Abstract
Patients with chronic kidney disease (CKD) have a disproportionate burden of coronary artery disease and commonly undergo revascularization. The role and safety of percutaneous coronary intervention (PCI) using drug eluting stents (DES) verses bare metal stents (BMS) in CKD patients not on renal replacement therapy has not been fully evaluated. This study investigated the efficacy and safety of DES in patients with CKD not on renal replacement therapy. Patients where drawn from the National Heart, Lung, and Blood Institute Dynamic Registry and were stratified by renal function based on estimated glomerular filtration rate (GFR). Of the 4157 participants, 1108 had CKD (“low-GFR” <60 ml/min/1.73m2), while 3049 patients had normal renal function (“normal-GFR”≥60 ml/min/1.73m2). For each strata of renal function, we compared the risk of death, myocardial infarction (MI), or repeat revascularization between subjects who received DES and BMS at the index procedure. Patients with low-GFR had higher one-year rates of death and MI and a decreased rate of repeat revascularization when compared to patients with a normal-GFR. The use of DES was associated with a decreased need for repeat revascularization in the normal-GFR group (adjusted hazard ratio [HR] 0.63, 95% CI 0.50–0.79, p<0.001) but not in the low-GFR group (HR 0.69, 95% CI 0.45–1.06, p=0.09). The risks of death and MI were not different between the two stents in either patient population. In conclusion, the presence of CKD predicted poor outcomes after PCI with high rates of mortality regardless of stent type. The effect of DES in reducing repeat revascularization appeared to be attenuated in these patients.
Keywords: Drug-eluting, Bare metal, Stents, Renal dysfunction
INTRODUCTION
Chronic kidney disease (CKD) affects more than 26 million Americans and is associated with significant cardiovascular morbidity and mortality.1 Clinical studies have shown that patients with CKD have poor outcomes after percutaneous coronary interventions (PCI). Although this risk is highest in patients with end-stage renal disease (ESRD) on renal replacement therapy, patients with lesser degrees of renal dysfunction also appear to be at greater risk of cardiovascular morbidity and mortality after PCI.2 Prior small studies demonstrate that the risk of restenosis after PCI with balloon angioplasty and bare metal stent (BMS) insertion is higher in patients with CKD compared to patients with normal renal function.3–6 The beneficial effects of drug-eluting stents (DES) demonstrated in randomized clinical trials7,8 has led to their widespread use. However, there is limited data of their safety and efficacy in patients with renal dysfunction not on chronic renal replacement therapy. In addition to safety concerns, recent small observational studies and post-hoc analyses of clinical trials have shown conflicting results regarding the efficacy of DES compared to BMS.9–15 Accordingly, we sought to explore the safety and efficacy of PCI with DES as compared to BMS in patients with renal dysfunction not on chronic renal replacement therapy.
METHODS
Patients were drawn from the NHLBI Dynamic Registry. This study population is comprised of consecutive patients from multiple institutions undergoing elective and non-elective PCI. Study details and data collection methods have been documented previously.16. Briefly, patients were enrolled in 5 recruitment waves of approximately 2,000 patients. Our analysis includes patients recruited from wave 3 (October 2001 to March 2002, n=2047), wave 4 (February to May 2004, n=2112), and wave 5 (February to August 2006, n=2178). Patients enrolled during waves 1 and 2 were excluded as baseline serum creatinine values were not collected.
Patients were stratified by their estimated glomerular filtration rate (eGFR) at the time of angiography and stent type received during the intervention. The simplified 4-variable Modification of Diet in Renal Disease (MDRD) equation was used to estimate GFR using age, race, sex, and serum creatinine.
We stratified patients included in the present analysis into two groups based on baseline level of renal function. Patients with eGFR <60 ml/min/1.73m2 but ≥10 ml/min/1.73m2 were included in the “low-GFR” group, while patients with eGFR ≥60 ml/min/1.73m2 were included in the “normal-GFR” group. Patients with missing data on age (n=10), race (n=2) and serum creatinine (n=271) were excluded because of an inability to calculate GFR. Furthermore, patients with ESRD on renal replacement therapy or with a GFR<10 ml/min/1.73m2 were excluded (n=94). Patients from wave 3 not receiving a stent were excluded (n=273) as were patients from waves 4 and 5 who received a bare metal stent due to a substantial bias in stent selection (BMS vs. DES) (n=837). Additionally, patients presenting with an ST elevation myocardial infarction (STEMI) were excluded (n=693).
Data from 4157 patients was available for analysis. Baseline demographic, angiographic and procedural information was collected. The incidence of death, MI, repeat PCI and CABG during hospitalization and during follow-up were recorded. Follow-up was obtained at 1, 6 and 12 months after the index procedure by telephone interview and chart review. All clinical centers received approval from their respectiveinstitutional review boards. Data was compiled and interpreted at the Epidemiology Data Center of the University of Pittsburgh.
Death was defined as all-cause mortality. MI was defined by evidence of at least one of the two following criteria:(1) Evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous electrocardiogram (ECG) leads, or new or presumably new LBBB pattern on the ECG. (2) Biochemical evidence of myocardial necrosis manifested as (a) CK-MB≥3 times the upper limit of normal, (b) Total CK≥3 times the upper limit of normal or (c) Troponin level above the upper limit of normal. Repeat PCI was defined as any repeat PCI in the follow up period, including target lesion and target vessel revascularization. Repeat revascularization was defined as the combination of repeat PCI and/or CABG during the follow up period.
Patients were stratified by both GFR (low or normal) and stent type (BMS/wave 3 patients or DES wave 4 or 5 patients). Within each GFR strata, patient characteristics at index PCI were analyzed by stent type (BMS v. DES), including patient demographics, past medical history, initial cardiac presentation, peri-procedural medications, procedural characteristics, and in-hospital outcomes. Comparisons between groups were made using the student t-tests or Wilcoxon non-parametric tests for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Similar methods were used for lesion-level analyses. One-year cumulative incidence rates of individual clinical outcomes and composite outcomes were estimated by the Kaplan-Meier method and tested by the log-rank statistic. Patients who did not experience the outcome of interest were censored at the last known date of contact or at 1 year if contact extended beyond 1 year.
Cox proportional hazards modeling was used to estimate one-year hazard ratios for adverse clinical events in relation to stent type (DES vs. BMS) on the study endpoints for each GFR category. propensity score approach was used to balance factors associated with the nonrandom assignment of treatment time (earlier recruitment Wave versus later recruitment Wave). The estimated propensity score for treatment with a DES once these devices were available was obtained from the fit of individual logistic regression models by GFR category for which demographic, angiographic, clinical, and procedural characteristics were considered. A c-statistic was calculated. Covariate balance between DES-treated patients and BMS-treated patients in each GRF strata was assessed after adjustment for the propensity score as a continuous covariate using logistic regression. Proportional hazards assumptions were evaluated and met. For all analyses, a two-sided p <0.05 was considered statistically significant. All statistical analyses were performed using the SAS System (Cary, NC), version 9.1.
Results
Of the 4157 subjects, 1108 (27%) had low-GFR and 3049 had normal renal function. Within the low-GFR group, 345 (31%) patients received BMS and 763 (69%) received DES. Among patients with a normal GFR (normal-GFR, estimated GFR ≥60ml/min/1.73m2), 1036 (34%) underwent stenting with BMS and 2013 (66%) received DES.
Within the low-GFR strata, there was no difference in mean age, sex, race, history of prior MI or CABG, prevalence of hypertension, severe non-cardiac disease such as peripheral vascular disease, congestive heart failure, or smoking history by type of stent (Table 1). The prevalence of hypercholesterolemia and a history of PCI weresignificantly higher in patients who received DES compared to BMS. Among the normal-GFR strata, patients receiving DES were younger and more often male and non-white. The prevalence of co-morbid conditions including diabetes, hypertension, hypercholesterolemia and a history of both kidney disease and prior PCI were significantly higher in DES-treated patients while the frequency of congestive heart failure was lower. In both GFR groups, the primary indication for revascularization differed with more acute MI and less unstable angina in the DES-treated patients presenting with a normal-GFR and more stable angina coupled with less unstable angina in the DES patients with low-GFR.
Table 1.
Baseline Characteristics of the Normal Glomerular Filtration Rate and Low Glomerular Filtration Rate Groups.
| Variable | Normal Glomerular Filtration Rate | Low Glomerular Filtration Rate | ||||
|---|---|---|---|---|---|---|
| BMS | DES | p-value | BMS | DES | p-value | |
| Number of Patients | 1036 | 2013 | 345 | 763 | ||
| Age (mean) (years) | 63.1% | 62.1% | 0.03 | 71% | 70.5% | 0.65 |
| Female | 31.5% | 26.8% | 0.007 | 48.1% | 46.9% | 0.71 |
| White | 80.5% | 74.7% | 79.4% | 79.7% | ||
| Black | 12.1% | 16.3% | 12.5% | 12.1% | ||
| Hispanic | 4% | 5.6% | 3.5% | 4.7% | ||
| Asian | 3.5% | 3% | 4.7% | 3.3% | ||
| Diabetes Mellitus | 27.1% | 31.2% | 0.04 | 41.4% | 47.6% | 0.06 |
| Hypertension | 74.1% | 78% | 0.01 | 87.1% | 87.8% | 0.75 |
| Hypercholesterolemia | 73.9% | 80.1% | <0.001 | 70.7% | 84.4% | <0.001 |
| Renal disease | 0.7% | 1.7% | 0.03 | 25.3% | 25.6% | 0.92 |
| Peripheral Vascular | ||||||
| Disease | 7.4% | 7.6% | 0.92 | 17.4% | 13% | 0.92 |
| Previous MI | 27.5% | 24.9% | 0.13 | 31.3% | 28.3% | 0.32 |
| Prior PCI | 29.3% | 35.8% | <0.001 | 29.6% | 39.1% | 0.002 |
| Prior CABG | 16.3% | 17.6% | 0.36 | 31.6% | 29.5% | 0.48 |
| CHF | 9.5% | 6.9% | 0.01 | 24.6% | 20.1% | 0.10 |
| LVEF % (mean) | 53.8% | 53.4% | 0.58 | 50% | 51.3% | 0.16 |
| Number of Coronary arteries narrowed | 0.42 | 0.94 | ||||
| 1 | 39.7% | 36.8% | 29% | 27.5% | ||
| 2 | 33.5% | 34.2% | 27.5% | 29.1% | ||
| 3 | 26.5% | 28.8% | 42.9% | 42.9% | ||
| Primary Indication | <0.001 | 0.005 | ||||
| Acute MI | 14.5% | 16.5% | 15.7% | 16% | ||
| USA | 49.3% | 39.6% | 48.4% | 39.4% | ||
| Stable Angina | 26.3% | 24.6% | 16.2% | 24.6% | ||
| Asymptomatic | 8.4% | 14.7% | 15.1% | 13.6% | ||
Abbreviations: BMS- Bare metal stent, DES= drug eluting stent, MI= Myocardial infarction, PCI= Percutaneous coronary intervention, CABG= Coronary artery bypass grafting, CHF= Congestive heart failure, USA= Unstable Angina.
The angiographic and procedural data for all groups are shown in Table 2. Within the low-GFR group, patients receiving BMS were more often treated urgently or emergently compared to DES-treated subjects. Emergent and elective interventions were more common in DES-treated patients compared to the BMS group among those with normal renal function. Intervened lesions were similar in their coronary distribution and their ACC/AHA classification in both stent types within the low-GFR strata whereas more Class-C lesions were treated in the DES patients with normal-GFR. The mean lesion length of DES-treated lesion, however, was significantly longer compared to those treated with BMS regardless of renal function. Total angiographic success was similar across all groups.
Table 2.
Lesion and Procedural Characteristics
| Variable | Normal Glomerular Filtration Rate | Low Glomerular Filtration Rate | ||||
|---|---|---|---|---|---|---|
| BMS | DES | p-value | BMS | DES | p-value | |
| N= | 1036 | 2013 | 345 | 763 | ||
| Timing of procedure | <0.001 | 0.004 | ||||
| Elective | 54.7% | 63.1% | 56.8% | 66.5% | ||
| Urgent | 42.9% | 33.4% | 38.3% | 31.1% | ||
| Emergent | 2.4% | 3.5% | 4.3% | 2.4% | ||
| Cardiogenic Shock | 0.5% | 0.1% | 0.09 | 0.3% | 0.1% | 0.56 |
| # of lesions attempted (mean) | 1.4% | 1.4% | 0.11 | 1.5% | 1.4% | 0.39 |
| N= | 1492 | 2803 | 510 | 1070 | ||
| Left Anterior Descending lesions | 34.9% | 38.3% | 0.07 | 31.6% | 34% | 0.67 |
| Ref vessel size (mean, mm) | 3% | 3% | 3.1% | 3% | 0.79 | |
| Lesion length (mean,mm) | 13.1% | 16.6% | <0.001 | 13.5% | 16.7% | <0.001 |
| ACC/AHA Classification | <0.001 | 0.90 | ||||
| A | 19.4% | 11.8% | 12.5% | 11.6% | ||
| B1 | 37.3% | 35.2% | 30.8% | 30% | ||
| B2 | 29.4% | 28.7% | 32% | 32.5% | ||
| C | 13.8% | 24.2% | 24.7% | 25.9% | ||
| DES type Sirolimus | 0% | 65.7% | n/a | 0% | 36.7% | n/a |
| Paclitaxel | 0% | 37.3% | n/a | 0% | 66.2% | n/a |
| Angiographic success | 97.9% | 98.3% | 0.30 | 95.9% | 97.1% | 0.37 |
Within the low-GFR group, the incidence of in-hospital mortality was significantly higher among those treated with BMS compared to DES-treated subjects (2.9% vs. 1.1%, p=0.02). There was no difference in in-hospital mortality by stent type in patients with normal-GFR (0.1% for both, p=0.98). The incidence of in-hospital MI was similar by stent type regardless of renal function (normal-GFR: 2.1% for both, p=0.98 and low-GFR: 2.0% for BMS and 2.4% for DES, p=0.73).
There were no differences in the incidence of death at 1-year or MI between DES- and BMS-treated patients, respectively (Table 3). However, the absolute rates of these outcomes were significantly greater in the low-GFR group, compared with those with normal-GFR (Death: 6.5% vs. 2.0%, p<0.001; MI: 5.7% vs. 4.2%, p=0.05). Within the normal-GFR group, the incidence of repeat revascularization was significantly lower among DES-treated subjects as compared to the BMS-treated individuals (10.7% versus 16.4%, p<0.001). In contrast, there was no reduction in the need for repeat revascularization among DES-treated patients compared to BMS-treated patients in those with impaired renal function (9.9% versus 12.2%, p=0.23).
Table 3.
Incidence of One-year Outcomes
| Event | Low Glomerular Filtration Rate
|
Normal Glomerular Filtration Rate
|
||||
|---|---|---|---|---|---|---|
| BMS (n=345) | DES (n=763) | p-value | BMS (n=1036) | DES (n=2013) | p-value | |
| Death | 8.0% | 5.8% | 0.16 | 2.0% | 2.2% | 0.71 |
| MI | 6.9% | 5.1% | 0.27 | 4.4% | 4.0% | 0.66 |
| Death/MI | 13.8% | 10.2% | 0.08 | 6.3% | 5.9% | 0.65 |
| Repeat PCI | 10.2% | 8.7% | 0.39 | 14.0% | 9.4% | <0.001 |
| Repeat | 12.2% | 9.9% | 0.23 | 16.4% | 10.7% | <0.001 |
| Revascularization MACE |
21.6% | 16.6% | 0.051 | 20.2% | 14.8% | <0.001 |
Abbreviations: MI= myocardial infarction, PCI= Percutaneous coronary intervention, MACE= Major adverse cardiac events
Although adjusted hazard ratios (HR) showed a significant benefit of DES in reducing the need for repeat revascularization in the normal-GFR group (Figure 1a), this was not observed in the low-GFR group (Figure 1b) There were no differences in the safety endpoints of death, MI, or death/MI based on stent type in either renal function stratum (Figures 1a and 1b). These endpoints were not different when propensity score adjustment was performed (data not shown). The independent predictors of 1-year mortality among the low-GFR subjects included age, history of heart failure, emergent indication for intervention, pulmonary disease at the time of presentation and the development of contrast-induced acute kidney injury (CI-AKI).
Figure 1.
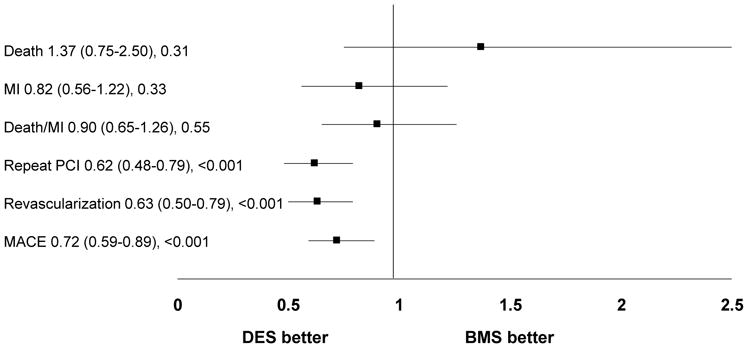
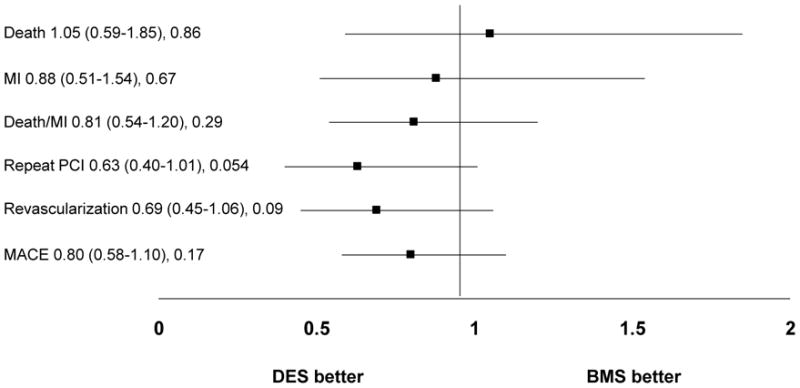
Figure 1a: Adjusted hazard ratios and 95% CIs for 1-year adverse outcomes by stent type in patients with normal glomerular filtration rate
Abbreviations: MI= myocardial infarction, PCI= Percutaneous coronary intervention, MACE= Major adverse cardiac events
Figure 1b: Adjusted hazard ratios and 95% CIs for 1-year adverse outcomes by stent type in patients with low glomerular filtration rate
Abbreviations: MI= myocardial infarction, PCI= Percutaneous coronary intervention, MACE= Major adverse cardiac events
Discussion
Our study shows that while patients with non-dialysis dependent CKD are at high risk for adverse outcomes after PCI, the beneficial effect of DES over BMS in reducingthe need for repeat revascularization is significantly attenuated when compared to patients with normal renal function. This effect appears to be predominantly driven by the large short term and long-term mortality that occurs in this patient sub-population, which is many times higher than a patient with a normal-GFR and CAD. Given that CKD is a growing problem worldwide, with percutaneous coronary interventions being the most common modality for treating coronary artery disease in this patient population, our findings have important clinical implications.
First, our study confirms the results of prior reports that have examined the association of renal dysfunction and worse clinical outcomes after PCI compared to patients with normal renal function.7,9,16–23 Recent studies have shown that these findings are not limited to those with ESRD, but rather that they extend to those with lesser degrees of renal dysfunction.24 Our study echo’s the recent findings by Charytan et al. and by Latif et al.25–27 in which both of these studies found an increased rate of mortality among patients with CKD when compared to patients with normal renal function. Our study also echoes the mortality findings from the recent extract from the SIRIUS trails, in which Garg et al. found a modest beneficial effect of DES vs. BMS in patients with mild to moderate renal insufficiency. Unlike our trial (but like the EVENT investigators), Garg et al. estimated GFR using the Cockcroft-Gault formula as opposed to the MDRD. The MDRD may be a more accurate measure of true GFR, particularly among patients with more advanced levels of CKD.28,29 They also included only relatively mild to moderate renal insufficiency and not patients with severe renal insufficiency, with the mean creatinine clearance level of 75.7 ml/min and 47.2 ml/min respectively. This could explain some of the benefit that Garg et al. saw from DES stenting, given that the level of renal dysfunction that was examined in their patient population was relatively mild and thus their patients were less likely to die and potentially more likely to benefit from the protective effects of DES. In addition, there is growing interest in subcategorizing patients with stage 3 CKD (eGFR 30–60) into those with more advanced CKD (i.e., eGFR 30–45) and those with less advanced CKD (i.e., eGFR 45–60) based, in part, on the observation that patients with more advanced stage 3 CKD experience more adverse outcomes.30 Thus it is quite possible that studies such as that conducted by the SIRIUS group would identify a benefit to DES that is not seen in studies such as ours, which analyzed a considerably higher risk patient population. This possibility seems to be in part validated by the recent analysis of the Mass-DAC registry done by Charytan et al.26,27 In their patient population with severely decreased GFRs (with 23.6% of their population on renal replacement therapy), they found a non-statistically significant decrease in target-vessel revascularization when using DES vs. BMS, with high rates of mortality regardless of stent type.
The observed increase in mortality among those with low GFR seen in the above trials, compared to those with normal GFR, may be explained by several factors.27 One explanation is that although restenosis has been regarded as a “benign” phenomenon, it has been described to present clinically as myocardial infarction and/or death. It is possible that aggressive restenosis in this patient population may manifest itself with catastrophic consequences such as death. Additionally, it is unclear if the vascular pathobiology in patients with CKD differs from that of patients with normal renal function. It may be that the drugs used in DES are less efficacious in the vascular beds of patients with severely reduced GFR due to derangements in hemostasis and vascular biology.
Second, our study compares the effectiveness of DES vs. BMS in reducing need for repeat revascularization in patients with renal dysfunction. Unlike all previous reports, our study found that the overall rates of need for repeat revascularization in patients with renal dysfunction are, as a whole, low as compared to those with normal renal function, and that the beneficial effects of DES in reducing the need for repeat revascularization was significantly attenuated and not significantly different compared to BMS. There are several potential explanations for these findings. There where low overall rates of repeat revascularization in our patient population which could be related to a referral bias given their underlying renal dysfunction, making it less likely for physicians to refer these patients for repeat procedures. In addition, competing risks may account for the differences in outcomes seen in this patient population. Specifically, given their high short-term mortality, it is possible that the highest-risk patients die before they develop either restenosis or progression of disease requiring repeat revascularization, hence resulting in “lower rates” at one year compared to those with normal renal function.
In terms of the lack of beneficial effect of DES as compared to BMS in reducing the need for repeat procedures in the low-GFR group, it is possible that we did not see a significant difference between the two types of stents due to insufficient statistical power given the relatively low rates of need for repeat revascularization at one year in both stent groups. Given the trends observed, however, one cannot discount the possibility that with a larger sample size, a beneficial effect could be seen. Alternatively, one could hypothesize that this patient population is one with more advanced and less responsive coronary artery disease, where the anti-restenotic effects of DES are outweighed by the concomitant factors associated with this patient population. Despite the failure to see a statistically significant benefit between the stent types it is important to note that DES use in this patient population did not appear to be associated with an increased hazard of death or MI when compared to BMS. This is a significant finding considering the theoretical increased risk of stent thrombosis presented by the use of DES in a patient population at high risk of clotting.
Our study has certain limitations. Our results reflect an analysis of registry data and are thus observational in nature and not drawn from patients in a randomized clinical trial. While we adjusted for several variables, it is possible that residual confounding could account for some of the observed differences between BMS and DES. However, despite being observational in nature, the baseline, angiographic, and procedural characteristics of our study population were reasonably well balanced between BMS and DES. Nevertheless, we feel the presentation of non-randomized, real world registry data from a large cohort of consecutive patients recruited from multiple centers provides important epidemiological data. Lastly, the length of follow-up for this study was limited to one year and therefore, the longer-term performance of DES in the setting of renal dysfunction is still not fully known.
Acknowledgments
This study was supported in part by grant HL033292 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Funding Sources: This study was supported in part by grant HL033292 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Footnotes
Disclosers: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Yeo FE, Villines TC, Bucci JR, Taylor AJ, Abbott KC. Cardiovascular risk in stage 4 and 5 nephropathy. Adv Chronic Kidney Dis. 2004;11:116–133. doi: 10.1053/j.arrt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Al Suwaidi J, Reddan DN, Williams K, Pieper KS, Harrington RA, Califf RM, Granger CB, Ohman EM, Holmes DR., Jr Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 4.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, Cleman M, Heuser R, Almond D, Terirstein P, Fish D, Colombo A, Brinker J, Moses J, Shanknovich A, Hirshfeld J, Bailiey S, Elis S, Rake R, Goldberg S. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 6.Attallah N, Yassine L, Fisher K, Yee J. Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol. 2005;64:412–418. doi: 10.5414/cnp64412. [DOI] [PubMed] [Google Scholar]
- 7.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 8.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 9.Farb A, Boam AB. Stent thrombosis redux--the FDA perspective. N Engl J Med. 2007;356:984–987. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 10.Schampaert E, Cohen EA, Schluter M, Reeves F, Traboulsi M, Title LM, Kuntz RE, Popma JJ. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43:1110–1115. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005;45:2088–2092. doi: 10.1016/j.jacc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 12.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27:2784–2814. doi: 10.1093/eurheartj/ehl282. [DOI] [PubMed] [Google Scholar]
- 14.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. discussion 1455. [DOI] [PubMed] [Google Scholar]
- 15.Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997;29:6–12. doi: 10.1016/s0735-1097(96)00452-4. [DOI] [PubMed] [Google Scholar]
- 16.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, 3rd, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 17.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 18.Gruberg L, Dangas G, Mehran R, Mintz GS, Kent KM, Pichard AD, Satler LF, Lansky AJ, Stone GW, Leon MB. Clinical outcome following percutaneous coronary interventions in patients with chronic renal failure. Catheter Cardiovasc Interv. 2002;55:66–72. doi: 10.1002/ccd.10103. [DOI] [PubMed] [Google Scholar]
- 19.Szczech LA, Best PJ, Crowley E, Brooks MM, Berger PB, Bittner V, Gersh BJ, Jones R, Califf RM, Ting HH, Whitlow PJ, Detre KM, Holmes D. Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation. 2002;105:2253–2258. doi: 10.1161/01.cir.0000016051.33225.33. [DOI] [PubMed] [Google Scholar]
- 20.Dixon SR, O’Neill WW, Sadeghi HM, Stone GW, Brodie B, Cox DA, Garcia E, Mattos L, Grines LL, Boura JA, Morice MC, Grines CL. Usefulness of creatinine clearance in predicting early and late death after primary angioplasty for acute myocardial infarction. Am J Cardiol. 2003;91:1454–1457. A6. doi: 10.1016/s0002-9149(03)00396-5. [DOI] [PubMed] [Google Scholar]
- 21.Schwertz DW, Vaitkus P. Drug-eluting stents to prevent reblockage of coronary arteries. J Cardiovasc Nurs. 2003;18:11–16. doi: 10.1097/00005082-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Reis SE, Olson MB, Fried L, Reeser V, Mankad S, Pepine CJ, Kerensky R, Merz CN, Sharaf BL, Sopko G, Rogers WJ, Holubkov R. Mild renal insufficiency is associated with angiographic coronary artery disease in women. Circulation. 2002;105:2826–2829. doi: 10.1161/01.cir.0000021597.63026.65. [DOI] [PubMed] [Google Scholar]
- 24.Garg P, Charytan DM, Novack L, Cutlip DE, Popma JJ, Moses J, Leon MB, Schofer J, Breithardt G, Schampaert E, Mauri L. Impact of moderate renal insufficiency on restenosis and adverse clinical events after sirolimus-eluting and bare metal stent implantation (from the SIRIUS trials) Am J Cardiol. 2010;106:1436–1442. doi: 10.1016/j.amjcard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ, Saucedo JF. In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: a report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc Interv. 2009;2:37–45. doi: 10.1016/j.jcin.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Charytan DM, Varma MR, Silbaugh TS, Lovett AF, Normand SL, Mauri L. Long-term clinical outcomes following drug-eluting or bare-metal stent placement in patients with severely reduced GFR: Results of the Massachusetts Data Analysis Center (Mass-DAC) State Registry. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;57:202–211. doi: 10.1053/j.ajkd.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Alexander P, David S, McCullough PA. Drug-eluting coronary stents in patients with kidney disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;57:188–189. doi: 10.1053/j.ajkd.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Liu C, Chauveau P, Baillet-Blanco L, Beauvieux MC, Combe C, Gin H. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of Diet in Renal Disease study equation? Diabetes Care. 2005;28:838–843. doi: 10.2337/diacare.28.4.838. [DOI] [PubMed] [Google Scholar]
- 29.Berman N, Hostetter TH. Comparing the Cockcroft-Gault and MDRD equations for calculation of GFR and drug doses in the elderly. Nat Clin Pract Nephrol. 2007;3:644–645. doi: 10.1038/ncpneph0627. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]


