Summary
While endometrial neutrophils and plasma cells are criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease (PID) research, plasma cell misidentification and nonspecificity may limit the accuracy of these criteria. Herein, we examined: 1) the identification of endometrial plasma cells with conventional methyl green pyronin-based methodology versus plasma cell-specific (CD138) immunostaining; 2) the prevalence of endometrial plasma cells among women at low risk for PID; and 3) endometrial leukocyte subpopulations among women diagnosed with acute or chronic histologic endometritis by conventional criteria. We observed an absence of CD138+ cells in 25% of endometrial biopsies in which plasma cells had been identified by conventional methodology, while additional immunohistochemical analyses revealed indistinguishable inflammatory infiltrates among women diagnosed with acute or chronic endometritis by conventional criteria. Among women considered at lower risk for PID development, flow cytometric analyses detected plasma cells in 30% of endometrial biopsy specimens, suggesting that these cells, even when accurately identified, only nonspecifically identify upper genital tract inflammatory processes. Combined, our findings underscore the limitations of the criteria used to diagnose histologic endometritis in PID-related research and suggest that satisfactory understanding of PID pathogenesis, treatment, and prevention is hindered by continued use of these criteria.
Keywords: Histologic endometritis, Plasma cell, Syndecan-1, Pelvic inflammatory disease
Introduction
Infection of the uterus, fallopian tubes, and adjacent structures that is not associated with pregnancy or surgery is termed pelvic inflammatory disease (PID) [15]. Migration of Chlamydia trachomatis or Neisseria gonorrhoeae from the lower to upper genital tract is the most frequently identified cause of this disease whose long-term sequelae include ectopic pregnancy and infertility [4, 16, 30]. Being less invasive and expensive than laparoscopy [22], trans-cervical endometrial sampling and histology have been employed in most recent epidemiologic PID investigations to identify endometritis/upper genital tract inflammation [2, 6, 10]. Such studies diagnosed acute histologic endometritis upon identification of neutrophils and plasma cells in hematoxylin and eosin (H&E) and methyl green pyronin (MGP)-stained endometrial biopsy sections, while the diagnosis of chronic endometritis was typically based on plasma cell detection alone. Comparison of results between many studies is hampered, however, by their use of slightly variable diagnostic criteria [20, 29, 31].
In a recent PID investigation, one such variation of these criteria was used to define histologic endometritis as detection of ≥ 5 neutrophils in endometrial epithelium and/or ≥ 2 plasma cells in endometrial stroma [19]. While nearly 95% of women in this study with an enrollment diagnosis of endocervical C. trachomatis or N. gonorrhoeae infection cleared the infection one month after completion of antimicrobial therapy, almost half failed to resolve histologic endometritis (as defined by this study). Moreover, the diagnosis of histologic endometritis was not associated with increased risk for the development of adverse reproductive sequelae [9]. It is possible, however, that imprecise identification of endometrial leukocytes or imprecise diagnostic criteria for histologic endometritis confounded results from this research. Indeed, prior work suggests that there may be problems associated with these diagnostic criteria. For example, endometritis cases have been reported in which neither endometrial neutrophils nor plasma cells were detected [33]. Plasma cells were also detected in 5%–10% of women undergoing endometrial biopsy for irregular vaginal bleeding, suggesting that these cells may nonspecifically identify endometrial inflammation [8, 23]. Of greatest concern, plasma cells are detected in endometria of healthy women and have been misidentified as endometrial stromal cells (and vice versa) [5]. These limitations weaken the precision of current diagnostic criteria for histologic endometritis, and further suggest that essential questions regarding PID epidemiology, etiology, therapy, and adverse sequelae will likely remain unanswered until more accurate identification of upper genital tract inflammation is achieved [26].
For the current investigation, we hypothesized that H&E/MGP-based methodologies will have inferior performance characteristics compared to immunohistochemical staining of endometrial biopsy sections with a plasma cell-specific monoclonal antibody. We also hypothesized that flow cytometric analysis of endometrial tissue from women considered to have low risk for current genital tract infection will frequently detect the presence of plasma cells. Finally, as plasma cells appear to be only nonspecific indicators of an upper genital tract inflammatory process, we hypothesized that immunohistochemical characterization of endometrial leukocyte subpopulations among women previously diagnosed with acute or chronic histologic endometritis would demonstrate inflammatory infiltrates that are indistinguishable from one another.
Materials and Methods
Patient population for immunohistochemical analyses
Stored, paraffin-embedded, endometrial specimens were selected from women between 15 and 30 years of age when they had participated in a cross-sectional investigation of the association between lower genital tract infection and PID [32]. Endometrial biopsies had been collected from each study participant, and a portion of the tissue had been paraffin-embedded, sectioned, and stained with H&E or MGP. As previously demonstrated, MGP stain is superior to H&E stain for accurate identification of endometrial plasma cells [7]. A single surgical pathologist (A.J.A) had previously evaluated all endometrial biopsy specimens [32]. Acute histologic endometritis had been defined as ≥ 5 neutrophils per 400x field of endometrial epithelium and ≥ 1 plasma cell per 120x field of stromal endometria, while chronic endometritis had been defined by the presence of ≥ 1 plasma cell alone [13]. Among stored endometrial specimens chosen for the current study, 26 were from women previously diagnosed with normal endometrium while 37 each were from women previously diagnosed with acute or chronic endometritis. Selected specimens were excluded from further work-up if the tissue-block size was deemed too scant or if H&E-stained slides demonstrated endometrial changes consistent with menses or a late secretory stage, as these cycle points are considered more problematic for the interpretation of inflammatory changes [24, 25].
Immunohistochemical analyses
The monoclonal antibodies used in the current investigation were HLA-DP, DQ, DR; CD3; CD8; CD20; CD56; CD68; and CD138. Immunostaining for HLA-DP, DQ, DR (Dako, Carpinteria, CA), a class II major histocompatibility complex (class II MHC) molecule, was performed without antigen retrieval. For evaluation of CD8 (Dako), CD56 (Neomarkers, Fremont, CA), and CD138 (AbD Serotec, Raleigh, NC) expression, microwave antigen retrieval in 10 mmol/L citrate buffer and indirect immunolabeling were performed. After antigen retrieval, slides were blocked for 15 minutes with Blue Block (Shandon, Pittsburgh, PA), and incubated for 1 h at room temperature with appropriate primary antibody. Slides were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20, incubated for 30 minutes at room temperature with Impress Reagent (MP-7500, Vector Laboratories, Burlingame, CA), stained with liquid DAB (SK-4100, Vector Laboratories), and counterstained with Shandon Hematoxylin (Thermo Fisher Scientific, Pittsburgh, PA). CD3, CD20, and CD68 immunostaining was performed using Ventana pre-diluted antibodies and the Ventana Benchmark XT (Ventana, Tucson, AZ). Positive control slides (paraffin-embedded sections of tonsilar tissue for every immunostain except CD56, which used paraffin-embedded neuroblastomal tissue) were used to confirm antibody activity (representative results for CD138 shown in Fig. 1A), while absence of reactivity among negative controls (produced by replacement of a primary antibody with PBS solution) confirmed specificity of positive staining. Two pathologists (M.C., U.K) that were unaware of the enrollment presentation and original histologic diagnosis independently assessed all slides for the current investigation. Results were concordant in more than 90% of cases, and discrepancies were resolved by secondary review (R.D.V.M). Cells of interest were graded using a 0–4 semi-quantitative scale: grade 0 = no positive cells; grade 1 = single scattered positive cells; grade 2 = loosely scattered cells or occasional small aggregates; grade 3 = densely scattered cells; grade 4 = dense collections of positive cells throughout entire specimen.
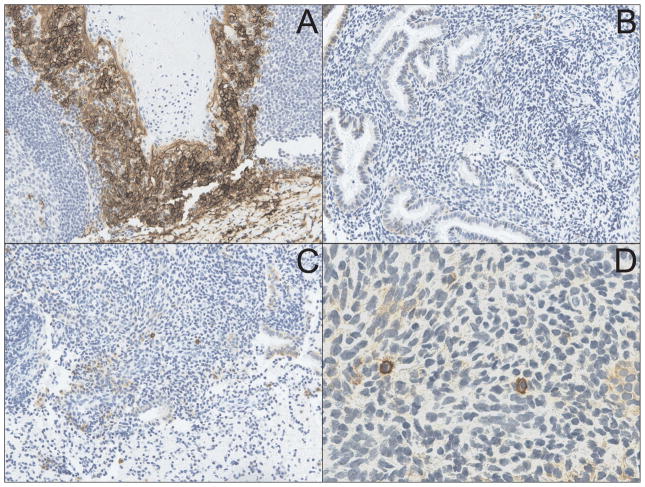
Fig. 1.
CD138 immunostaining is superior to MGP staining of endometrial biopsy sections for identification of plasma cells. (A) Immunohistochemical staining of tonsilar tissue (used as positive controls) with the plasma cell-specific marker CD138 (syndecan-1) shows characteristic membrane staining (X200). (B) Endometrial stroma from a woman originally diagnosed with chronic endometritis (upon identification of plasma cells with MGP staining) demonstrates complete absence of CD138-positive cells (X200). (C) Endometrial biopsy sample from a woman originally diagnosed with normal endometrial tissue shows multiple CD138-positive cells in the endometrial stroma (X200) (D). CD138 positive endometrial cells from section show in Fig. 1C display cell membrane staining characteristically similar to that seen in tonsilar tissue (X600).
Patient population and methods for flow cytometric analyses
Endometrial sampling for flow cytometric analysis of leukocyte subpopulations was performed on freshly excised surgical specimens from 10 pre-menopausal women undergoing hysterectomy for dysfunctional uterine bleeding. These women were considered to be at lower risk for PID development as they denied history of any sexually transmitted infection and were without evidence of lower genital tract inflammation (an absence of leukorrhea). However, no diagnostic testing for genital tract pathogens was performed. To simulate the amount, type, and quality of tissue obtained from trans-cervical biopsies, endometrial sampling devices were used to collect tissue from each excised uterus. Specimens were digested with collagenase and processed into single cell suspensions. Aliquots were stained with fluorochrome-conjugated monoclonal antibodies against CD3, CD11b, CD14, CD15, CD19, CD27, CD38, CD45, CD68, CD69, CD138, Granzyme B (all BD Pharmingen, San Diego, CA), and LIVE/DEAD® fixable aqua dead cell stain (Invitrogen, Carlsbad, CA). Appropriate isotype control antibodies were included with each experiment. Stained specimens were examined by flow cytometry using a FACSAria cytometer (Becton Dickinson, San Jose, CA). Flow cytometric analyses were performed using FACS DiVA (Becton Dickinson) and FlowJo (Tree Star, Ashland, OR) software. Both the parent and current investigation were approved by the University of Pittsburgh’s Institutional Review Board.
Statistical considerations
Statistical analyses were performed using GraphPad Prism 5 software (San Diego, California). Calculations of medians and percentile distributions were used to compare differences in the number of various leukocyte subpopulations among women diagnosed with normal endometrial tissue or women diagnosed with acute or chronic histologic endometritis using conventional diagnostic criteria. Comparisons of scores between these groups were made using Kruskal-Wallis test on ranks and Dunn’s multiple comparison post hoc test (p values ≤ 0.05 were considered statistically significant).
Results
We first used the stored, endometrial biopsy specimens to compare the accuracy of plasma cell identification with MGP (as performed in the parent investigation) to the results obtained by immunostaining sections with antibodies specific for CD138 (syndecan-1). CD138 is a plasma cell-specific surface molecule that has been previously employed as an adjunct stain in the work-up of suspected cases of endometritis [3]. As hypothesized, we detected a wide discrepancy between results reported for plasma cell identification with MGP and results obtained upon CD138 staining of the same endometrial specimens. In fact, 25% of cases originally diagnosed with endometritis had no detectable CD138+ cells (representative results shown in Fig. 1B), while CD138 immunostaining identified plasma cells in 35% of the cases originally diagnosed as normal endometrial tissue (representative results shown in Fig. 1C and Fig. 1D). Using CD138 immunostaining as the new diagnostic gold standard, we found sensitivity and specificity of MGP for plasma cell identification to be 78% and 65%, respectively.
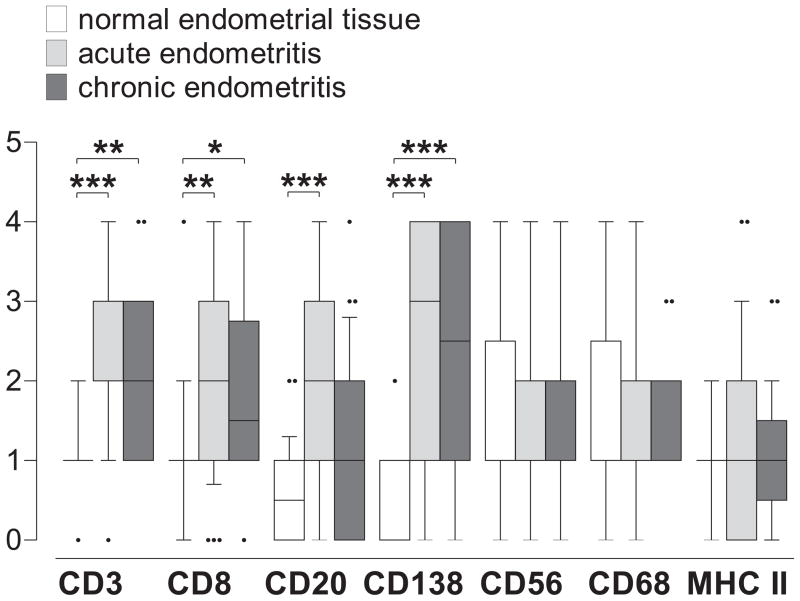
As prior reports suggest that infrequent detection of endometrial plasma cells may only nonspecifically identify upper genital tract inflammation (as opposed to being necessary and sufficient for endometritis diagnosis), we posited that stratification of endometritis into acute and chronic forms (as performed in the parent investigation using conventional histologic criteria) would not delineate endometrial inflammatory responses distinctive of either form. To test this hypothesis, we used a semi-quantitative immunohistochemical scoring system to compare the number of CD3, CD8, CD20, CD56, CD68, CD138, and MHC class II-positive cells from stored, endometrial biopsy specimens that had been diagnosed with acute or chronic endometritis to the numbers present among women identified with normal endometrial tissue. We observed that diagnosis of acute and chronic endometritis was associated with statistically significant increases in endometrial T cell, B cell, and plasma cell numbers compared to numbers detected in normal endometrial tissue (Fig. 2), suggesting that conventional histologic criteria correctly identified at least a subset of women with endometrial leukocytic infiltrates that were consistent with increased upper genital tract inflammation. Evaluation of the number of CD3, CD8, CD20, CD56, CD68, CD138, and MHC class II complex positive cells that were associated with acute or chronic endometritis, on the other hand, demonstrated endometrial leukocytic infiltrates nearly indistinguishable from one another (Fig. 2).
Fig. 2.
Acute and chronic endometritis identified by conventional histologic criteria have indistinguishable leukocytic infiltrates. Stored endometrial biopsy sections identified as normal tissue or acute or chronic endometritis were immunostained for CD3, CD8, CD20, CD138, CD56, CD68, and MHC II. Each stained section was scored for number of positive cells using the semi-quantitative scoring system described in text, and group comparisons made using Kruskal-Wallis test and Dunn’s multiple comparison test (* p < 0.05; ** p < 0.005; *** p< 0.001). Box and whiskers indicate median ± 10–90 percentile.
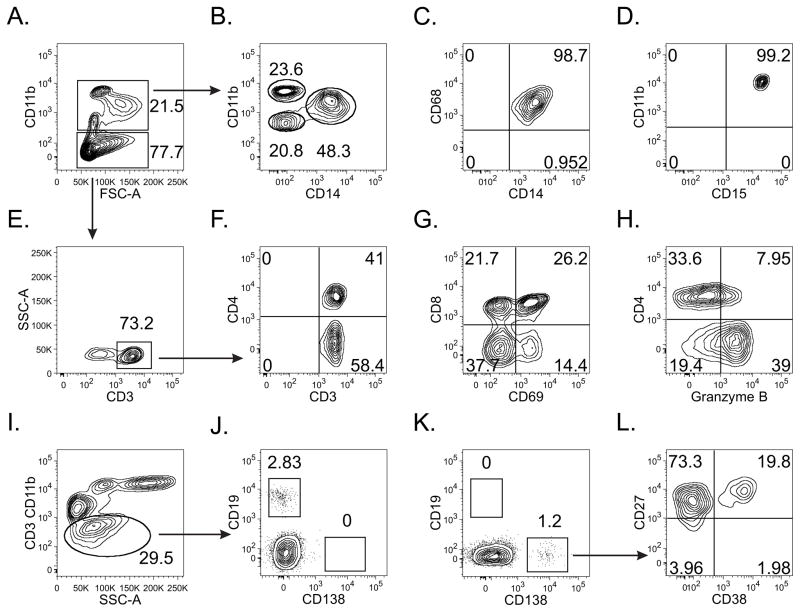
While the above findings suggested that a more accurate identification of endometrial plasma cells can be achieved via immunostaining of biopsy specimens with the plasma cell-specific surface molecule CD138 (Fig. 1), we had not fully addressed the possibility that isolated detection of endometrial plasma cells may be unreliable for diagnosis of chronic endometritis and, by extension, PID case definition. To best characterize the leukocyte subpopulations found among women at lower risk for PID development, we completed flow cytometric analyses on endometrial samplings from 10 women that had undergone elective hysterectomy for irregular vaginal bleeding. Similar to the results from previous immunohistochemically-based characterizations [12, 18], we detected CD45+ cells in all specimens that were consistent with macrophage and lymphocyte populations (Fig. 3). In addition, a distinct plasma cell population was present in 30% (3/10) of endometrial samples (Fig. 3). This latter finding corroborates earlier reports that used H&E/MGP stains to identify endometrial plasma cells among women at low risk for upper genital tract inflammation [1, 7]. It also provides an argument that even when highly accurate endometrial plasma cell identification is achieved, these cells represent only a nonspecific criterion for the diagnosis of histologic endometritis, if not interpreted in the context of additional indices of inflammation.
Fig. 3.
Flow cytometric analysis of endometrial biopsies represents a powerful approach for identification of upper genital tract leukocytes (representative contour plots shown). After defining the CD45+ cell population (not shown), a large percentage was identified as (A) CD11b+, and (B, C) further identified as macrophages (CD11int/hiCD14+CD68+) (B, D) neutrophils (CD11bhiCD14-CD15+), and (B) another myeloid cell subpopulation (CD11bloCD14−CD11−). (E) To characterize T cell populations, CD11b− cells were interrogated for CD3 expression. Both CD4+ and CD8+ cell populations were clearly defined (F), allowing exploration of their functional activation status, as indicated by the levels of CD69 (G) and granzyme B (H) expression. For plasma cell identification, CD3 and CD11b− cells were interrogated for CD19 (J) and CD138 (K) expression. All CD138+ cells were CD27+ while a significant portion were CD38+ (L), strong evidence for the proper identification of a plasma cell population.
Discussion
PID is a poorly understood disease affecting millions of young women worldwide. When antimicrobial treatment of PID is delayed, the risks for ectopic pregnancy and infertility are increased [11]. Completion of research that provides better understanding of PID epidemiology, pathogenesis, and treatment, however, is handicapped by fundamental problems with disease diagnosis and case definition. While women with PID often present with leukorrhea and pelvic organ tenderness, the high frequency of subclinical PID cases lowers the accuracy of the diagnostic algorithms reliant upon a constellation of signs and symptoms [27]. Although laparoscopic diagnosis of overt disease has been considered the diagnostic standard, it is invasive and perhaps less accurate than previously realized [28]. Newer techniques, such as trans-vaginal Doppler ultrasound and magnetic resonance imaging, are less invasive modalities that provide acceptable diagnostic accuracy, but remain impractical for large-scale PID investigations [21]. Trans-cervical endometrial sampling is less expensive, less invasive, and safer to perform than laparoscopy, and has become the diagnostic modality of choice in many recent PID epidemiologic investigations. There are, however, limitations associated with its use as well. In particular, it is currently unknown if endometritis and PID represent distinct clinical entities, if endometritis represents an intermediary stage among women that will develop PID, or if endometritis and PID are different aspects of the same disease sharing similar risks for the development of adverse reproductive outcomes [22]. Moreover, prior results from PID studies utilizing trans-cervical, endometrial biopsies suggest that current histologic diagnostic criteria may lack sufficient precision to yield consistent results.
Our study took several methodological approaches to better define the limitations of criteria currently used to diagnose histologic endometritis. We demonstrated that more accurate endometrial plasma cell identification occurs upon the use of the plasma cell-specific molecule CD138. H&E or MGP staining does not allow easy discrimination between endometrial plasma cells and stromal cells, as both cell types often display an eccentric nuclei and a plasmacytoid appearance [14, 17]. By comparing results of MGP versus CD138 staining of the same endometrial biopsy, our investigation provides direct evidence of the difficulties of plasma cell identification with conventional methodology. However, even if accurately identified, our data does not support continued reliance on endometrial plasma cell detection as criterion sufficient for PID diagnosis. Using flow cytometric analyses, we detected endometrial plasma cells among 30% of the women undergoing hysterectomy for dysfunctional uterine bleeding. This provided additional support for the accumulating body of evidence, suggesting that these cells should be regarded as nonspecific indicators of upper genital tract inflammation. As dysfunctional uterine bleeding can occur among women with subclinical genital tract infection, further work will be needed to determine the utility of flow cytometric analyses of leukocyte subpopulations in PID-related research. Immunohistochemical characterization of the leukocyte subpopulations associated with acute or chronic endometritis (as diagnosed by conventional criteria) also revealed endometrial inflammatory responses that were essentially indistinguishable from one another. This implies stratification of endometritis into acute and chronic forms of disease, defines neither magnitude of the inflammatory response nor chronicity of the disease, and does little to inform on the understanding of PID pathogenesis. Taken together, our results suggest that continued reliance on current methodology and criteria for histologic identification of upper genital tract inflammation in PID-related research may be unwarranted. If trans-cervical, endometrial sampling is employed in future epidemiologic PID investigations, it seems that additional research is needed to improve the performance characteristics of the criteria used for histologic diagnosis of endometritis and to delineate the upper genital inflammatory responses and inflammatory cell infiltrates most closely associated with PID development and adverse gynecologic outcomes.
Acknowledgments
The authors thank Kathleen Cieply, Kimberly Fuhrer, Jamie Haggerty, George Michalopoulos, W. Allen Hogge, Lorna Rabe, and Robert Schreiner for their assistance in the execution of this study.
This study was supported by funds provided by the University of Pittsburgh School of Medicine’s Department of Obstetrics, Gynecology, and Reproductive Sciences and National Institutes of Health grants K23AI064396 and U19AI084024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of these results were presented at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Meeting (Washington D.C.; October, 2008).
References
- 1.Achilles SL, Amortegui AJ, Wiesenfeld HC. Endometrial plasma cells: do they indicate subclinical pelvic inflammatory disease? Sex Transm Dis. 2005;32:185–188. doi: 10.1097/01.olq.0000154491.47682.bf. [DOI] [PubMed] [Google Scholar]
- 2.Andrews WW, Hauth JC, Cliver SP, Conner MG, Goldenberg RL, Goepfert AR. Association of asymptomatic bacterial vaginosis with endometrial microbial colonization and plasma cell endometritis in nonpregnant women. Am J Obstet Gynecol. 2006;195:1611–1616. doi: 10.1016/j.ajog.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Bayer-Garner IB, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with Syndecan-1. Mod Pathol. 2001;14:877–879. doi: 10.1038/modpathol.3880405. [DOI] [PubMed] [Google Scholar]
- 4.Chow JM, Yonekura ML, Richwald GA, Greenland S, Sweet RL, Schachter J. The association between Chlamydia trachomatis and ectopic pregnancy. A matched-pair, case-control study. JAMA. 1990;263:3164–3167. [PubMed] [Google Scholar]
- 5.Crum CP, Egawa K, Fenoglio CM, Richart RM. Chronic endometritis: the role of immunohistochemistry in the detection of plasma cells. Am J Obstet Gynecol. 1983;147:812–815. doi: 10.1016/0002-9378(83)90045-5. [DOI] [PubMed] [Google Scholar]
- 6.Crum CP, Hornstein MD, Steward EA. Evaluation of cyclic endometrium and benign endometrial disorders. In: Crum CP, Lee KR, editors. Diagnostic Gynecologic and Obstetric Pathology. 1. Elsevier Saunders; Philadelphia: 2006. pp. 441–491. [Google Scholar]
- 7.Gilmore H, Fleischhacker D, Hecht JL. Diagnosis of chronic endometritis in biopsies with stromal breakdown. Hum Path. 2007;38:581–584. doi: 10.1016/j.humpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981;58:176–184. [PubMed] [Google Scholar]
- 9.Haggerty CL, Ness RB, Amortegui A, et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol. 2003;188:141–148. doi: 10.1067/mob.2003.87. [DOI] [PubMed] [Google Scholar]
- 10.Haggerty CL, Totten PA, Astete SG, Ness RB. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2006;2006:30184. doi: 10.1155/IDOG/2006/30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillis SD, Joesoef R, Marchbanks PA, Wasserheit JN, Cates W, Jr , Westrom L. Delayed care of pelvic inflammatory disease as a risk factor for impaired infertility. Am J Obstet Gynecol. 1993;168:1503–1509. doi: 10.1016/s0002-9378(11)90790-x. [DOI] [PubMed] [Google Scholar]
- 12.Kamat BR, Isaacson PG. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol. 1987;127:66–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Kiviat NB, Wølner-Hanssen P, Eschenbach DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990;14:167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 14.McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. J Clin Pathol. 2006;59:801–812. doi: 10.1136/jcp.2005.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack WM. Pelvic inflammatory disease. N Engl J Med. 1994;330:115–119. doi: 10.1056/NEJM199401133300207. [DOI] [PubMed] [Google Scholar]
- 16.Moore DE, Spadoni LR, Foy HM, et al. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to distal tubal disease. Lancet. 1982;2:574–577. doi: 10.1016/s0140-6736(82)90659-6. [DOI] [PubMed] [Google Scholar]
- 17.Mount S, Mead P, Cooper K. Chlamydia trachomatis in the endometrium: can surgical pathologists identify plasma cells? Adv Anat Pathol. 2001;8:327–329. doi: 10.1097/00125480-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Morris H, Edwards J, Tiltman A, Emms M. Endometrial lymphoid tissue: an immunohistological study. J Clin Path. 1985;38:644–652. doi: 10.1136/jcp.38.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929–937. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 20.Paukku M, Puolakkainen M, Paavonen T, et al. Plasma cell endometritis is associated with Chlamydia trachomatis infection. Am J Clin Path. 1999;112:211–215. doi: 10.1093/ajcp/112.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Ross JD. An update on pelvic inflammatory disease. Sex Transm Infect. 2002;78:18–19. doi: 10.1136/sti.78.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JDC. What is endometritis and does it require treatment? Sex Transm Infect. 2004;80:252–253. doi: 10.1136/sti.2004.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotterdam H. Chronic endometritis. Pathol Annu. 1978;13:209–231. [PubMed] [Google Scholar]
- 24.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6:16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- 25.Sherman ME, Mazur MT, Kurman RJ. Benign diseases of the endometrium. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. 5. Springer-Verlag; New York: 2002. pp. 421–466. [Google Scholar]
- 26.Simms I, Stephenson JM. Pelvic inflammatory disease epidemiology: what do we know and what do we need to know? Sex Transm Infect. 2000;76:80–87. doi: 10.1136/sti.76.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184:856–863. doi: 10.1067/mob.2001.113847. [DOI] [PubMed] [Google Scholar]
- 28.Sellors J, Mahony J, Goldsmith C, et al. The accuracy of clinical findings and laparoscopy in pelvic inflammatory disease. Am J Obstet Gynecol. 1991;164:113–120. doi: 10.1016/0002-9378(91)90639-9. [DOI] [PubMed] [Google Scholar]
- 29.Smith M, Hagerty KA, Skipper B, Bocklage T. Chronic endometritis: A combined histopathologic and clinical review of cases from 2002 to 2007. Int J Gynecol Path. 2010;29:44–50. doi: 10.1097/PGP.0b013e3181ae81bb. [DOI] [PubMed] [Google Scholar]
- 30.Stacey CM, Munday PE, Taylor-Robinson D, et al. A longitudinal study of pelvic inflammatory disease. Br J Obstet Gynaecol. 1992;99:994–999. doi: 10.1111/j.1471-0528.1992.tb13705.x. [DOI] [PubMed] [Google Scholar]
- 31.Stern RA, Svoboda-Newman SM, Frank TS. Analysis of chronic endometritis for Chlamydia trachomatis by polymerase chain reaction. Hum Path. 1996;27:1085–1088. doi: 10.1016/s0046-8177(96)90288-9. [DOI] [PubMed] [Google Scholar]
- 32.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100:456–463. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 33.Yörükolu K, Kuyucoulu F. Chronic nonspecific endometritis. Gen Diagn Pathol. 1998;143:287–290. [PubMed] [Google Scholar]