Abstract
Docosahexaenoic acid (DHA), the n-3 essential fatty acid that is highly enriched in the brain, increases neurite growth and synaptogenesis in cultured mouse fetal hippocampal neurons. These cellular effects may underlie the DHA-induced enhancement of hippocampus-dependent learning and memory functions. We found that N-docsahexaenoylethanolamide (DEA), an ethanolamide derivative of DHA, is a potent mediator for these actions. This is supported by the observation that DHA is converted to DEA by fetal mouse hippocampal neuron cultures and a hippocampal homogenate, and DEA is present endogenously in the mouse hippocampus. Furthermore, DEA stimulates neurite growth and synaptogenesis at substantially lower concentrations than DHA, and it enhances glutamatergic synaptic activities with concomitant increases in synapsin and glutamate receptor subunit expression in the hippocampal neurons. These findings suggest that DEA, an ethanolamide derivative of DHA, is a synaptogenic factor, and therefore we suggest utilizing the term ‘synaptamide’. This brief review summarizes the neuronal production and actions of synaptamide and describes other N-docosahexaenoyl amides that are present in the brain.
Keywords: N-Docosahexaenoylethanolamide, Synaptamide, DHA, Hippocampus, Neuron, Anandamide, N-Docosahexaenoyl-amino acylamide
Introduction
Docosahexaenoic acid (DHA, 22:6n-3) is the n-3 polyunsaturated fatty acids that is highly enriched in the brain, including the hippocampus [1]. DHA accumulates in the brain during development, and this is associated with an increase in the hippocampus-related learning and memory functions [2]. Survival of the neurons, neurite development, synapse formation and glutamatergic synaptic activity are increased by DHA in embryonic hippocampal neuron cultures [3], suggesting that these cellular effects may underlie the DHA-induced enhancement in cognitive function. DHA also increases neurite growth in dorsal root ganglia neurons cultured from young and older rats [4], indicating that these actions of DHA are not limited to the hippocampus or the fetal period. It is important to determine the mechanism of these effects in order to gain further insight into the role of DHA in neurological development and function.
Most of the DHA in the tissues is esterified in phosphatidylserine (PS) and phosphatidylethanolamine (PE), phospholipids localized primarily in the inner leaflet of cell membranes. Some actions of DHA are due to effects on membrane lipid properties. These include effects of the DHA-enriched phospholipids on lipid domain structure [5], on proteins embedded in these membrane lipid domains [6,7], or signaling pathways activated by interacting with these domains [8-11]. Alternatively, DHA can be hydrolyzed from phospholipids by the Ca2+-independent phospholipase A2 (iPLA2) [12], and other functional effects are produced by metabolites synthesized from the released DHA. These include 10,17(S)-docosatriene that reduces neutrophil entry into inflammatory exudates [13], neuroprotectins that limit brain injury [14,15] and cyclopentenone neuroprostanes that have anti-inflammatory effects [16].
N-docosahexaenoylethanolamide (DEA), another metabolite synthesized from DHA, is a member of the N-acylated amino acid or neurotransmitter class of lipid signaling molecules [17]. A number of these compounds have important bioactive properties in the brain; for example, the endocannabinoid N-arachidonoylethanolamide (AEA, anandamide) that modulates synaptic transmission and affects memory and behavior patterns [18,19]. DEA is present in the brain, and the amount in mouse brain increases when the diet is supplemented with DHA [20,21]. Our findings indicate that DEA is the mediator of the DHA-induced increase in neurite growth and synaptogenesis in hippocampal neurons, resulting in enhanced synaptic activity [22]. Therefore, we coin the term ‘synaptamide’ for DEA, a synaptogenic amide derivative of DHA. This brief review summarizes the formation and potent action of synaptamide in neurodevelopment and describes the currently available information about other N-docosahexaenoyl derivatives of amino acids and neurotransmitters that have been detected in the brain.
Neurite growth and synaptogenesis mediated by an amide form of DHA
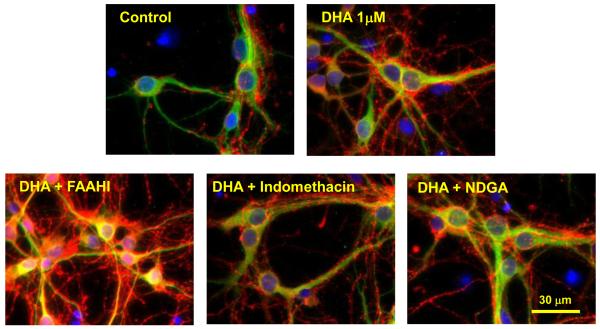
DHA uniquely promotes neurite growth, synaptogenesis, synaptic protein expression and synaptic function in hippocampal neuron cultures obtained from day 18 (E-18) mouse fetuses [3]. The treatment of hippocampal neurons with DHA significantly increases the density of synapsin puncta (synapsins associated with synaptic vesicles) on neurites, suggesting improved synaptogenesis in developing neurons. Synapsins are a family of neuron-specific phosphoproteins associated with the membranes of synaptic vesicles, and have been identified as a molecular component involved in synaptogenesis, synaptic maturation and synaptic function [23-25]. Synapsins have also been shown to regulate neurotransmitter release by controlling the number of vesicles available for the neurotransmitter release during the action potential [26]. Increases in neurite growth and synaptogenesis produced by 1 μM DHA after 7 days in culture are due to at least in part its transformation to an amide form. As shown in Fig. 1, the effect of DHA was substantially greater when treated with 1 μM URB597, a fatty acid amide hydrolase (FAAH) inhibitor, while inhibitors of either cyclooxygenase (indomethacin) or lipoxygenase (NDGA) exerted little effect [22]. The DHA-induced expression of glutamate receptors as measured by the NR2B and GluR1 antibodies also increased when the FAAH inhibitor was added [22], indicating the involvement of an amide derivative of DHA in the expression of these synaptic proteins.
Fig. 1.
Effects of inhibitors on DHA-induced development of mouse hippocampal neurons. Hippocampal cultures were prepared from fetuses obtained on embryonic day 18 (E18) from timed pregnant C57/BL6 mice and maintained in Neurobasal medium with 2 % B27 supplement as previously described [3,22]. The cultures then were treated with DHA (1 μM) for 7 days in the presence or absence of the fatty acid amide hydrolase inhibitor URB597 (FAAHI, 1 μM), cyclooxygenase inhibitor indomethacin (50 μM) or lipoxygenase inhibitor NDGA (10 μM). Representative photomicrographs are shown at 60× magnification. Corresponding control cultures were included. After incubation, neurons were stained with MAP2 (green, a neuron-marker protein), DAPI (blue, nuclear-marker) and synapsin 1 (red). These photomicrographs are taken from reference 22.
Biosynthesis of synaptamide in hippocampal neurons
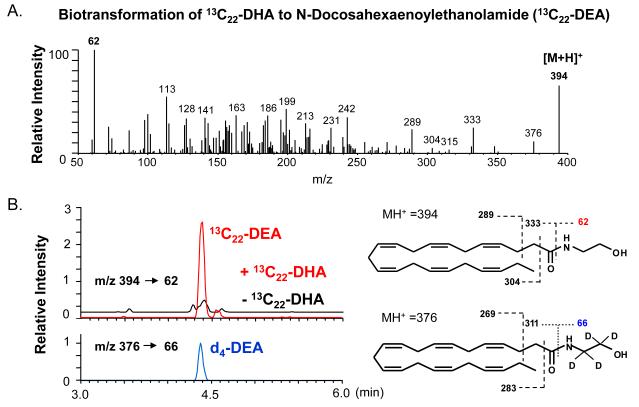
The hippocampal neuron cultures were found to synthesize synaptamide from DHA [22], consistent with the inhibitor profile indicating that an amide metabolite of DHA mediates the DHA-induced effects on neurite growth and synaptogenesis. Fig. 2 contains mass spectrometry data showing that hippocampal cultures convert 13C22-DHA to a metabolite detected at m/z 394. The DEA standards, synthesized from DHA reacted with d4- or unlabeled ethanolamine, are detected at m/z 376 or m/z 372, respectively. The mass spectrometric fragmentation pattern in Fig. 2A, in comparison to that of the DEA and d4-DEA standards, revealed the identity of the 13C22-metabolite produced by cultures as 13C22-N-docosahexaenoylethanolamide. Monitoring multiple transitions of molecular ions to specific fragments (multiple reaction monitoring) confirmed the production of 13C22-DEA from 13C22-DHA in E-18 hippocampal neuron cultures. As shown in Fig. 2B, the chromatographic retention times of standard DEA and the 13C22-metabolite are identical [22]. Homogenates of E-18 hippocampi also converted 13C22-DHA to 13C22-DEA. In contrast, no docosahexaenoylamide (DHA-amide) production was detected, and addition of DHA-amide to the cultures did not affect hippocampal neurite growth or synaptogenesis.
Fig. 2.
Conversion of DHA to DEA in hippocampal cultures. A. The tandem mass (MS/MS) spectrum was obtained from [M+H]+ of 13C22-DEA produced by mouse E-18 hippocampal neurons cultured for 3 days in the presence of 1 μM 13C22-DHA. The characteristic fragmentation pattern indicates that 13C22-DEA is produced by the cells. B. The MRM chromatograms using characteristic mss transitions from [M+H]+ to the ethanolamine moiety at m/z 62 confirmed the production of DEA in E-18 hippocampal cultures after supplementation with 1 μM 13C22-DHA or for 3 days. The retention time of 13C22-DEA is identical to that of the standard DEA. The mass spectrum is taken from reference 22
Synaptamide production was quantitatively determined by multiple reaction monitoring of E-18 hippocampal cultures. The cellular synaptamide level after 3 days of supplementation with 1 μM DHA was estimated to be approximately 13.5 nM, or 240 nM when its hydrolysis was blocked by URB597 [22].
No information is available regarding the mechanism of synaptamide synthesis. Because of the structural similarity to AEA, however, it may be postulated that AEA and synaptamide synthesis occur through the same mechanism. An intermediate in this biosynthetic pathway is N-acylphosphatidylethanolamine (NAPE) [27]. NAPE-dependent AEA synthesis has been observed in rat cortical neurons cultured from day17 fetuses and in rat brain, including the hippocampus [28,29]. One possible mechanism for the production of N-acylethanolamide from NAPE is hydrolysis by a phospholipase D [27]. Another involves an initial double O-deacylation of NAPE by the α/β-hydrolase 4 (ABH4), forming a glycerophospho-N-acylethanolamine intermediate that is hydrolyzed by glycerophosphodiesterase 1 (GDE1) to release the N-acylethanolamide group [30]. NAPE synthesis is presumed to occur through transfer of the sn-1 fatty acid of phosphatidylcholine (PC) to PE [31]. This is a potential problem with regard to synaptamide synthesis because the sn-1 position of PC contains mostly saturated fatty acids and very little, if any, DHA. However, a Ca2+- independent N-acyltransferase (iNAT) that can transfer either the sn-1 or sn-2 fatty acyl group of PC to PE has been recently described (32), and this reaction could be a source of the DHA required to form the N-docosahexaenoyl group of NAPE. A possible alternative pathway for synaptamide synthesis is direct condensation of DHA and ethanolamine, a reversal of the hydrolysis reaction catalyzed by FAAH. Synthesis of AEA by such a direct condensation mechanism has been observed in bovine and rabbit brain preparations [33-35]. However, the fatty acid and ethanolamine concentrations required for this reaction are high, and thus the physiological relevance of this process is questionable [27]. Currently, it is uncertain if any of these reported pathways are applicable to the biosynthesis of synaptamide.
Synaptamide modulates neuritogenesis, synaptogenesis and synaptic function
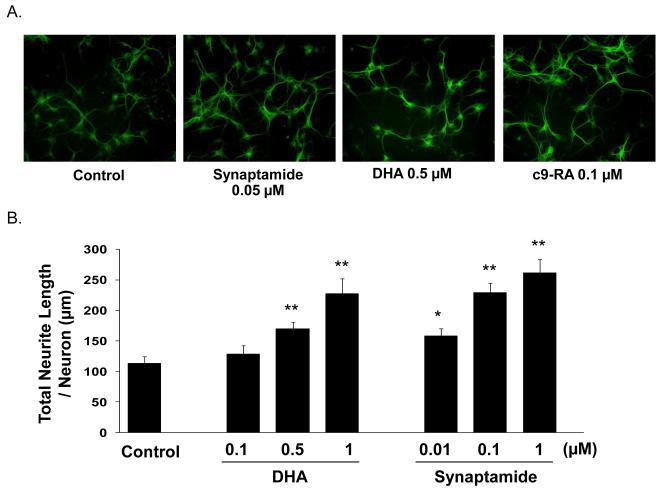
Synaptamide is a potent mediator for the neuritogenic and synaptogenic effects of DHA. Fig. 3A shows the comparative effectiveness of synaptamide and DHA on neurite growth in 3 days-in-vitro (DIV) cultures. Although a small increase in neurite growth relative to the control culture was noted with 0.5 μM DHA, a larger effect was produced by a 10-fold lower concentration of synaptamide. Fig. 3B shows the quantitative difference in neurite growth induced by synaptamide and DHA during 3 days in culture. A statistically significant increase was produced by 0.01 μM synaptamide, and further increases occurred when the concentration was raised to 0.1 μM and 1 μM. In contrast, a DHA concentration of 0.5 μM was required to produce a significant increase.
Fig. 3.
Comparative effects of DHA and synaptamide on hippocampal neurite growth. A. Mouse E-18 hippocampal neurons were incubated for 3 days with 0.05 μM synaptamide, 0.5 μM DHA, or 0.1 μM 9-cis-retinoic acid (9C-RA), and immunostained for MAP2. Representative photomicrographs from at least 8 fields in each case are shown at 20× magnification. B. Total neurite length per neuron was measured in the neurons cultured for 3 days with DHA or synaptamide. The concentration range for DHA and synaptamide was 0.1 - 1 μM and 0.01 - 1 μM, respectively. Measurements of 60 neurons in each culture were made, and the statistical analysis was performed with the student’s t-test. The data presented are the mean values ± standard deviation, and the significant differences were compared to the control cultures: *, p < 0.05, and **, p < 0.05.
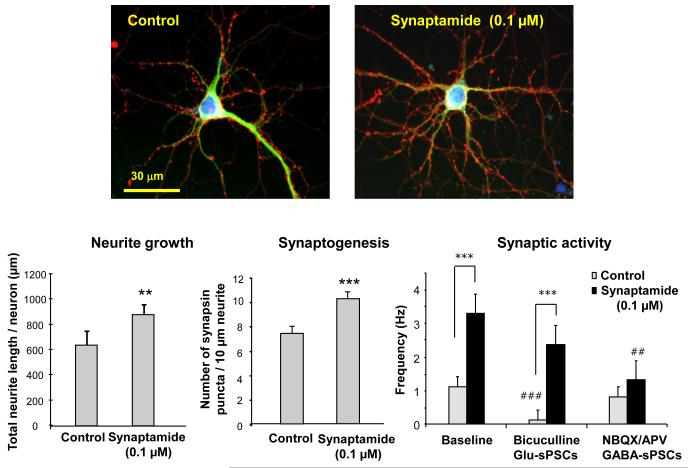
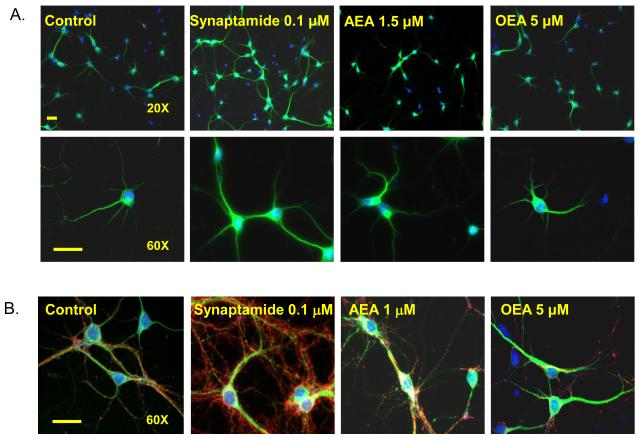
Synaptamide is also a potent synaptogenic factor. In addition to promoting neurite growth, 0.1 μM synaptamide included in the E-18 hippocampal neuron culture for 7 days significantly increased synaptogenesis measured by the number of synapsin puncta per a given neurite length (Fig. 4). Functional evidence for the observed improvement in synpatogenesis was provided electrophysiologically. Supplementation of hippocampal neurons with 0.1 μM synaptamide promoted synaptic transmission, concomitantly increasing expression of synapsins and glutamate receptors [22]. The spontaneous postsynaptic current frequency (sPSC), which represents the number of active synapses and relative levels of presynaptic release, was increased 2.9-times by treatment of the hippocampal cultures with 0.1 μM synaptamide for 10 days. The magnitude of the effects on synaptogenesis and synaptic activity produced by 0.1 μM synaptamide was similar to, if not higher than, that produced by 1 μM DHA. Synaptamide did not affect the GABAergic component of sPSCs which was measured in the presence of both AMPA and NMDA receptor antagonists. On the contrary, glutamatergic sPSCs isolated using a GABA receptor antagonist was significantly higher in neurons treated with synaptamide. These data demonstrates that synaptamide enhances specifically glutamatergic synaptic activity as in the case with DHA-treated neurons. Synaptamide acutely added to the target neurons produced no effects on synaptic activity, indicating that prolonged treatment that allows development of synapses and synaptic protein expression is required for the synaptamide-induced improved synaptic activity. Synaptamide action on development of hippocampal neurons is unique and distinctive, as other fatty acyl ethanolamides including AEA and oleylethanolamide (OEA) did not affect neurite growth (Fig. 5A) or synaptogenesis even at significantly higher concentrations (Fig. 5B).
Fig. 4.
Effects of synaptamide (0.1 μM) on neurite growth, synaptogenesis and synaptic activity. Representative photomicrographs are shown at 60× magnification. Synaptogenesis was evaluated by the number of synapsin puncta / 10μm neurite. **, p<0.01; ***, p<0.001. For synaptic activity, spontaneous postsynaptic currents (sPSCs) including glutamatergic (Glu-sPSCs) and GABAergic (GABA-sPSCs) components were recorded in hippocampal neurons cultured with or without 0.1 μM synaptamide for 10 days. To isolate the GABAergic sPSCs, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium (NBQX, 5 μM) and D-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) were used. For measurement of glutamatergic sPSCs, bicuculline (20μM) was superfused onto the neuron. Paired t-tests were performed against the baseline value of each group (##, p<0.01; ###, p<0.001) or between indicated groups (***, p<0.001). The bar graphs are taken from reference 22.
Fig. 5.
Effects of N-acylethanolamides on neurite growth and synaptogenesis. Embryonic hippocampal neurons were cultured for 3 (A) or 7 days (B) with 0.1 μM synaptamide, AEA or OEA at concentrations from 1 to 5 μM. Representative photomicrographs are shown for neurons triple-stained with MAP2 (green), synapsin (red) and nuclei (blue). Scale bar: 30μm. While 0.1 μM synaptamide significantly increased neurite growth and synaptogenesis, AEA or OEA showed no effects at substantially higher concentrations.
No conclusive information is available regarding the mechanism of action for synaptamide. The fact that synaptamide was effective when added to the culture medium suggests that it might act through a membrane receptor mechanism. The functional effects of AEA are mediated through binding to cell surface cannabinoid (CB) receptors [36]. As a structural analogue of AEA, synaptamide may also function through a CB1-mediated mechanism. AEA has been observed to promote hippocampal neurogenesis by binding to CB1 receptors [37], while other evidence indicates that AEA binding to CB1 receptors inhibits the differentiation of neuronal progenitor cells [38]. Although a role for CB1 receptors in the synaptamide-mediated effect cannot be excluded, this seems unlikely for several reasons. AEA binds much more strongly than synaptamide to CB1 receptors [39]. Yet, it was not effective in stimulating neurite growth or synaptogenesis in the E-18 hippocampal neuron cultures at a concentration of 1 μM (Fig. 5B), 10-times higher than the effective concentration of synaptamide (Fig. 4). The selectivity for synaptamide is consistent with the possibility that it functions through a mechanism that differs from that of AEA.
Another possibility is that synaptamide acts through an intracellular mechanism although whether and how extracellular synaptamide transfers into the intracellular compartment in neuronal cells are not known. Neuronal cells have been shown to take up AEA by a high-affinity transport system [36,40]. It has been also reported that intact molecules of AEA can be transported into mouse cortical neurons without hydrolysis [41]. Based on the structural similarity, it is reasonable to assume that neurons also can take up synaptamide and that intact synaptamide might be present inside the cell for a period of time prior to its hydrolysis by FAAH. The possibility of an intracellular mechanism of action is suggested by the finding that N-oleoylethanolamide promotes neurite growth by a PPARα-dependent process [42]. Nevertheless, the N-oleoylethanolamide concentration required for this effect is relatively high. In addition, PPARα downstream protein expression in the hippocampal neuron cultures is not affected by synaptamide [22], indicating that PPARα does not mediate synaptamide activity. Synaptamide increases neurite growth in E-18 hippocampal neurons to the similar extent as 9-cis-retinoic acid which activates the retinoid X receptor (RXR), a nuclear transcription factor. DHA is also known to activate RXR in brain tissues [43]. These findings suggest the possibility that synaptamide also functions through a RXR-dependent or a related transcriptional mechanism.
Effects of dietary n-3 fatty acid deficiency on hippocampal N-acylethanolamides
Hippocampal neurite growth and synaptogenesis are impaired due to DHA depletion in offspring of female mice maintained on an n-3 fatty acid deficient diet [3,44]. The deficient diet produced approximately 75 % decrease in hippocampal DHA. This was accompanied by a 10-fold increase in the n-6 docosapentaenoic acid (DPAn-6). Accordingly, changes occurred in the levels of synaptamide and N-docosapentaenoyl ethanolamide (DPEA) in the hippocampi obtained from E-18 fetuses, as well as from offspring that were continued on the respective diet for 18 days. In both cases, the n-3 fatty acid deficient diet substantially reduced the synaptamide content, but it had no appreciable effect on the AEA content. The decrease in synaptamide was partially replaced by DPEA, consistent with the fact that DPAn-6 replaces much of the decrease in DHA when n-3 fatty acids are deficient. The dependence of the hippocampal synaptamide level on the DHA content in the tissue indicates that DHA-derived synaptamide synthesis is an endogenous process of in vivo relevance in hippocampal neuronal development. The effect of synaptamide on synapse formation in vivo remains to be determined. However, the impairment of synaptic function that occurred in the DHA-deficient mice when the hippocampal synaptamide level decreased suggests that synaptamide is likely to promote synapse formation in vivo. However, we have shown the impairment of synaptic function in the DHA-deficient mice when the hippocampal synaptamide level decreased [3], suggesting that synaptamide is likely to promote synapse formation in vivo.
N-Docosahexaenoylamide derivatives
In addition to the N-acylethanolamides, more than 70 N-acylamide derivatives of amino acids and neurotransmitters have been detected in the brain [45,46]. A number of these N-acylamides have bioactivity. For example, N-arachidonoylglycine inhibits pain [47,48], is an insulin secretagogue [49], a ligand for the orphan receptors GPR18 and GPR92 [50,51], and a reversible inhibitor of the glycine transporter GLYT2a [52]. N-palmitoylglycine increases Ca2+ influx in a dorsal root ganglion-like cell line and is antinociceptive [53], and N-arachidonoylGABA also suppresses pain [47]. N-arachidonoyldopamine and N-oleoyldopamine activate the vanilloid type 1 receptor [54-56]. Six of the N-acylamides detected in brain are DHA derivatives, including N-docosahexaenoylglycine, N-docosahexaenoylglutamic acid, N-docosahexaenoylglutamine, N-docosahexaenoylGABA, N-docosahexaenoylhistidine and N-docosahexaenoylphenylalanine [45,46]. It remains to be determined whether these DHA derivatives, like synaptamide, also have biological activity in the nervous system.
Highlights.
N-docsahexanoylethanolamide (DEA) is a potent synaptogenic factor, and thus termed synapamide.
Docosahexaenoic acid (DHA) is converted to DEA by fetal mouse hippocampal neurons.
DEA is present in the mouse hippocampus.
DEA stimulates neurite growth, synaptogenesis and glutamatergic synaptic activities.
DEA increases synapsin and glutamate receptor subunit expression in the hippocampal neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Medicine. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–81. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- [3].Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–21. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurons throughout development and in aged animals. Neurobiol Aging. 2010;31:678–87. doi: 10.1016/j.neurobiolaging.2008.05.027. [DOI] [PubMed] [Google Scholar]
- [5].Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid differences in lipid matrix properties from the loss of one double bond. J Am Chem Soc. 2003;125:6409–21. doi: 10.1021/ja029029o. [DOI] [PubMed] [Google Scholar]
- [6].Litman B, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways Visual transduction. J Molec Neurosci. 2001;16:237–42. doi: 10.1385/JMN:16:2-3:237. [DOI] [PubMed] [Google Scholar]
- [7].Niu S, Mitchell D, Lim SY, Wen Z, Salem N, Jr, Kim HY, Litman B. N-3 Deficiency in rats reduces G-protein coupled signal transduction in retinal rod outer segment membranes. J Biol Chem. 2004;279:31098–104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- [8].Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3): Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- [9].Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–5. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- [11].Kim HY, Akbar M, Kim SY. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostagl Leukot Essen Fatty Acids. 2010;82:165–72. doi: 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, Greenstein D, Wohltmann M, Turk J, Rapoport SI. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2β (VIA)-deficient mice. J Lipid Res. 2010;51:3166–73. doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autocoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- [14].Lukiw WJ, Cui JG, Marcheselli VL, Bodker A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–87. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bazan NG, Calandria JM, Cerhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin BA, Morrow JD. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. J Biol Chem. 2008;283:19927–35. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Connor M, Vaughan CW, Vandenberg RJ. N-acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets. Brit J Pharmacol. 2010;160:1857–71. doi: 10.1111/j.1476-5381.2010.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target for pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- [20].Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamides in piglets. Proc Natl Acad Sci USA. 2001;98:6402–06. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–23. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim HY, Moon SY, Cao D, Lee J, Jun SB, Lovinger DM, Abkar M, Huang BX. N-docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011;435:327–36. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Südhof TC, Czernik AJ, Kao HT, Takei K, Johnston PA, Horicuhi A, Kanazir SD, Wagner MA, Perin MS, De Camilli P, Greengard P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–80. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- [24].Lu B, Greengard P, Poo MM. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992;8:521–29. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- [25].Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci USA. 1995;92:9230–34. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci. 2007;27:13520–31. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schmid HHO, Schmid PC, Natarajan V. The N-acylation phosphodiesterase pathway and cell signaling. Chem Phys Lipids. 1996;80:133–42. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- [28].Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–42. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–42. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schmid HHO, Schmid PC, Natarajan N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- [32].Jin HA, Uyama T, Wang J, Okamoto Y, Tonai T, Ueda N. cDNA cloning and characterization of human and mouse Ca2+-independent phosphatidylethanolamine N-acyltransferases. Biochim Biophys Acta. 2009;1791:32–38. doi: 10.1016/j.bbalip.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [33].Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–49. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- [34].Kruszka KK, Gross RW. The ATP- and CoA-independent synthesis of arachidonoylethanolamide. A novel mechanism underlying the synthesis of the endogenous ligand of the cannabinoid receptor. J Biol Chem. 1994;269:14345–48. [PubMed] [Google Scholar]
- [35].Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Ishima Y, Waku K. Transacylation-mediated and phosphodiesterase-mediated synthesis of N-arachidonoyletholamine, an endogenous cannabinoid ligand, in rat brain microsomes: Comparison and synthesis from free arachidonic acid and ethanolamine. Eur J Biochem. 1996;240:53–62. doi: 10.1111/j.1432-1033.1996.0053h.x. [DOI] [PubMed] [Google Scholar]
- [36].Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- [37].Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–16. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–50. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- [39].Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40:659–67. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- [40].Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–8. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by hydrolysis-resistent inhibitor AM1172. Proc Natl Acad Sci USA. 2004;101:8756–61. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bento-Abreu A, Tabernero A, Medina JM. Peroxisome proliferator activated receptor-α is required for the neurotrophic effect of oleic acid in neurons. J Neurochem. 2007;103:871–81. doi: 10.1111/j.1471-4159.2007.04807.x. [DOI] [PubMed] [Google Scholar]
- [43].de Urquiza A Mata, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mousebrain. Science. 2000;290:2140–44. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- [44].Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–88. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- [45].Bradshaw H, Lee SH, McHugh D. Orphan endogenous lipids and orphan GPCRs:A good match. Prostagl Other Lipid Mediat. 2009;89:131–4. doi: 10.1016/j.prostaglandins.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tan B, O’Dell DK, Yu YW, Monn MF, Hughes HV, Burstein S, Walker JM. Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J Lipid Res. 2010;51:112–19. doi: 10.1194/jlr.M900198-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Madea DY, DePetrocellis L, Burstein S, DiMarzo V, Walker JM. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–44. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- [48].Prusakiewicz JJ, Kingsley PJ, Kozak KR, Marnett LJ. Selective oxygenation of N-arachidonoylglycine by cyclooxygenase-2. Biochem Biophys Res Commun. 2002;296:612–7. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- [49].Ikeda Y, Iguchi H, Nakata M, Ioka RX, Tanaka T, Iwasaki S, Magoori K, Takayasu S, Yamamoto TT, Kodama T, Yada T, Sakurai T, Yanagisawa M, Sakai J. Identification of N-arachidonoylglycine, U18666A and 4-androstene-3,17-dione as novel insulin secretogogues. Biochem Biophys Res Commun. 2005;333:778–86. doi: 10.1016/j.bbrc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- [50].Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Identification of N-arachidonoylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347:827–32. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- [51].Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O’Dell DK, Walker JM, Na HS, Lee MG, Kwon B, Kim K, Seong JY. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–64. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wiles AL, Pearlman RJ, Rosvall M, Aubrey KR, Vanderberg RJ. N-Arachidonoyl-glycine inhibits the glycine transporter GLYT2a. J Neurochem. 2006;99:781–6. doi: 10.1111/j.1471-4159.2006.04107.x. [DOI] [PubMed] [Google Scholar]
- [53].Rimmerman N, Bradshaw HB, Hughes HV, Chen JSC, Hu SSJ, McHugh D, Vefring E, Jahnsen JA, Thompson EL, Masuda K, Cravatt BF, Burstein S, Vasko MR, Prieto AL, O’Dell DK, Walker JM. N-Palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol Pharmacol. 2008;74:213–24. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with a high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–5. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chu CJ, Huang SM, DePetrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, DiMarzo V, Walker JM. N-Oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–39. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- [56].Zhong B, Wang DH. N-oleoyldopamine, a novel endogenous capsaicin-like lipid, protects against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol. 2008;295:H728–35. doi: 10.1152/ajpheart.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]