Abstract
Exercise, decompensated heart failure, and exposure to high altitude have been shown to cause symptoms of pulmonary edema in some, but not all, subjects, suggesting a genetic component to this response. Epithelial Na+ Channels (ENaC) regulate Na+ and fluid reabsorption in the alveolar airspace in the lung. An increase in number and/or activity of ENaC has been shown to increase lung fluid clearance. Previous work has demonstrated common functional genetic variants of the α-subunit of ENaC, including an A→T substitution at amino acid 663 (αA663T). We sought to determine the influence of the T663 variant of αENaC on lung diffusion at rest and at peak exercise in healthy humans. Thirty healthy subjects were recruited for study and grouped according to their SCNN1A genotype [n= 17vs.13, age=25±7vs.30±10yrs., BMI= 23±4vs.25±4kg/m2, V̇O2peak= 95±30vs.100±31%pred., mean±SD, for AA (homozygous for αA663) vs. AT/TT groups (at least one αT663), respectively]. Measures of the diffusing capacity of the lungs for carbon monoxide (DLCO), the diffusing capacity of the lungs for nitric oxide (DLNO), alveolar volume (VA), and alveolar-capillary membrane conductance (DM) were taken at rest and at peak exercise. Subjects expressing the AA polymorphism of ENaC showed a significantly greater percent increase in DLCO and DLNO, and a significantly greater decrease in systemic vascular resistance from rest to peak exercise than those with the AT/TT variant (DLCO=51±12vs.36±17%, DLNO=51±24vs.32±25%, SVR=−67±3vs.−50±8%, p<0.05). The AA ENaC group also tended to have a greater percent increase in DLCO/VA from rest to peak exercise, although this did not reach statistical significance (49±26vs.33±26%, p=0.08). These results demonstrate that genetic variation of the α-subunit of ENaC at amino acid 663 influences lung diffusion at peak exercise in healthy humans, suggesting differences in alveolar Na+ and, therefore, fluid handling. These findings could be important in determining who may be susceptible to pulmonary edema in response to various clinical or environmental conditions.
Keywords: DLCO, DLNO, polymorphism, lung fluid balance, epithelial sodium channel
1. Introduction
Certain clinical and environmental conditions can challenge the ability of the lungs to handle fluid. In a healthy lung, optimal lung fluid balance is regulated by both forces that control lung fluid accumulation and those that influence lung fluid removal. Given similar clinical and environmental parameters, exposure to intense prolonged exercise, exposure to high altitude, or development of heart failure can all lead to pulmonary edema in some, but not all, individuals (Caillaud et al., 1995; Hopkins et al., 1997). However, not all sojourners to high altitude, athletes, or patients with heart failure show evidence for pulmonary edema, suggesting a genetic component to one’s susceptibility to pulmonary edema formation (Guenette et al., 2007; Hodges et al., 2007; Hopkins et al., 1997; McKenzie et al., 2005). While lung fluid accumulation is primarily passive and follows Starling’s law, lung fluid clearance is an active process involving several pathways including activation of the amiloride sensitive epithelial sodium channel (ENaC).
ENaC is a heteromeric sodium channel that is composed of three distinct subunits, alpha, beta, and gamma (Canessa et al., 1994). ENaC is most functional as a heteromer consisting of two α, β, and γ subunits, however the α-subunit is also able to create a functional channel (Canessa et al., 1994). The α-subunit of ENaC has shown the greatest functional effect on channel activity in oocyte expression, knock out, and RNA interference models (Canessa et al., 1994; Hummler et al., 1996; Li and Folkesson, 2006). ENaC is expressed on the apical membrane of principal cells in the distal tubule and collecting duct in the kidney, as well as in the apical membrane of the type I and II alveolar cells in the lung (Garty and Palmer, 1997). The primary function of ENaC is to allow for the passive transport of Na+ down its electrochemical gradient from the apical side of the cell into the cell, where Na+ is actively extruded to the interstitial space by the Na+/K+ ATPase. In the kidney, ENaC reabsorbs Na+ from the glomerular filtrate and, as water follows salt, is a key player in the regulation of blood volume. As a result, changes in activity of ENaC are associated with changes in blood pressure (Ambrosius et al., 1999). In the lungs, the ENaC is located in the proximal and distal airways as well as in the alveoli, and plays a major role in the regulation of lung fluid balance (Eaton et al., 2004).
Diffusion of oxygen and carbon dioxide across the respiratory membrane requires tight regulation of airway surface fluid, as increases or decreases in airway surface fluid will affect gas transfer across the alveoli (Aarseth et al., 1975; Snyder et al., 2006b; Wallin and Leksell, 1994). In the alveoli, ENaC mediates the movement of Na+, and therefore fluid, from the airway surface fluid layer into the alveolar epithelial cell, where it is then pumped into the interstitial space by the Na+/K+-ATPase. Water in the interstitial space is subsequently returned to the circulation via the lymphatics. The importance of the ENaC in lung fluid clearance is especially evident at birth, where ENaC knockout mice die within forty hours of birth due to their inability to clear amniotic fluid from the lungs (Hummler et al., 1996). Dysfunction of the ENaC has been associated with several pathological states. Over-expression or over-activity of the ENaC can lead to a Cystic Fibrosis-like dry lung, whereas low-Na+ affinity, low-activity or low-expression of the ENaC can lead to alveolar flooding (Eaton et al., 2004).
Several functional polymorphisms of the ENaC α subunit have been reported, including a common alanine to threonine substitution at amino acid 663 in the C-terminus of the ENaC. The wildtype ENaC (αA663) variant shows decreased ENaC activity as compared to the αT663 variant in oocyte models, due to decreased expression of ENaC in the membrane (Samaha et al., 2004; Tong et al., 2006). Interestingly, αA663 ENaC has been shown to be protective against hypertension, further supporting decreased channel activity and therefore decreased Na+ reabsorption in the kidneys (Ambrosius et al., 1999).
There has been much debate as to whether pulmonary edema occurs (Caillaud et al., 1995; Zavorsky, 2007) or does not occur (Guenette et al., 2007; Hodges et al., 2007; MacNutt et al., 2007) during high-intensity sea level exercise. The development of acute exercise-induced pulmonary edema likely depends on the balance between fluid accumulation and alveolar fluid clearance via ENaC-mediated transcellular Na+ transport. β-adrenergic stimulation has been shown to increase ENaC mediated lung fluid clearance (Dumasius et al., 2001; Li and Folkesson, 2006). Exercise results in a 1000 fold increase in epinephrine, which is the primary agonist for β2-adrenergic stimulation (Snyder et al., 2006a). Additionally, exercise has been shown to increase fluid clearance from interstitial space of the lungs by the lymphatic system (Coates et al., 1993; Newman et al., 1988). Increased ENaC activity during exercise, caused by β-adrenergic stimulation, may increase lung fluid clearance and counteract the challenge of lung fluid accumulation. Currently, it is unknown whether ENaC polymorphisms, such as the αA663T variant, have functional effects on lung diffusion, as they do in the kidney.
Using exercise to stimulate catecholamine release (which activates β2-adrenergic receptors), we sought to determine the influence of the αT663 polymorphism of ENaC on lung diffusion at rest and at peak exercise in healthy humans. Due to increased ENaC activity seen in the αT663 polymorphism in previous work, we predicted that those with at least one allele encoding theαT663 variant (AT/TT) would show increased lung diffusion capacity at peak exercise compared to those homozygous for the αA663 variant (AA) due to enhanced ENaC-mediated lung fluid clearance.
2. Methods
2.1 Subjects
The protocol was reviewed and approved by the Institutional Review Board of the University of Arizona. All participants provided written informed consent before participating and all aspects of the study were performed according to the Declaration of Helsinki. Thirty healthy subjects with no exclusion criteria (smoking, cardiovascular or pulmonary abnormalities, inability to exercise, or pregnancy) were recruited for study (Table 2). All subjects were given a complete blood count to rule out anemia, and females were tested for pregnancy. Cardiovascular and respiratory abnormalities were ruled out using maximal exercise and pulmonary function tests. Subjects were genotyped and, following data collection, grouped according to their SCNN1A genotype. Heterozygous individuals and those homozygous for the αT663 were categorized into the AT/TT group. Individuals homozygous for the αA663 variant were categorized into the AA group.
Table 2.
Subject Characteristics
| AT/TT | AA | |
|---|---|---|
| n | 13 | 18 |
| Age (years) | 30±10 | 25±7 |
| Gender (% female) | 46 | 39 |
| Height (cm) | 171±3 | 173±3 |
| Weight (kg) | 73±4 | 69±3 |
| BMI (kg/m2) | 25±1 | 23±1 |
| VO2 peak (% predicted) | 100±9 | 95±7 |
| FVC (% predicted) | 100±6 | 95±3 |
| FEV1 (% predicted) | 95±5 | 95±3 |
| FEF25–75 (% predicted) | 87±7 | 92±5 |
Values are mean ±SD; BMI, body mass index; VO2, volume of oxygen consumed; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FEF25–75, forced expiratory flow 25–75%.
2.2 Protocol
Upon arrival in a two hour fasted state, subjects were consented and genotyped. A venous blood sample was drawn for quantification of hemoglobin. Baseline diffusing capacity of the lungs for carbon monoxide (DLCO), diffusing capacity of the lungs for nitric oxide (DLNO), pulmonary function, and oxygen saturation (SaO2, Nellcor N-600 Pulse oximeter Boulder, CO) were measured in triplicate. Subjects then completed a subject-specific maximal exercise capacity test that was based on their reported type, speed and intensity of exercise, as well as bodysize, predicted V̇O2 and training background (Hansen et al., 1984). Subjects began exercising on a cycle ergometer (Corival Lode B.V., The Netherlands) at an appropriate initial workload, which was increased by the initial workload every three minutes until exhaustion (ie. initial workload of 50 watts with a 50 watt increase in workload every three minutes). The exercise test ended with a three-minute recovery period at the initial workload. Exhaustion was determined by an inability to maintain a pedal rate of 60–80 revolutions per minute, a rating of perceived exertion (RPE) of at least 18 out of 20, or a respiratory exchange ratio (RER) greater than or equal to 1.15. The initial workload and incremental increases per stage were determined through interviewing the subject’s exercise habits and also based on height, weight, and age of the subjects. We sought to complete each exercise test in 12–15 minutes. Volume of oxygen consumed (V̇O2), volume of carbon dioxide produced (V̇CO2), respiratory rate, tidal volume, and minute ventilation were continuously monitored and averaged every three seconds during rest, exercise, and recovery using a Medical Graphics CPX/D metabolic cart (St. Paul, MN) interfaced with a Perkin Elmer MGA-1100 mass spectrometer (Perkin Elmer MGA-1100, Wesley, MA). Heart rate (HR) was also continuously monitored during rest, exercise, and recovery by a Marquette Electronics 12 lead electrocardiogram (Milwaukee, WI), which was also interfaced with the Medical Graphics CPX/D metabolic cart. DLCO, DLNO, blood pressure and SaO2 were measured during each stage of exercise.
2.3 Diffusing Capacity of the Lung and Cardiac Output
Pulmonary capillary blood volume, DM, and cardiac output (Q̇) can be determined accurately by measuring the disappearance of nitric oxide (NO) in tandem with carbon monoxide (CO) and acetylene, as described previously (Snyder et al., 2007; Snyder et al., 2005; Snyder et al., 2008; Tamhane et al., 2001). Triplicate maneuvers of the diffusing capacity of the lungs for carbon monoxide (DLCO), and nitric oxide (DLNO) were performed in conjunction with the acetylene wash-in method for determining Q̇ at baseline and during recovery. Single maneuvers of DLCO, DLNO, and Q̇ were performed during each stage of exercise.
DLCO, DLNO, and Q̇ were measured in an upright and seated position using the rebreathe technique, with gases sampled by a mass spectrometer (Perkin Elmer MGA-1100, Wesley, MA) and NO analyzer (Seivers Instruments, Boulder, CO) integrated with custom analysis software, as described previously(Snyder et al., 2006b; Snyder et al., 2005; Wheatley et al., 2011a; Wheatley et al., 2011b). Subjects breathed into a five-liter rebreathe bag containing 0.7% acetylene, 9% helium, 0.3% carbon monoxide (C18O), 40 PPM NO, and 35% O2 with a respiratory rate controlled at 32 breaths per minute via metronome. The 40 PPM NO was diluted in the anesthesia bag immediately before each maneuver from an 800 PPM NO tank. C18O was used instead of the more common C16O to enable the mass spectrometer to distinguish between C16O and N2, which have similar molecular weights. The total volume of gas in the rebreathe bag was determined by the tidal volume of the subject during exercise, and was standardized at 1575mL at rest. Consistent bag volumes were ensured using a timed switching circuit which resulted in the desired volume, given a constant rate of flow from the tank. The switching circuit and tank were calibrated for accurate volumes before each test. At the end of a normal expiration (EELV, end-expiratory lung volume) subjects were switched into the rebreathe bag and breathed the test gas for eight to ten breaths. Following each maneuver, the rebreathe bag was emptied via vacuum and refilled immediately before the next maneuver.
Membrane conductance and binding of carbon monoxide to hemoglobin contribute to the diffusion capacity of the lungs for carbon monoxide (Tamhane et al., 2001). Acetylene does not bind to hemoglobin, and therefore its disappearance is limited by the delivery of a new volume of blood to the lungs, providing a measure of cardiac output. Custom software was used to calculate the rate of disappearance of the gases with each breath calculated from the slope of the exponential disappearance for each gas with respect to helium (Snyder et al., 2005). Unlike DLCO, DLNO is based primarily on membrane conductance (DMNO) as nitric oxide is scavenged 280 times faster by hemoglobin than oxygen, causing the uptake of NO to be essentially instantaneous (DLNO≈DMNO). Therefore, DLNO is considered a direct measure of membrane conductance as the diffusion resistance of the blood is insignificant (Hsia, 2002; Hsia and Raskin, 2005; Roughton and Forster, 1957; Tamhane et al., 2001). DMNO was then used to calculate the DM for carbon monoxide (DMCO) by correcting for their different diffusion constants based on molecular weight and solubility as described previously (Hsia, 2002; Hsia and Raskin, 2005; Roughton and Forster, 1957; Tamhane et al., 2001). Recently, Ceridon et al. has demonstrated that a correction factor of 2.11 is most appropriate during exercise (Ceridon et al., 2010). Pulmonary-capillary blood volume was then calculated from the DLCO measured by subtracting the resistance to diffusion associated with alveolar-capillary barrier (DMCO). Finally, we corrected for individual differences in the rate of uptake and binding to hemoglobin due to differences in hemoglobin concentrations (14.6 g/dl used as the normal hemoglobin concentration) and the alveolar pressure of oxygen.
2.4 Genotyping
Subjects were instructed to swab the inside of each cheek, and place the swab into a stabilizing buffer solution for storage purposes. Samples were sent to the University of Arizona Genetics Core Laboratory for genotyping of the αENaC amino acid at position 663 of both alleles using a Taqman SNP assay for rs#2228576. Briefly, initial DNA quantitation and QC was performed using PicoGreen (Life Technologies). Pre-validated primers and probe sets for TaqMan Allelic Discrimination Assay were obtained from Life Technologies. Reactions were run at 10uL, containing TaqMan Universal PCR Master Mix, No AmpEraseR UNG (Life Technologies), 10ng total DNA, and 1X Assay Mix. All samples processed and analyzed on 7900 Real-Time PCR System (Life Technologies) with cycling conditions (95°C for 10 minutes, 50 cycles of 92°C for 15 seconds and 60°C for 1 minute) and Genotyper software (SDS system, version 2.3).
2.5 Hemoglobin Concentration
Hemoglobin was measured at the University of Arizona Medical Center Pathology Laboratory using a cyanide-free hemoglobin method on an ADVIA 2120 Hematology system.
2.6 Calculation of Systemic Vascular Resistance and Mean Arterial Pressure
Mean arterial pressure (MAP) was calculated from systolic and diastolic blood pressures obtained using the auscultation technique with the same technician performing all measures. Systolic and diastolic blood pressure measures were taken during each stage of exercise, rest, and recovery. Mean Arterial Pressure was calculated as follows:
Systemic vascular resistance was calculated as follows:
Where 10 is the estimated pulmonary artery wedge pressure and 80 is a numerical constant which compensates for the units used in the calculation.
2.7 Statistical Analyses
The SPSS statistical software package (v.18; SPSS, Inc., Chicago, IL) was used for all statistical analyses. After confirming equality of variance with a Levene’s Test, a t-test was used to determine significance between genotype groups at rest and peak exercise as well as for percent change from rest to peak exercise. An α level of 0.05 or lower was considered significant. All data are presented as mean ± SD, unless otherwise stated.
3. Results
The amino acid at position 663 was first reported to most commonly be threonine, however the consensus sequence at amino acid position 663 more recently is believed to be alanine (Ambrosius et al., 1999; Pratt, 2003). Therefore, threonine at position 663 is the polymorphism and in this study wildtype individuals (A663) were compared to nonwildtype individuals (at least one allele encoding T663). Table 1 shows the sequence alignments from several established mammalian sequences around amino acid 663.
Table 1.
αENaC sequence is well conserved in mammals
| αENaC Amino Acid Sequence 663 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | A | G | A | S | S | S | T | C | P | L | G | G | P | - |
| Guinea Pig | A | G | A | S | S | A | A | C | A | P | R | E | P | - |
| Cow | A | E | A | S | T | S | A | H | A | P | G | E | P | - |
| Mouse | A | V | P | G | S | S | A | C | A | P | A | M | A | L |
| Rat | A | A | P | D | C | S | A | C | A | L | A | A | L | - |
| Rabbit | A | - | - | - | - | - | - | C | A | P | G | E | P | - |
|
| ||||||||||||||
| Consensus | A | |||||||||||||
Residue number refers to human αENaC; Human, guinea pig, cow, mouse, rat, and rabbit sequences from NCBI loci P37088.1, Q9R1N2.1, NP_777023.1, NP_035454.2, NP_113736.1, and NP_001076197.1, respectively.
Healthy subjects with at least one SCNN1A allele resulting in a threonine at amino acid 663 (AT/TT group) or with a homozygous SCNN1A genotype resulting in an alanine at amino acid 663 (AA group) were similar in gender, age, height, weight, and pulmonary function (Table 2). At rest, there were no observed differences in V̇O2, HR, MAP, SaO2, DLCO, DLNO, and DM between AT/TT and AA groups (Table 3, Table 4).
Table 3.
Cardiopulmonary response to exercise
| AT/TT
|
AA
|
|||
|---|---|---|---|---|
| Rest | Peak Exercise | Rest | Peak Exercise | |
| Workload (Watts) | 0 | 178±72 | 0 | 185±61 |
| Heart Rate | 83±19 | 178±16 | 79±12 | 180±13 |
| Cardiac Output (L/min) | 6±2 | 17±5 | 5±2 | 16±6 |
| SBP (mmHg) | 110±9 | 157±15 | 109±11 | 157±24 |
| DBP (mmHg) | 73±7 | 61±29 | 69±8 | 59±21 |
| MAP (mmHg) | 85±7 | 93±19 | 83±8 | 91±14 |
| VO2 (mL/kg/min) | 6±1 | 34±12 | 6±2 | 36±12 |
| RR (breaths/min) | 19±13 | 41±12 | 18±4 | 42±11 |
| VT BTPS (mL) | 889±388 | 2381±783 | 785±238 | 2199±587 |
| VE BTPS (L/min) | 15±6 | 93±33 | 14±4 | 92±30 |
Values are mean±SD; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; VO2, volume of oxygen consumed; RR, respiratory rate, VT BTPS, tidal volume standardized for temperature, pressure, and humidity, VE BTPS, minute ventilation standardized for temperature, pressure, and humidity.
Table 4.
Lung diffusion measures
| AT/TT
|
AA
|
|||||
|---|---|---|---|---|---|---|
| Rest | Peak Exercise | Recovery | Rest | Peak Exercise | Recovery | |
| DLCO (mL/min/mmHg) | 25±6 | 34±9* | 25±7 | 24±5 | 36±7* | 24±5 |
| DLNO (mL/min/mmHg) | 82±21 | 106±29* | 84±23 | 78±14 | 117±26* | 79±18 |
| DLCO/VA (mL/min/mmHg/L) | 9±1 | 11±2* | 9±2 | 8±2 | 12±3* | 9±1 |
| DM (mL/min/mmHg) | 39±10 | 50±14* | 40±11 | 38±7 | 55±12* | 38±8 |
| SaO2 (%) | 98±2 | 97±2 | 97±1 | 98±2 | 97±2 | 93±13 |
Values are mean±SD; DLCO, diffusing capacity of the lungs for carbon monoxide; DLNO, diffusing capacity of the lungs for nitric oxide; DLCO/VA, diffusing capacity of the lungs for carbon monoxide corrected for alveolar volume; DM, alveolar-capillary conductance; SaO2, peripheral oxygen saturation.
P<0.05 from rest to peak exercise within groups
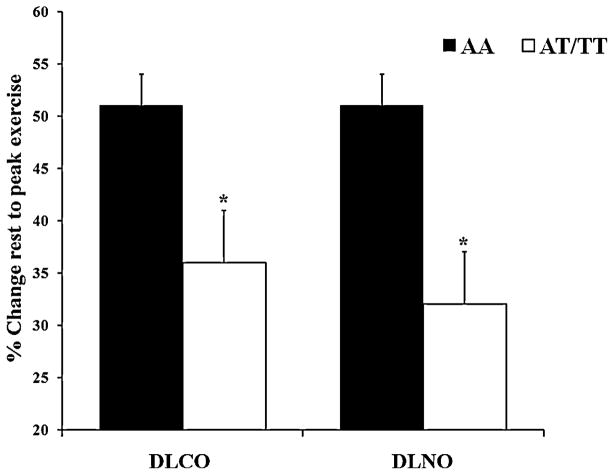
Exercise resulted in similar increases in HR, systolic BP, V̇O2, respiratory rate, tidal volume, and ventilation for AA and AT/TT groups (Table 3). The exercise tests were completed in 15 minutes on average. Subjects with the AA variant demonstrated a significantly greater percent increase in DLCO and DLNO as compared to the AT/TT variant (DLCO=51±12 vs. 36±17%, DLNO=51±24 vs. 32±25%, Figure 1, p<0.05). No outliers were found upon comparing each individual’s percent change in DLCO, DLNO, or DM from rest to peak exercise to the rest of their group (data not shown). A significantly greater decrease in systemic vascular resistance (SVR) was seen in the AA variant than the AT/TT variant from rest to peak exercise (SVR=−67±3 vs. −50±8%, Figure 2, p<0.05). The AA ENaC group tended to have a greater percent increase in DLCO/VA from rest to peak exercise, although this trend did not reach significance (DLCO/VA=49±26 vs. 33±26%, p=0.08). The increase in DM from rest to peak exercise was similar between AA and AT/TT polymorphisms of ENaC, although higher in the AA (DM=45±21 vs. 32±26%). Exercise tended to cause a greater increase in VC for the AA ENaC group as compared to the AT/TT group (VC=87±13 vs. 59±11%). No differences in DM/VC were seen during recovery.
Figure 1.
Percent increase in the diffusing capacity of the lung for carbon monoxide (DLCO) and the diffusing capacity of the lung for nitric oxide (DLNO) in response to peak exercise. The filled in bars represent the AA group (homozygous for SCNN1A resulting in alanine at amino acid 663) and the open bars represent the AT/TT group (at least one copy of SCNN1A resulting in threonine at amino acid 663). The error bars represent the SE of the mean. *P <0.05 between groups.
Figure 2.
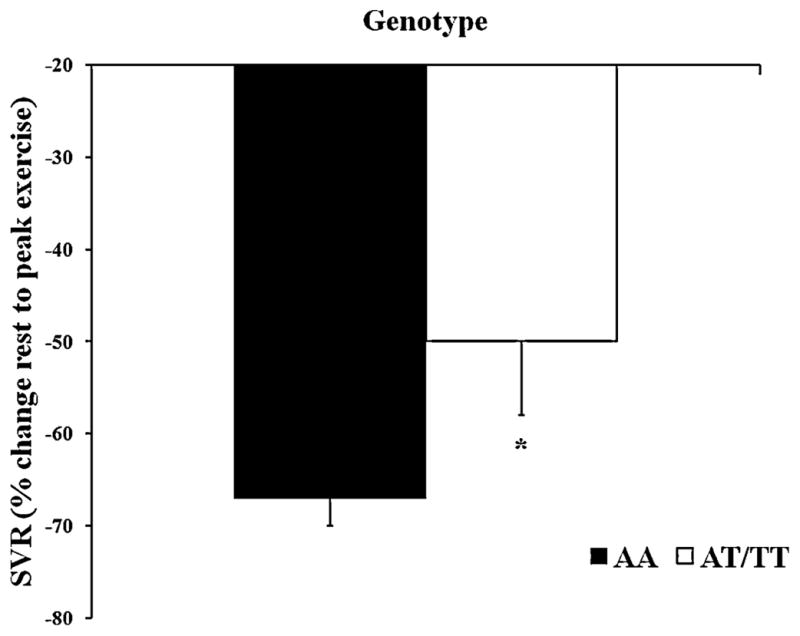
Percent decrease in systemic vascular resistance (SVR) in response to peak exercise. The filled in bars represent the AA group (homozygous for SCNN1A resulting in alanine at amino acid 663) and the open bars represent the AT/TT group (at least one copy of SCNN1A resulting in threonine at amino acid 663). The error bars represent the SE of the mean. *P<0.05 between groups.
4. Discussion
In the present study we demonstrate that genetic variation of the alpha subunit of ENaC at amino acid 663 influences lung diffusion in response to peak exercise in untrained individuals. The diffusing capacity of the lung corresponds to the conductance of gas, oxygen or carbon monoxide (DLCO), from the alveolar airspace to capillary hemoglobin expressed as mL/min/mmHg. During exercise, the diffusing capacity of the lung for oxygen, DLCO, and DLNO increase with cardiac output. These increases result from alveolar recruitment as lung volume increases, recruitment of unevenly perfused or unperfused capillary beds as cardiac output increases, or through lung fluid clearance (Hsia, 2002; Snyder et al., 2006b). The diffusing capacity of the lungs for carbon monoxide is determined by its components: membrane conductance (DM) and pulmonary capillary blood volume (VC). While DLCO can be influenced by both DM and VC, DLNO is thought to be a surrogate of DM, given the affinity of hemoglobin for NO. Similar to previous studies, we have demonstrated an increase in both DLCO and DLNO with exercise in both groups (Hsia, 2002; Tamhane et al., 2001; Wheatley et al., 2011a; Wheatley et al., 2011b). We found that the AA group (which has previously been shown to have reduced ENaC activity) had a greater percent increase in DLCO, DLNO, and DLCO/VA with exercise (endogenous β2-adrenergic stimulation) as compared to the AT/TT group. This result was opposite of what we had expected, possibly indicating a greater response in the AA group to β-adrenergic receptor-mediated alterations in channel function. Upon separating DLCO into its components, DM and VC, we observed non-significant increases in both DM and VC which together lead to the differences seen between groups in DLCO. We suspect that the difference in VC seen between groups is due to differences in ENaC mediated Na+ retention in the kidney and therefore an overall larger plasma volume seen in the AA group. Previous work has found that increasing plasma volume in healthy humans tends to lower peripheral resistance, while maintaining similar blood pressure and similar cardiac output in response to exercise (Hopper et al., 1988). The significantly larger percent decrease in systemic vascular resistance from rest to peak exercise in the AA group may also be indicative of a larger plasma volume in the AA group as compared to the AT/TT group. No difference was seen in cardiac output or blood pressure between the groups. At peak exercise there is a significant negative correlation between VC and SVR, suggesting that individuals with a lower SVR also tend to have higher VC (−.468, p<0.01). Although we found an increase in DLCO, DLNO, and a trend towards an increase in DLCO/VA, VC and DM, we did not demonstrate differences in VO2 or SaO2 at peak exercise suggesting that this difference in lung diffusion has limited functional consequences in untrained subjects.
Regulation of ENaC can occur through stimulation of the β2-adrenergic receptor by epinephrine. Additionally, ENaC can be regulated through direct activation as a result of increases in shear stress in response to increased ventilation (Eaton et al., 2004; Fronius et al.). Shear forces on the alveolar epithelium increase during exercise due to increased airflow through the airways (Tarran et al., 2006; Wirtz and Dobbs, 2000). The differences seen in DLCO and DLNO from rest to peak exercise between the genetic variants at amino acid 663 of ENaC may have resulted from either the response to adrenergic stimulation or the response to sheer stress.
Lung fluid balance is controlled by factors that influence both lung fluid accumulation (including pulmonary arterial pressure changes) and those that influence lung fluid removal (including lymphatic drainage and ion removal from the alveolar airspace). Previous research has demonstrated that exercise, particularly in elite athletes and animals, can challenge the ability of the lungs to regulate fluid (Caillaud et al., 1995; Schaffartzik et al., 1993; Zavorsky, 2007). Although there have been studies that have demonstrated evidence for lung fluid accumulation, and even pulmonary edema, in subjects who undertake prolonged intense exercise, conclusive data that all athletes develop pulmonary edema with exercise is controversial (Manier et al., 1999; McKenzie et al., 2005). Red blood cells have been found in bronchoalveolar lavage fluid following intense exercise in athletes, suggesting movement from the pulmonary circulation into the airways (Hopkins et al., 1997). Similarly, prolonged exercise has shown radiographic evidence for pulmonary edema using chest x-ray (McKechnie et al., 1979). In addition, studies utilizing CT-scanning have demonstrated an increase in lung density following normoxic exercise, although this was likely due to an increase in pulmonary blood volume, rather than lung water (Caillaud et al., 1995; Guenette et al., 2007). Other research using normoxic and hypoxic exercise as a potential stimulus for lung fluid accumulation has demonstrated an increase in alveolar-capillary membrane conductance, even when corrected for changes in pulmonary capillary blood volume, and a decrease in lung density when corrected for changes in pulmonary blood volume, suggesting a loss of lung water (Hodges et al., 2007; Snyder et al., 2006b).
The findings of the present study clearly indicate the activity of a known lung fluid clearance pathway is important during exercise. Research has shown that stimulation of the β2- adrenergic receptors via an endogenous or exogenous agonist results in an increase in the number and activity of ENaC on the apical side of alveolar cells (Johnson et al., 2006; Minakata et al., 1998). Increasing ENaC activity allows for the movement of Na+ from the alveolar airspace into type-I and type-II alveolar cells where Na+ is subsequently removed from the cell via the Na+/K+-ATPase. As water follows salt, water is also moved into the interstitial space and then cleared by the lymphatic system. Exercise causes an increase in lymphatic drainage from the lungs. Maximal exercise results in an increase in epinephrine (an endogenous β2-adrenergic receptor agonist) up to 1000-fold that of rest (Snyder et al., 2006a). Because of exercise-induced increases in epinephrine, along with the SCNN1A genotype-related change in lung diffusion from rest to peak exercise, it is likely that baseline ENaC activity level and the response to activation of ENaC was the primary driving force for the differences observed in the present study.
In a companion paper in this issue of the journal we provide evidence that the administration of an exogenous β2-agonist decreases exhaled Na+ differentially according to these same genetic variants of ENaC. Combined, these papers suggest that both exogenous and endogenous activation of ENaC can result in a decrease in exhaled Na+ at rest and increase in lung diffusion with exercise.
5. Limitations
Several factors may have limited this study. Subjects were grouped into AA and AT/TT groups due to sample size. The heterozygous individuals were grouped with those homozygous for threonine at amino acid 663 as the wildtype gene encodes alanine at position 663. A larger sample size would have allowed the groups to be split into AA, TT, and AT groups, and future studies will allow for this separation. Only one DLCO/DLNO maneuver was completed during peak exercise as the subjects would not able to maintain peak exercise long enough to complete multiple DLCO and DLNO trials. Also, healthy untrained subjects who are not likely prone to alveolar flooding because of relatively low cardiac outputs were used in this study. These findings may be particularly important in athletes who participate in prolonged high intensity exercise and are more prone to alveolar flooding due to high pulmonary arterial pressures that untrained individuals are unable to reach.
6. Conclusion
We found that genetic variation of the α-subunit of ENaC at amino acid 663 affects lung diffusion with exercise. Those in the AA group had a larger percent increase in DLCO and DLNO from rest to peak exercise than those in the AT/TT group. The larger percent increase for those in the AA group was likely attributable to lower basal ENaC activity and a larger increase in ENaC activity in response to β2-adrenergic stimulation as compared to the AT/TT group. These results may be particularly important in explaining why some, but not all, individuals develop pulmonary edema in response to various clinical and environmental conditions.
Acknowledgments
We are sincerely grateful to the subjects who willingly donated their time and effort to participate in this study. Funding for this study was provided by HL108962-01 and the University of Arizona Clinical Scholars program.
Footnotes
8. Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Aarseth P, Karlsen J, Bo G. Effects of catecholamine-infusions and hypoxia on pulmonary blood volume and extravascular lung water content in cats. Acta Physiol Scand. 1975;95:34–40. doi: 10.1111/j.1748-1716.1975.tb10021.x. [DOI] [PubMed] [Google Scholar]
- Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34:631–637. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- Caillaud C, Serre-Cousine O, Anselme F, Capdevilla X, Prefaut C. Computerized tomography and pulmonary diffusing capacity in highly trained athletes after performing a triathlon. J Appl Physiol. 1995;79:1226–1232. doi: 10.1152/jappl.1995.79.4.1226. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple-and single-inspired oxygen tension methods. J Appl Physiol. 2010;109:643–653. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates G, O’Brodovich H, Goeree G. Hindlimb and lung lymph flows during prolonged exercise. J Appl Physiol. 1993;75:633–638. doi: 10.1152/jappl.1993.75.2.633. [DOI] [PubMed] [Google Scholar]
- Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. {beta}2-Adrenergic Receptor Overexpression Increases Alveolar Fluid Clearance and Responsiveness to Endogenous Catecholamines in Rats. Circ Res. 2001;89:907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ Channels in Lung Alveolar Type II Epithelial Cells. Proc Am Thorac Soc. 2004;1:10–16. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- Fronius M, Bogdan R, Althaus M, Morty RE, Clauss WG. Epithelial Na+ channels derived from human lung are activated by shear force. Respir Physiol Neurobiol. 170:113–119. doi: 10.1016/j.resp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Sporer BC, MacNutt MJ, Coxson HO, Sheel AW, Mayo JR, McKenzie DC. Lung density is not altered following intense normobaric hypoxic interval training in competitive female cyclists. J Appl Physiol. 2007;103:875–882. doi: 10.1152/japplphysiol.00247.2007. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- Hodges AN, Sheel AW, Mayo JR, McKenzie DC. Human Lung Density is not Altered Following Normoxic and Hypoxic Moderate-Intensity Exercise: Implications for Transient Edema. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.01087.2006. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Schoene RB, Henderson WR, Spragg RG, Martin TR, West JB. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med. 1997;155:1090–1094. doi: 10.1164/ajrccm.155.3.9116992. [DOI] [PubMed] [Google Scholar]
- Hopper MK, Coggan AR, Coyle EF. Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol. 1988;64:404–408. doi: 10.1152/jappl.1988.64.1.404. [DOI] [PubMed] [Google Scholar]
- Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest. 2002;122:1774–1783. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118:205–211. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci U S A. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Folkesson HG. RNA interference for alpha-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;290:L649–L660. doi: 10.1152/ajplung.00205.2005. [DOI] [PubMed] [Google Scholar]
- MacNutt MJ, Guenette JA, Witt JD, Yuan R, Mayo JR, McKenzie DC. Intense hypoxic cycle exercise does not alter lung density in competitive male cyclists. Eur J Appl Physiol. 2007;99:623–631. doi: 10.1007/s00421-006-0388-1. [DOI] [PubMed] [Google Scholar]
- Manier G, Duclos M, Arsac L, Moinard J, Laurent F. Distribution of lung density after strenuous, prolonged exercise. J Appl Physiol. 1999;87:83–89. doi: 10.1152/jappl.1999.87.1.83. [DOI] [PubMed] [Google Scholar]
- McKechnie JK, Leary WP, Noakes TD, Kallmeyer JC, MacSearraigh ET, Olivier LR. Acute pulmonary oedema in two athletes during a 90-km running race. S Afr Med J. 1979;56:261–265. [PubMed] [Google Scholar]
- McKenzie DC, O’Hare TJ, Mayo J. The effect of sustained heavy exercise on the development of pulmonary edema in trained male cyclists. Respir Physiol Neurobiol. 2005;145:209–218. doi: 10.1016/j.resp.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of beta-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol. 1998;275:L414–422. doi: 10.1152/ajplung.1998.275.2.L414. [DOI] [PubMed] [Google Scholar]
- Newman JH, Butka BJ, Parker RE, Roselli RJ. Effect of progressive exercise on lung fluid balance in sheep. J Appl Physiol. 1988;64:2125–2131. doi: 10.1152/jappl.1988.64.5.2125. [DOI] [PubMed] [Google Scholar]
- Pratt JH. Erratum to genetic variants in the epithelial sodium channel in relation to aldosterone and potassium secretion adn risk for hypertension. Hypertension. 2003;41:e1. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- Schaffartzik W, Arcos J, Tsukimoto K, Mathieu-Costello O, Wagner PD. Pulmonary interstitial edema in the pig after heavy exercise. J Appl Physiol. 1993;75:2535–2540. doi: 10.1152/jappl.1993.75.6.2535. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Dietz NM, Joyner MJ, Turner ST, Johnson BD. Influence of {beta}2-Adrenergic Receptor Genotype on Airway Function During Exercise in Healthy Adults. Chest. 2006a;129:762–770. doi: 10.1378/chest.129.3.762. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006b;101:1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic variation of the beta2-adrenergic receptor is associated with differences in lung fluid accumulation in humans. J Appl Physiol. 2007;102:2172–2178. doi: 10.1152/japplphysiol.01300.2006. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol. 2005;99:1985–1991. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Olson TP, Johnson BD, Frantz RP. Influence of sildenafil on lung diffusion during exposure to acute hypoxia at rest and during exercise in healthy humans. Eur J Appl Physiol. 2008;103:421–430. doi: 10.1007/S00421-008-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- Wallin CJ, Leksell LG. Estimation of extravascular lung water in humans with use of 2H2O: effect of blood flow and central blood volume. J Appl Physiol. 1994;76:1868–1875. doi: 10.1152/jappl.1994.76.5.1868. [DOI] [PubMed] [Google Scholar]
- Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: alveolar-capillary membrane conductance in type 1 diabetes. European Journal of Applied Physiology. 2011a;111:567–578. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- Wheatley CM, Foxx-Lupo WT, Cassuto NA, Wong EC, Daines CL, Morgan WJ, Snyder EM. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. Journal of Cystic Fibrosis: official journal of the European Cystic Fibrosis Society. 2011b;10:45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS. Evidence of pulmonary oedema triggered by exercise in healthy humans and detected with various imaging techniques. Acta Physiol (Oxf) 2007;189:305–317. doi: 10.1111/j.1748-1716.2006.01660.x. [DOI] [PubMed] [Google Scholar]