Abstract
We have proposed that neuropathic pain engages emotional learning, suggesting the involvement of the hippocampus. Since cytokines in the periphery contribute to induction and maintenance of neuropathic pain, but might also participate centrally, we used two neuropathic pain models, chronic constriction injury (CCI) and spared nerve injury (SNI), to investigate the temporal profile of hippocampal cytokine gene expression in two rat strains that show different post-injury behavioral threshold sensitivities. SNI induced long-lasting allodynia in both strains, while CCI induced allodynia with time-dependent recovery in Sprague-Dawley (SD) and no allodynia in Wistar Kyoto (WK) rats. In WK rats, only SNI induced sustained upregulation of hippocampal IL-1β, while IL-6 expression was transiently increased and no significant changes in IL-1ra expression were detected. Conversely, in SD rats, SNI resulted in sustained and robust increased hippocampal IL-1β expression, which was only transient in rats with CCI. In this strain, IL-6 expression was not affected in any of the two injury models and IL-1ra expression was significantly increased in rats with SNI or CCI at late phases. The main findings reported are that: 1) the degree and development of neuropathic pain depend on the specific nerve injury model and rat strain; 2) hippocampal IL-1β mRNA levels correlate with neuropathic pain behavior; 3) in contrast to sham-operated animals, there are no correlations between hippocampal IL-1β and IL-1ra or IL-6 in neuropathic rats; and 4) alterations in cytokine expression are restricted to the hippocampus contralateral to the injury side, again implying that the observed changes reflect nociception.
Keywords: neuropathic pain, hippocampus, cytokines, IL-1, IL-1ra, lateralization
1. Introduction
We have advanced a general model for the role of supraspinal circuitry in the transition from acute to chronic pain [2, 4]. The model proposes that sub-cortical limbic circuitry play a critical role in re-organizing cortical circuitry and transitioning the latter from monitoring sensory properties to a state where pain becomes internalized and emotional in nature. The amygdala, ventral striatum, and hippocampus are envisioned as critical limbic components of this transition. Here we concentrate on the role of the hippocampus in chronic neuropathic pain behavior. Moreover, we have proposed that chronic pain can be reformulated as a state of continuous associative learning with no opportunity for extinction [3, 4], implicating hippocampal-cortical learning processes.
Prefrontal cortex undergoes rapid reorganization in animals with neuropathic pain [40], which also exhibits increased levels of cytokine expression [5]. As the hippocampus tightly interacts with the prefrontal cortex, it may also reorganize with neuropathic pain. In fact, accumulating evidence shows abnormal hippocampal function in chronic pain, see review [38]. A recent study indicates that neuropathic animals exhibit working memory deficits and decreased long-term potentiation (LTP) mediated by upregulation of hippocampal TNF [50], yet these changes were not related to the pain behavior of the animals. Neuropathic rats also show bilateral hippocampal increase in NF-kappaB [17] and in cytokines [1], differential regulation of gene expression [25, 33, 55], and impaired neurogenesis [26, 54]. Given that the hippocampus is critically involved in anxiety and depression [10] and since neuropathic pain is also commonly associated with anxiety and depression, it remains unclear to what extent these reported changes reflect secondary stress-related effects and if any are a direct modulation of hippocampal processing by neuropathic pain.
The expression of cytokines, such as TNF, IL-1β and IL-4, is decreased in the hippocampus hours after peripheral nerve injury [55], yet again the extent to which these changes reflect general stress or effects of pain remains unclear. On the other hand, we have shown that in vivo and in vitro LTP induction in the hippocampus results in a long lasting increase in IL-1β and IL-6 expression [9, 52]. Furthermore, blockade of IL-1 signaling impairs the maintenance of LTP [52] while blockade of endogenous IL-6 prolongs it [9]. Also, both cytokines can affect learning of hippocampus-dependent tasks [9, 23, 60]. Thus, it is possible that the regulation of cytokine expression by neuropathic pain underlies hippocampal reorganization. As a first step to address this hypothesis, we studied hippocampal IL-1β the endogenous antagonist of IL-1 (IL-1ra), and IL-6 gene expression in two models of neuropathic pain: chronic constriction nerve injury (CCI) and spared nerve injury (SNI), which display different degrees of pain behavior in response to nerve injury [12, 20]. Since different strains of rats display variable manifestation of neuropathic pain behaviors after peripheral nerve injury [39, 61], we compared changes in cytokine expression related to the maintenance of neuropathic pain between Wistar-Kyoto (WK) and Sprague-Dawley (SD) rats.
2. Material and methods
2.1. Animals
Male Wistar Kyoto and Spague Dawley rats (250-350 grams) were obtained from Harlan, Indianapolis, IN. All procedures were approved by the Animal Care and Use Committee (ACUC) at Northwestern University, Chicago, and were in accordance with the NIH guidelines for the ethical use of laboratory animals.
2.2. Surgical procedures
Animals were anesthetized with isofluorane 5%, and a mixture of 30% N2O and 70% O2. Two different models were used to induce neuropathic injury on the left hind paw. In the chronic constriction injury model (CCI), the left sciatic nerve was exposed above the level of trifurcation, and four loose knots were carefully applied to the nerve using absorbable chromic gut [12]. In the spare nerve injury (SNI) model, the left sciatic nerve was exposed at the level of its trifurcation into the sural, tibial and common peroneal nerves, and each of the tibial and common peroneal nerves was tightly ligated by two knots 4 mm apart and then completely severed in between, leaving the sural nerve intact [20]. In sham operated animals, the sciatic nerve was exposed but not manipulated. After surgery, wounds were sutured using a non-absorbable surgical suture, and treated with a topical antibiotic ointment.
2.3. Von Frey test
Tactile thresholds were monitored in all operated animals (CCI, SNI and sham) prior to and at different time points post-injury, using the Von Frey test. Mechanical sensitivity of the hind paw was measured by determining withdrawal thresholds to Von Frey filaments. All tests were performed on the right (uninjured) and left (injured) hind paws. The 50% threshold for each paw withdrawal was calculated as described by Chaplan et al. [15]. The behavioral assessment of signs for neuropathic pain was evaluated only by this test because the procedure is minimally stressful. All measures were done in a blinded fashion, where right and left paw data were collected separately to minimize expectation bias.
2.4. Tissue collection
Animals were not handled for 48 hours prior to sacrifice. At the times indicated in the figures, Wistar Kyoto rats from the three experimental groups were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused with RNA-ase free saline (Accugene PBS). Sprague Dawley rats were anesthetized using Isofluorane (inhalation) and then decapitated. In both cases, the brains were removed from the skull and placed on a cold glass surface previously sprayed with Rnaze. A perpendicular cut was made to separate the cerebral hemispheres and the cerebellum. The cerebral hemispheres were then glued to the cutting platform with the rostral tip upwards; thick (600-1000 μm) coronal slices containing the hippocampus (approximately from Bregma -2.52 to -6.12 mm; [46]) were then cut, and the slices from the right and left hemisphere were collected in separate petri dishes filled with ice cold PBS. The hippocampus was then carefully dissected out under a stereo microscope, and immediately frozen and stored at -80°C until PCR analysis was performed for the determination of cytokine gene expression.
2.5. Determination of cytokine gene expression
Total RNA was extracted from the hippocampus using TRIzol Reagent (Invitrogen Life Technologies) according to a standard protocol [16]. The RNA was treated with 2U DNaseI (Epicentre technologies) in 10x Buffer Y+/Tango (MBI Fermentas) followed by purification using RNeasy Mini Spin Columns (Qiagen) according to the manufacturer's instruction and eluted in 30μl RNase free water. Reverse transcription (RT) was performed as previously described [5]. Briefly, 1μg total RNA was reversed transcribed using 40U MMLV reverse transcriptase (Invitrogen Life Technologies) and 0.5mg/ml oligop(dT) 12-18 primers (Amersham Biosciences) in a total volume of 20 μl. RT was performed at 42°C for 60 min and 70°C for 15 min. PCR was performed in a volume of 25 μl with the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems) using optical reaction tubes. A master mix was prepared containing 12.5μl 2x PCR buffer (100mMKCl, 20mM Tris HCl pH 8.3, 0.02mM EDTA, 0.1% gelatin 0.02% Tween20), nucleotides dATP, dCTP, dGTP (200μM each), 400μM dUTP, 1.0μl 25mM MgCl2, 0.625 U AmpliTaqGold (PE Applied Biosystems), 0.25 U Uracil-DNA-Glycosylase (UDG) (New England Biolabs), 200 nM of each primer, 100 nM of the corresponding probe, and Rox dye in a final concentration of 300 nM (TIB MOLBIOL). 21μl of the master mix were added to each well of 96 well-plates followed by addition of 4μl cDNA. PCR products were verified by cloning using the PCR 2.1Topo® Vektor from Invitrogen GmbH, Life Technologies (Karlsruhe, Germany), followed by sequencing. The sequences were confirmed using the NCBI gene data bank. Standard curves were generated for all genes analyzed. The efficiency of the PCR reaction was between 96% and 100 %. All PCR reactions were performed 3 to 4 times at least in triplicate using the following conditions: initial 50°C for 2 min and 95°C for10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The average of the PCR reaction was the outcome measure for each sample. Primer and fluorogenic probes were designed using the automated primer analysis software Primer Express (PE Applied Biosystems). Primer and probes were chosen to bind in different exons or to span exon junctions to prevent amplification of genomic DNA. The forward primers for the genes evaluated, the fluorogenic internal probes and the reverse primers used were exactly as previously described [21, 22]. The comparative CT method described by Fink et al. [27] was used to calculate relative gene expression since we have previously determined that the amplification efficiencies of the target and reference genes are approximately the same. Thus, cytokine mRNA levels were normalized to the reference gene in each sample. The value of sham animals was arbitrarily set at 1.0.
2.6. Statistical analysis
Results are expressed as mean ± SE. Data were analyzed with StatView 5.0 using one-way ANOVA followed by Fisher's PLSD test for multiple comparisons. Regression analysis was also performed using StatView 5.0. Differences were considered significant for p<0.05.
3. Results
The CCI and SNI models of neuropathic pain were used to study the profile of pain-associated cytokine expression in the rat hippocampus. Two strains of rats (SD and WK) were used in order to exploit their different behavioral response to these surgeries [39]. Mechanical allodynia, which is widely used as a behavioral readout of neuropathic pain, was evaluated using the Von Frey test before and after experimental nerve injury, starting 4 days after the operation.
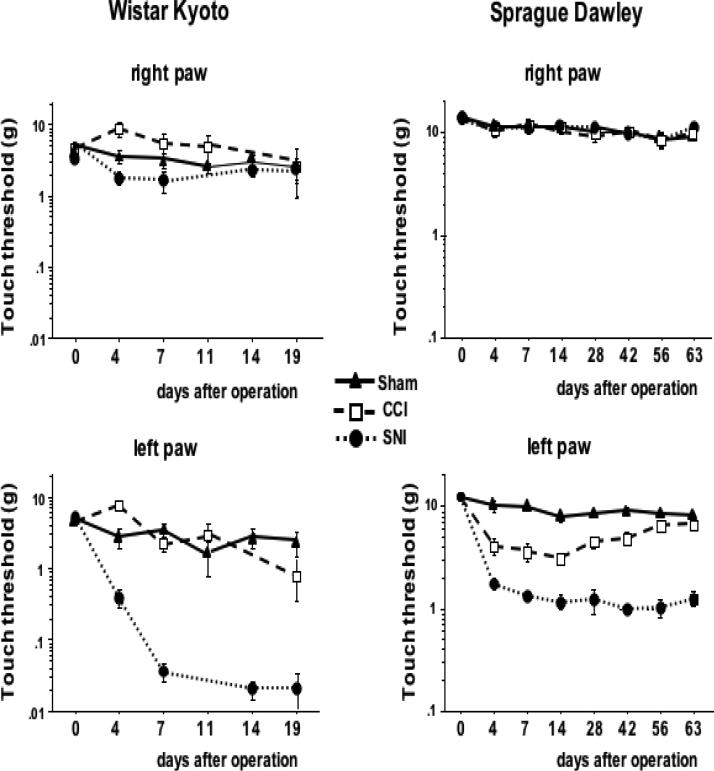
In WK rats, SNI-induced mechanical allodynia in the left paw was already present on day 4 and was maintained up to day 19 after the neuropathic injury (Fig. 1). On the contrary, CCI did not produce detectable significant effects on tactile thresholds, consistent with previous findings [5]. In SD rats, both SNI and CCI resulted in mechanical hypersensitivity in the hind paw ipsilateral to the peripheral nerve injury and was also already present on day 4 after surgery, while no difference of tactile threshold was observed in the contralateral hind paw. Interestingly, while SNI-induced allodynia remained constant for up to 9 weeks after surgery, CCI-induced allodynia gradually recovered, starting 28 days after surgery (Fig. 1).
Figure 1. Tactile allodynia shows dependence on strain and nerve injury model.
Neuropathic injury on the left hind paw was performed by chronic constriction (CCI) or spare nerve injury (SNI) of the left sciatic nerve in Wistar Kyoto (WK) and Sprague Dawley (SD) rats. Sham-operated rats served as control. Tactile thresholds were monitored in all operated animals prior to and at different time points post-injury using the Von Frey test. In WK rats, consistent and statistically significant decreases in touch thresholds were observed only for the paw with nerve damage in the SNI model at times longer than 4 days past injury in contrast to rats with CCI and sham-operated animals (n ranged from 14 to 4 as different animals were sacrificed at varying time points for cytokine analyses), In SD rats, significantly decreased touch thresholds were observed in both, SNI and CCI, models as compared to sham (n=9 per group and time point). Data were analyzed by ANOVA. Results are expressed as mean ± SE.
Pro-inflammatory cytokines, such as IL-1β and IL-6, have been shown to be involved in the peripheral mechanism of neuropathic pain development [45, 53]. Very recently, it has also been reported that acute or chronic peripheral administration of IL-1ra attenuates the allodynic response in mouse models of partial nerve injury or complete nerve cut [29] but dual effects of this antagonist have been reported in a model of spinal cord injury-induced neuropathic pain in morphine-treated animals [31]. Having determined that the behavioral effects of the surgical nerve injuries differed in intensity and duration in WK and SD rats, we evaluated IL-1β, IL-6, and IL-1ra gene expression in the hippocampus contralateral to the injured side in both strains of rats and at different times after surgery in the two models of neuropathic injury and compared with the expression in sham-operated animals. Tactile thresholds were determined 48 hrs before sacrifice.
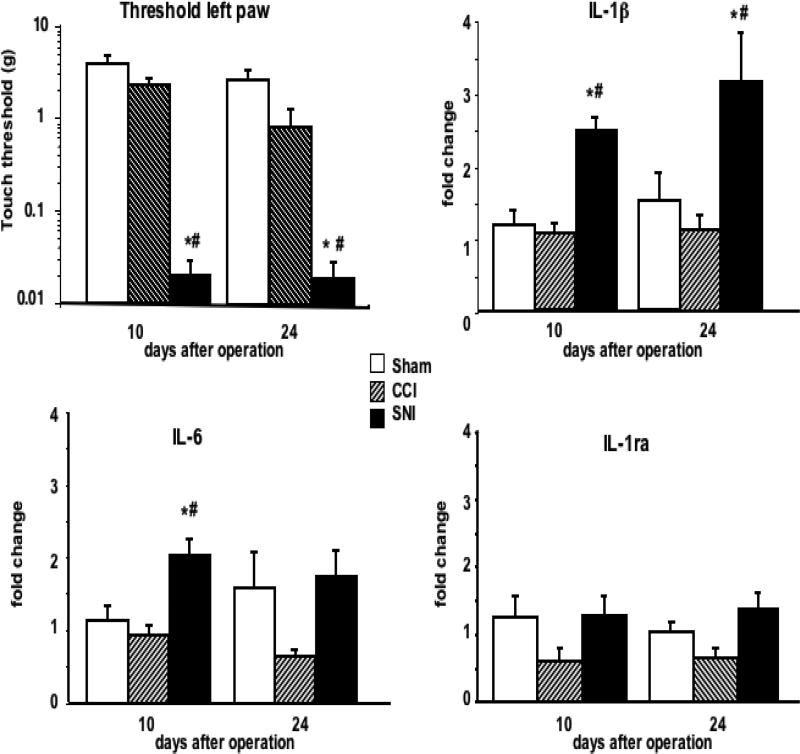
In agreement with the lack of behavioral effects, CCI surgery failed to induce significant changes in IL-1β, IL-6, and IL-1ra expression in the hippocampus of WK rats over the times tested, as compared to the sham-operated controls (Fig. 2). Conversely, IL-1β mRNA levels were 2.5-3-fold elevated in the contralateral hippocampus of WK rats in which tactile thresholds were decreased as consequence of the SNI performed 10 days before and were still elevated 24 days after surgery (Fig. 2). IL-6 expression was significantly increased only on day 10. On day 24, 2 animals in the sham group had higher levels of IL-6 expression than the other 6 rats, thus resulting in a larger SE in this group than on day 10. In any case, no statistically significant differences in IL-6 expression were detected either between CCI and SNI on day 24, suggesting that the increase in the expression of this cytokine in WK with SNI was transient. Although there was a tendency to decreased IL-1ra mRNA in the hippocampus of rats with CCI, this difference was not statistically significant in WK rats (Fig. 2).
Figure 2. Tactile thresholds of the injury paw and cytokine gene expression in the contralateral hippocampus of WK rats at different times after nerve injury.
Groups of CCI, SNI or sham-operated WK rats were killed 10 and 24 days after operation and cytokine gene expression in the right side (contralateral to the lesion) of the hippocampus was evaluated. Tactile thresholds were determined 2 days before sacrifice. Results are expressed as mean ± SE of 8 (sham-operated) or 4 rats (CCI or SNI) per group and per time point. * p < 0.05 vs. sham; # p< 0.05 vs. CCI.
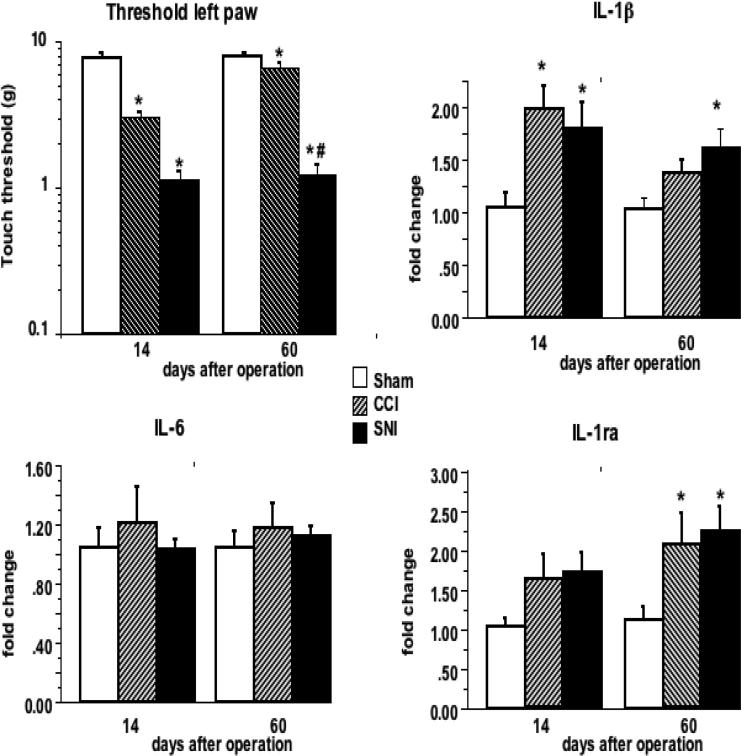
In SD rats, both SNI and CCI resulted in decreased tactile thresholds 14 days after surgery (Fig. 3). Hyperalgesia was still maintained 60 days after the operation in rats that had undergone SNI, while the tactile threshold was nearly recovered in rats with CCI. In agreement with the observed behavioral effects, IL-1β mRNA expression in the contralateral hippocampus was significantly increased 14 days after surgery in both pain models. However, although a trend toward higher IL-1β expression could be detected in rats with CCI, IL-1β mRNA remained significantly elevated up to 60 days only in rats with SNI, i.e. in those in which the decreased tactile threshold persisted.
Figure 3. Tactile thresholds of the injury paw and cytokine gene expression in the contralateral hippocampus of SD rats at different times after nerve injury.
Groups of CCI, SNI or sham-operated SD rats were killed 14 and 60 days after operation and cytokine gene expression in the right side (contralateral to the lesion) of the hippocampus was evaluated. Tactile thresholds were determined 2 days before sacrifice. Results are expressed as mean ± SE of 8 rats per group and time point. * p < 0.05 vs. sham; # p< 0.05 vs. CCI
Interestingly, the effect of SNI and CCI on IL-6 and IL-1ra expression in the hippocampus was different in SD as compared to that in WK rats. While SNI and CCI failed to produce any detectable effect on the expression of IL-6 mRNA level in the hippocampus, IL-1ra expression tended to increase 14 days after SNI and CCI, and it was significantly increased in both pain models after 60 days, reaching levels that were about 2-fold of those of the sham-operated controls.
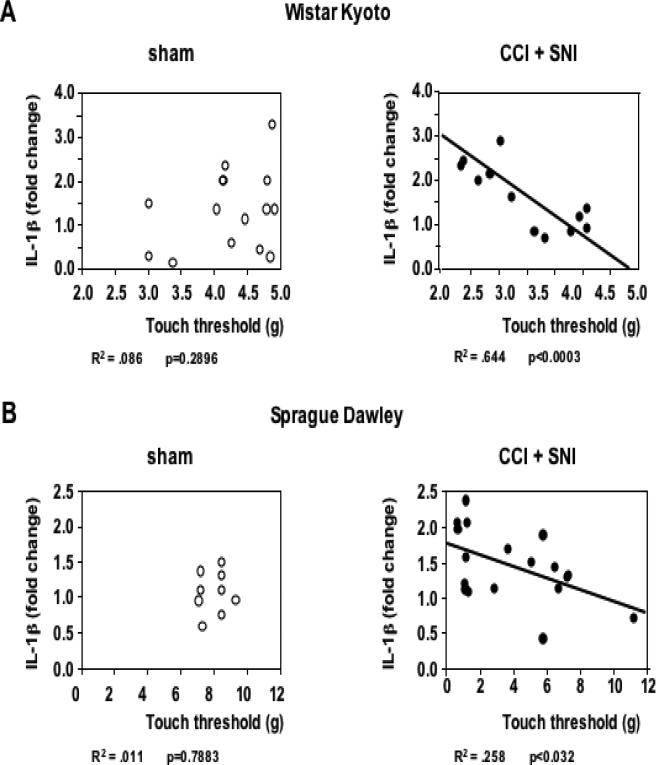
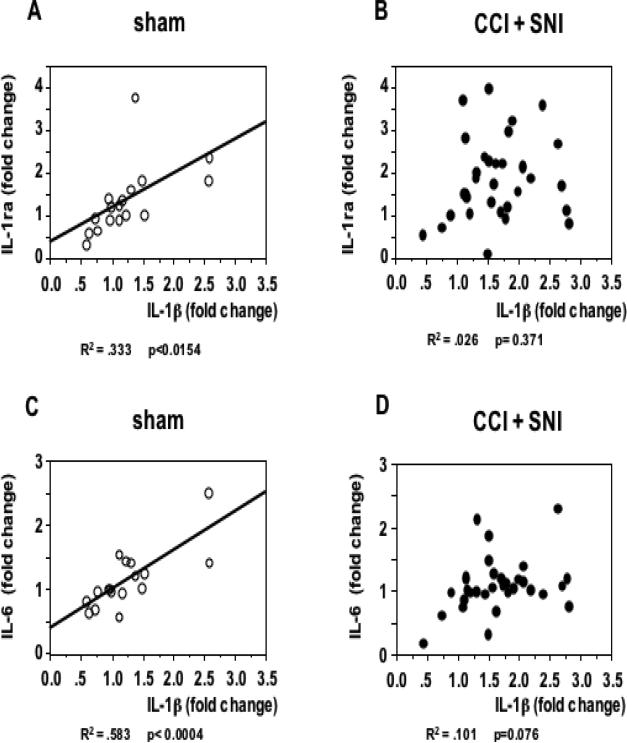
It is worth noting that IL-1β expression in the hippocampus was highly correlated with the degree of mechanical allodynia. As shown in Fig. 4, there was a linear correlation between the tactile threshold in the injured (left) leg and IL-1β mRNA levels in the contralateral hippocampus in both strains, for both nerve injury models (but not in sham operated animals), and at different time points after operation.
Figure 4. Correlation between pain perception and hippocampal IL-1β gene expression in the contralateral hippocampus.
A. Tactile thresholds in the left (injured) leg significantly correlated with IL-1β gene expression in the contralateral (right) side of the hippocampus of WK rats after CCI or SNI (n=12; data pooled from 10 and 24 days post nerve injury). No significant correlation between these parameters was observed in sham-operated rats (n=15). B. Similar results are seen in SD rats 60 days after CCI or SNI (n=16) but not in sham-operated animals (n= 9). The corresponding R2 and p values as determined by regression analysis are indicated below each corresponding panel.
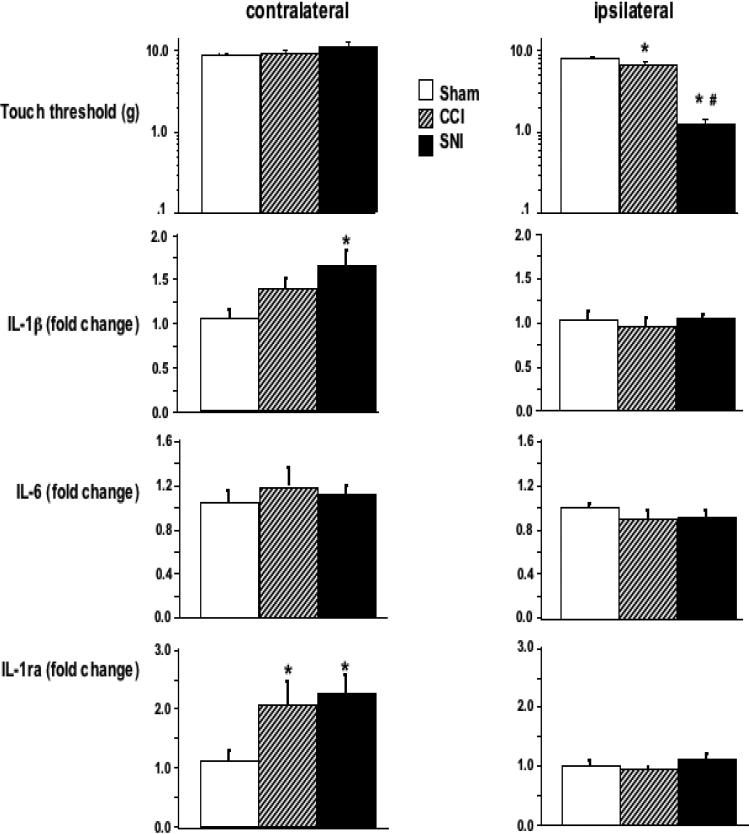
It has been known for long time that when the immune system is activated, particularly via Toll-like receptors, the production of IL-1β, IL-6, and IL-1ra is concomitantly increased [24]), and we have shown that these cytokines are simultaneously overexpressed in the brain and in the periphery following endotoxin challenge at doses that do not disturb the blood-brain barrier (BBB) and that have no apparent effects on animal behavior [21, 47]. Positive correlations between IL-1β and IL-1ra, and between IL-1β and IL-6 expression were observed in the hippocampus of sham-operated SD rats (fig. 5A, C). To the best of our knowledge, this is the first time that such positive correlations are reported, and, interestingly, they were not observed in animals with CCI and SNI (Fig. 5B, D). The results presented so far were obtained in the hippocampus contralateral to the lesion site and agree with our previous work in WK rats showing cytokine modulation in supraspinal regions to be lateralized and limited to contralateral areas [5]. As a next step we tested whether this lateralization holds true also for hippocampal expression. Thus, we further examined the expression of cytokines in the ipsilateral hippocampus on day 60 after the neuropathic injury in SD rats. As compared to the sham-operation, neither SNI nor CCI treatment led to any significant change in the expression of the cytokines evaluated in the ipsilateral hippocampus (Fig. 6). These data suggest a clear hemispheric lateralization of cytokine expression modulation in the hippocampus of SD rats with neuropathic pain.
Figure 5. Lack of correlation between cytokine gene expression in the hippocampus of SD rats with chronic pain.
The expression of IL-1β on the side of the hippocampus contralateral to the operated paw 14 and 60 days after sham (A, C; n=16) or CCI and SNI (B, D; n= 32) in SD rats is plotted together with the IL-1ra and IL-6 values corresponding to each individual animal. While positive correlations were observed in sham-operated rats, no significant correlations were established in rats with chronic pain. The corresponding R2 and p values as determined by regression analysis are indicated below each corresponding pannel.
Figure 6. Changes in cytokine gene expression are observed only in the hippocampal side contralateral to the nerve lesion.
Tactile thresholds in the left (injured) and right paws and cytokine gene expression in both sides of the hippocampus of SD rats were determined 60 days after CCI, SNI or sham-operation. Results are expressed as mean ± SE of 8 rats per group. * p<0.05 vs. sham.
4. Discussion
The temporal course of neuropathic pain development and hippocampal cytokine gene expression were compared between two rat strains (WK versus SD) and between two peripheral nerve injury models (SNI versus CCI). Our results highlight strain-related differences in the development of allodynia in models that differ in the extent of nerve injury. SNI results in irreversible damage of the sciatic nerve while the effects of CCI are at least in part reversible [12, 36]. We show here that SNI-induced mechanical allodynia showed no reversibility in both SD and WK rats during the time tested, while in the CCI model SD rats showed partial recovery from hyperalgesia already four weeks after surgery, and WK rats did not develop mechanical allodynia at all. Differences in hyperalgesic responses have been described even within sub-strains of SD rats [39, 59], and, although the underlying mechanisms are not clear, they may be attributable to genetic differences that could result in different organization of the sciatic nerve [42, 43, 51, 61].
An interesting observation concerns the correlation between the allodynic response measured with von Frey filaments and changes in the hippocampus. Such correlation suggests that even an apparentaly simple nocifensive response is heavily regulated by hippocampal circuitry.
IL-1β is a pro-inflammatory cytokine that has been implicated in the induction and maintenance of neuropathic pain [30, 49]. We have previously reported that IL-1β induction in the brain stem and prefrontal cortex of WK rats following SNI is related to the presence of painful experience [5]. Here we show that, also in the hippocampus, the SNI- or CCI-induced upregulation of IL-1β is closely correlated with mechanical allodynia in both rat strains. Hippocampal involvement in chronic pain has been suggested previously; for example, LTP in the CA1 area is moderately decreased in brain slices from neuropathic animals [37]. On the other hand, acute (2 hrs) bee venom- or formalin-induced pain affects synaptic efficacy in the dentate gyrus and CA1 area [62]. In line with the hippocampal involvement suggested by these studies, cognitive function deficits have been reported in patients and animals with chronic pain [6, 33, 34]. Since IL-1 is involved in the regulation of synaptic plasticity and memory processes in the hippocampus [8, 11, 23, 35, 52, 60], the neuropathy-induced increased hippocampal expression of IL-1β may correlate with disturbance of hippocampal function. Recently published evidence that acute interference of IL-1 signals in the brain reduces the depressive-like symptoms observed during SNI [44] indicates that over-expression of IL-1 in the hippocampus may be relevant for these symptoms.
The findings that IL-1β is over-expressed in the hippocampus during chronic pain and that there is a significant correlation between pain phenotype and hippocampal IL-1 gene expression indicate that this cytokine plays an active role during this process. However, tentative conclusions about causality are limited. Injection of IL-1β in the brain can induce hyperalgesia or analgesia, depending on the dose and region where it is administered [13, 32]. Also, studies in animals with permanent impediment of IL-1 signaling [57] do not answer the question of whether an increase of endogenous IL-1 contributes to pain sensitivity or whether such increase is a consequence of hyperalgesia. Studies showing attenuation of the allodynic response by intrathecal IL-1ra injection several days after inflammatory neuropathy [41], and by peripheral administration of the antagonist four days after spinal nerve transection [29] suggest that the correlation between pain sensitivity and IL-1 gene expression indicates that increased IL-1βresults in lower tactile thresholds. However, this speculation has also to be taken cautiously since in none of these studies IL-1 effects were neutralized directly at supraspinal levels, and it is still debatable whether IL-1ra can cross the BBB [18, 41]. We are aware of only two publications using neuropathic pain models in which IL-1 was neutralized directly in the brain. Injection of an anti-IL-1 antibody in the Red nucleus 2 weeks after SNI in SD rats significantly attenuated mechanical allodynia [56]. However, they did not find changes in IL-1β immunoreactivity in the hippocampus. In the study by Norman et al. [44], using the SNI model in mice, IL-1ra was injected i.c.v., but the functional consequence of IL-1 neutralization on mechanical allodynia was not tested.
We have evaluated IL-6 in the hippocampus during pain because we have shown that this cytokine is overexpressed during LTP and that it plays a role in hippocampal-dependent learning [9]. Although less studied than IL-1, there are also reports indicating the involvement of IL-6 during pain. For example, IL-6 levels are increased in both ipsilateral and contralateral lumbar dorsal root ganglia and in the spinal cord following peripheral nerve injury [14]. Spinal administration of IL-6 elicits anti-nociceptive effects [28] and spinal IL-6 levels correlate with the intensity of nerve injury-induced mechanical allodynia [7]. On the other hand, intrathecal administration of an IL-6 neutralizing antibody significantly decreases allodynia [7, 19]. Also, spinal nerve lesion-induced mechanical, but not thermal, allodynia is reduced in IL-6 knockout mice [48], and other authors have reported that IL-6-deficient female mice exhibit signs of neuropathic pain significantly more frequently than IL-6-deficient male or wild type mice following peripheral nerve injury, suggesting that some effects of IL-6 in pain are sex-related [58]. Thus, our finding that IL-6 levels are not affected in the hippocampus of SD rats with SNI- or CCI-induced neuropathic pain was unexpected. On the other hand, SNI treatment did produce an increase in IL-6 expression in the hippocampus of WK rats, although the duration of the increase was shorter than that of IL-1β. These results may indicate that, as it happens with the different manifestation of pain behaviors, there might also be a strain-related dependence of hippocampal IL-6 expression after peripheral nerve injury.
IL-1ra is an endogenous cytokine that interferes with most of the effects of IL-1. Our results showing a concomitant over-expression of IL-1 and IL-1ra in SD rats with SNI is in line with the findings that this antagonist is generally produced following IL-1 induction. However, IL-1ra was not increased in WK with SNI, which had decreased threshold sensitivity and IL-1β over-expression in the hippocampus. Since, as already mentioned, mice with genetic impairment of IL-1 signaling display a decreased allodynic response [29] and exogenously administered IL-1ra reduces neuropathic pain in mice [29, 44], it is possible that endogenously produced IL-1ra contributes to some extent to modulate threshold sensitivity, but its production during pain may also be strain-dependent.
Interestingly, IL-1β mRNA levels in the hippocampus of sham-operated SD rats positively correlated with those of IL-1ra and IL-6. The fact that these correlations were not observed in animals with CCI and SNI indicates that the interactions between these cytokine are disrupted in the hippocampus of animals with chronic pain.
Finally, our data indicate a clear hemispheric lateralization of cytokine expression following SNI or CCI. While SNI or CCI-induced mechanical allodynia was observed only at the hind paw ipsilateral to the nerve injury, increased IL-1β and IL-1ra mRNA levels appeared specifically in the contralateral hippocampus, indicating that changes in cytokine expression are not due a stress response, as the latter is expected to show bilateral effects. The present results agree with our previous study showing lateralization of IL-1β expression in the pre-frontal cortex of WK rats 10 days after SNI [5]. However, they apparently contradict those reported by Al-Amin et al. [1]. These authors found bilateral increases in hippocampal IL-1β levels 14 days after SNI and CCI, and even more expression in the ipsilateral than in the contralateral side in the last model. Female SD rats were used in this study and SNI and CCI induced similar neuropathic manifestations over 3 weeks. Furthermore, the groups that could be compared to ours received daily i.p. injections of saline during 2 weeks. It is possible that these differences may account for the discrepancy in the results.
We have shown that the hippocampus is among the brain regions in which IL-1β transcripts are more increased following IL-1 production at peripheral levels [47]. Thus, one possibility is that IL-1β produced in the periphery as consequence of nerve damage induces its own “de novo” production in the hippocampus. However, our finding that the increased IL-1β expression is lateralized suggests that a different mechanism might operate during chronic pain. Given that nociceptive information is primarily conveyed to the contralateral hemisphere, we interpret the laterality of IL-1β expression as being driven by nociceptive inputs to the hippocampus, which is consistent with our evidence that its level of expression correlated with pain behavior.
In summary, our study shows that the degree and development of neuropathic pain are correlated to specific nerve injury models and rat strains, that hippocampal IL-1β expression is correlated with pain behavior, that neuropathic pain seems to disrupt a network of cytokine interactions in the hippocampus, and that the alterations in cytokine expression are restricted to the hippocampus contralateral to the injury side. Further studies are required to clarify the pathophysiological links between the induced cytokines and pain states as well as hippocampal function.
Acknowledgements
This study was funded by NIH NINDS RO1 NS057704 (AVA), and NINDS RO1 NS064091 (M.M). We thank E. Becker for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that no conflict of interest exists in connection with this study.
IL-1 gene overexpression in the contralateral hippocampus correlates with neuropathic pain behavior in rats, and its correlation with hippocampal IL-1ra or IL-6 are lost.
References
- 1.Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol. 2011;228:30–40. doi: 10.1016/j.expneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, del Rey A. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett. 2006;407:176–181. doi: 10.1016/j.neulet.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- 8.Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 9.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. Faseb J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 10.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 12.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi M, Dib B, Panerai AE. Interleukin-1 and nociception in the rat. J Neurosci Res. 1998;53:645–650. doi: 10.1002/(SICI)1097-4547(19980915)53:6<645::AID-JNR2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Brazda V, Klusakova I, Svizenska I, Veselkova Z, Dubovy P. Bilateral changes in IL-6 protein, but not in its receptor gp130, in rat dorsal root ganglia following sciatic nerve ligature. Cell Mol Neurobiol. 2009;29:1053–1062. doi: 10.1007/s10571-009-9396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Chou CW, Wong GT, Lim G, McCabe MF, Wang S, Irwin MG, Mao J. Peripheral nerve injury alters the expression of NF-kappaB in the rat's hippocampus. Brain Res. 2011;1378:66–71. doi: 10.1016/j.brainres.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 19.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96:1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. table of contents. [DOI] [PubMed] [Google Scholar]
- 20.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 21.del Rey A, Randolf A, Wildmann J, Besedovsky HO, Jessop DS. Re-exposure to endotoxin induces differential cytokine gene expression in the rat hypothalamus and spleen. Brain Behav Immun. 2009;23:776–783. doi: 10.1016/j.bbi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Rey A, Wolff C, Wildmann J, Randolf A, Hahnel A, Besedovsky HO, Straub RH. Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 2008;58:3090–3099. doi: 10.1002/art.23869. [DOI] [PubMed] [Google Scholar]
- 23.Depino AM, Alonso M, Ferrari C, del Rey A, Anthony D, Besedovsky H, Medina JH, Pitossi F. Learning modulation by endogenous hippocampal IL-1: blockade of endogenous IL-1 facilitates memory formation. Hippocampus. 2004;14:526–535. doi: 10.1002/hipo.10164. [DOI] [PubMed] [Google Scholar]
- 24.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 25.Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133:999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- 27.Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- 28.Flatters SJ, Fox AJ, Dickenson AH. Spinal interleukin-6 (IL-6) inhibits nociceptive transmission following neuropathy. Brain Res. 2003;984:54–62. doi: 10.1016/s0006-8993(03)03092-0. [DOI] [PubMed] [Google Scholar]
- 29.Gabay E, Wolf G, Shavit Y, Yirmiya R, Tal M. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. Eur J Pain. 2011;15:242–248. doi: 10.1016/j.ejpain.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Hook MA, Washburn SN, Moreno G, Woller SA, Puga D, Lee KH, Grau JW. An IL-1 receptor antagonist blocks a morphine-induced attenuation of locomotor recovery after spinal cord injury. Brain Behav Immun. 2011;25:349–359. doi: 10.1016/j.bbi.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hori T, Oka T, Hosoi M, Aou S. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci. 1998;840:269–281. doi: 10.1111/j.1749-6632.1998.tb09567.x. [DOI] [PubMed] [Google Scholar]
- 33.Hu Y, Yang J, Wang Y, Li W. Amitriptyline rather than lornoxicam ameliorates neuropathic pain-induced deficits in abilities of spatial learning and memory. Eur J Anaesthesiol. 2010;27:162–168. doi: 10.1097/EJA.0b013e328331a3d5. [DOI] [PubMed] [Google Scholar]
- 34.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 36.Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 37.Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol. 2007;574:127–132. doi: 10.1016/j.ejphar.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 38.Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25:237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovell JA, Stuesse SL, Cruce WL, Crisp T. Strain differences in neuropathic hyperalgesia. Pharmacol Biochem Behav. 2000;65:141–144. doi: 10.1016/s0091-3057(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 40.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 43.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 44.Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; Amsterdam: 2007. [DOI] [PubMed] [Google Scholar]
- 47.Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain. 1998;78:115–121. doi: 10.1016/S0304-3959(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 49.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral Nerve Injury Leads to Working Memory Deficits and Dysfunction of the Hippocampus by Upregulation of TNF-alpha in Rodents. Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Terada M, Kuzumaki N, Hareyama N, Imai S, Niikura K, Narita M, Yamazaki M, Suzuki T. Suppression of enriched environment-induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett. 2008;440:314–318. doi: 10.1016/j.neulet.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 55.Uceyler N, Tscharke A, Sommer C. Early cytokine gene expression in mouse CNS after peripheral nerve lesion. Neurosci Lett. 2008;436:259–264. doi: 10.1016/j.neulet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Wang J, Li X, Yuan Y, Fan G. Interleukin-1 beta of Red nucleus involved in the development of allodynia in spared nerve injury rats. Exp Brain Res. 2008;188:379–384. doi: 10.1007/s00221-008-1365-1. [DOI] [PubMed] [Google Scholar]
- 57.Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Xu XJ, Hao JX, Andell-Jonsson S, Poli V, Bartfai T, Wiesenfeld-Hallin Z. Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine. 1997;9:1028–1033. doi: 10.1006/cyto.1997.0243. [DOI] [PubMed] [Google Scholar]
- 59.Xu XJ, Plesan A, Yu W, Hao JX, Wiesenfeld-Hallin Z. Possible impact of genetic differences on the development of neuropathic pain-like behaviors after unilateral sciatic nerve ischemic injury in rats. Pain. 2001;89:135–145. doi: 10.1016/s0304-3959(00)00356-0. [DOI] [PubMed] [Google Scholar]
- 60.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Yoon YW, Lee DH, Lee BH, Chung K, Chung JM. Different strains and substrains of rats show different levels of neuropathic pain behaviors. Exp Brain Res. 1999;129:167–171. doi: 10.1007/s002210050886. [DOI] [PubMed] [Google Scholar]
- 62.Zhao XY, Liu MG, Yuan DL, Wang Y, He Y, Wang DD, Chen XF, Zhang FK, Li H, He XS, Chen J. Nociception-induced spatial and temporal plasticity of synaptic connection and function in the hippocampal formation of rats: a multi-electrode array recording. Mol Pain. 2009;5:55. doi: 10.1186/1744-8069-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]