-derived macrophages, A-THP-1 cells, and THP-1 cells. Growth rates differ depending on the extent of activation of THP-1 cells, the multiplicity of infection (50:1 versus 10:1), the presence or absence of serum during binding, and the growth period (1 to 7 days versus 1 to 14 days); however, the relationship among strains remain the same. The A-THP-1 cells are an attractive alternative to THP-1 cells, since no exogenous stimulators are needed to activate the cells and adherence of the monolayer takes place overnight, decreasing the assay time. The two clinical isolates we tested displayed clearly different capacities for growth within human macrophages, and the higher growth rate observed with TB282 (strain 210) correlates with its ability to spread in communities. One recognizes that the relationship between the intrinsic virulence of an isolate or strain measured as a growth rate in macrophages and rate of transmission among humans is obviously complex and is going to be difficult to define. Thus, having a reliable model for distinguishing clinical isolates on the basis of intracellular growth rates will be useful, with the possibility that this model will be used to elucidate differences in virulence among M. tuberculosis isolates.
Acknowledgments
This work was supported in part by a contract from the National Institutes of Health: AI-45244/AI-95383 (Tuberculosis Research Unit).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Barnes, P. F., Z. Yang, S. Preston-Martin, J. M. Pagoda, B. E. Jones, M. Otaya, K. D. Eisenach, L. Knowles, S. Harvey, and M. D. Cave. 1997. Patterns of tuberculosis transmission in central Los Angeles. JAMA 278:1159-1163. [PubMed] [Google Scholar]
- 2.Li, Q., C. C. Whalen, J. M. Albert, R. Larkin, L. Zukowski, M. D. Cave, and R. F. Silver. 2002. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 70:6489-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. J. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 4.Ordway, D. J., M. G. Sonnennberg, S. A. Donahue, J. T. Belisle, and I. M. Orme. 1995. Drug-resistant strains of Mycobacterium tuberculosis exhibit a range of virulence for mice. Infect. Immun. 63:741-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul, S., P. Laochumroonvorapong, and G. Kaplan. 1996. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J. Infect. Dis. 174:105-112. [DOI] [PubMed] [Google Scholar]
- 6.Riendeau, C. J., and H. Kornfeld. 2003. THP-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 71:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokes, R. W., and D. Doxsee. 1999. The receptor mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell Immunol. 197:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Tominga, T., M. Suzuki, H. Saeki, S. Matsuno, T. Tachibana, and T. Kudo. 1998. Establishment of an activated macrophage cell line, A-THP-1, and its properties. Tohoku J. Exp. Med. 186:99-119. [DOI] [PubMed] [Google Scholar]
- 9.Valway, S. E., M. P. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]
- 10.Zhang, M., J. Gong, Z. Yang, B. Samten, M. D. Cave, and P. F. Barnes. 1999. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J. Infect. Dis. 179:1213-1217. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, M., J. Gong, Y. Lin, and P. Barnes. 1998. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect. Immun. 66:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]