Abstract
The soluble epoxide hydrolase (sEH) enzyme regulates the levels of endogenous epoxygenated fatty acid (EFA) lipid metabolites by rapidly degrading these molecules. The EFAs have pleiotropic biological activities including the modulation of nociceptive signaling. Recent findings indicate that the EFAs, in particular the arachidonic acid (AA) derived epoxyeicosatrienoic acids (EETs), the docosahexaenoic acid (DHA) derived epoxydocosapentaenoic acids (EpDPEs) and eicosapentaenoic acid (EPA) derived epoxyeicosatetraenoic acids (EpETEs) are natural signaling molecules. The tight regulation of these metabolites speaks to their importance in regulating biological functions. In the past several years work on EFAs in regard to their activities in the nervous system evolved to demonstrate that these molecules are anti-inflammatory and anti-nociceptive. Here we focus on the recent advances in understanding the effects of sEH inhibition and increased EFAs on the nociceptive system and their ability to reduce pain. Evidence of their role in modulating pain signaling is given by their direct application and by inhibiting their degradation in various models of pain. Moreover, there is mounting evidence of EFAs role in the crosstalk between major nociceptive and anti-nociceptive systems which is reviewed herein. Overall the fundamental knowledge generated within the past decade indicates that orally bioavailable small molecule inhibitors of sEH may find a place in the treatment of a number of diverse painful conditions including inflammatory and neuropathic pain.
Keywords: Epoxygenated fatty acids, Nociception, Hyperalgesia, Allodynia, Arachidonic acid, Epoxyeicosatrienoic acid, CNS, Opioid, Cannabinoid
Introduction
The difficulty in working with bioactive lipids given their hydrophobicity, structural variability, and short half-lives has been a significant impediment to the investigation of their functional roles in biology. Thus discoveries indicating their major roles in signal transduction was delayed until recently [1]. Historically nucleic acids were viewed as being the blueprint of the cell and proteins as the machinery that ran it. Early views of lipids being merely structural components or fuels clearly reduced enthusiasm towards studying their functions. However, there is a growing body of literature that addresses the activity of the polyunsaturated fatty acids (PUFAs) and the resulting oxylipins in modulating signal transduction and neurotransmission [2–5]. This has been a shift in understanding because bioactive lipids such as endocannabinoids and epoxy fatty acids (EFAs) do not fit the classical definition of neurotransmitters as they are not stored in vesicles or released from them. However they can freely diffuse through cell membranes. In contrast to neurotransmitters, bioactive metabolites of fatty acids are often synthesized de novo or released from membrane bilayers upon cell stimulation [6]. Additionally unlike neurotransmitters there is a general lack of knowledge regarding their fate following the activation of receptors. The importance of several classes of bioactive lipid metabolites including those of arachidonic acid (AA) origin are now uncontested. The metabolism of free AA can result in several classes of lipid metabolites with opposing bioactivities. While the algogenic and pro-inflammatory prostanoids and leukotrienes drive and maintain inflammation the anti-inflammatory and analgesic EFAs reduce and resolve inflammation. Though the anti-inflammatory action of EFAs has been substantially studied, a number of more recent publications now indicate direct anti-nociceptive activity of these molecules. Thus, bioactive lipid metabolites have roles in the transmission of sensory information, specifically pain under pathological conditions. In most cases under physiological conditions the roles of these bioactive lipids are unclear. However upon the initiation of inflammation most algogenic lipids reduce the activation thresholds of pain specific neurons to stimuli, while others such as PGE2 can directly be painful. Although the pro-nociceptive roles of bioactive lipids are well studied, the anti-nociceptive roles of have traditionally attracted much less attention. In the past decade however our ability to modulate the levels of anti-nociceptive lipid metabolite pathways increased significantly. Here we will discuss recent developments in the area of bioactive EFAs and nociception which we argue establish a solid role for natural EFAs in the mediation of pain. We will use the knowledge of other bioactive lipid mediators such as the endocannabinoids for a comparison where appropriate, though the EFAs are unique in multiple aspects compared to all other mediators in nociception. Overall the emerging findings on the anti-nociceptive roles of EFAs indicate that targeting these molecules could become an effective strategy to treat various painful conditions, including neuropathic pain.
Tight regulation of epoxygenated fatty acids (EFAs)
Outside of adipose tissue the highest concentration of lipids are in the brain [7] with phospholipids constituting 45% of total brain dry weight [8]. Docosahexaenoic acid (DHA) is the predominant PUFA in the mammalian CNS accounting for 20–50% of the fatty acid concentration in the brain while AA is also abundant in the brain and the most prevalent fatty acid in all cell types studied [9–11]. These lipids play a significant role in development and normal homeostatic functioning of the CNS [10]. Cellular membranes are possibly the largest potential substrate pools of fatty acids in biological systems. Given their ubiquitous presence in the membranes it is not surprising that the release and subsequent metabolism of fatty acids to many types of bioactive lipid metabolites are highly regulated events.
EFA production
Both the parent long chain PUFAs and their EFA metabolites are linked at the sn-2 position to membrane phosphoglyceride subclasses [9, 11]. Therefore they are selectively liberated from plasma membranes by phospholipase A2 activity [5, 12]. Released free fatty acids and EFAs are rapidly reincorporated into cellular membranes contributing to their short half-life in vivo [5, 11, 13]. AA is quite efficiently reincorporated by up to 95% in the cell phospholipids with only a small amount (<5%) left unesterfied [12]. The minor percentage that is not incorporated is free to undergo further metabolism by the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 enzymes [14] into highly potent bioactive lipid mediators including prostaglandins, leukotrienes, and epoxyeicosatrienoic acids (Figure 1). Compared to the products of the COX and LOX branches of the AA cascade the cytochrome P450 generated epoxyeicosatrienoic acids (EETs) were the last to be discovered in the early 1980s. Thus less is known about these molecules. While the cytochrome P450 family of enzymes is often associated with xenobiotic metabolism, their endogenous roles include steroid metabolism and EETs formation. More recently, it became clear that epoxy fatty acids from a number of PUFAs are generated by cytochrome P450s. Epoxygenation of these endogenous PUFAs such as linoleic, linolenic, AA, DHA and eicosapentaenoic acid (EPA) lead to multiple regioisomers of epoxygenated metabolites of the parent lipids [15, 16]. While each PUFA can be metabolized to a number of different monoepoxy fatty acid regioisomers, each of the regioisomers also have two possible geometric isomers (cis or trans) and two possible enantiomers for each geometric isomer, thus comprising a large number of biological active lipid metabolites [17, 18]. Most natural PUFAs are in Z or cis isomers which yield cis epoxides. It was not clear until recently if this molecular diversity was paralleled in functional diversity. However the large number of biological processes that the EFAs mediate support the idea that different EFAs have non-overlapping functions [19–22]. With the exception of the epoxides of LA, the plasma and cellular levels of free EFAs are typically in the low single digit nM range suggesting that these molecules function at exceedingly low concentrations.
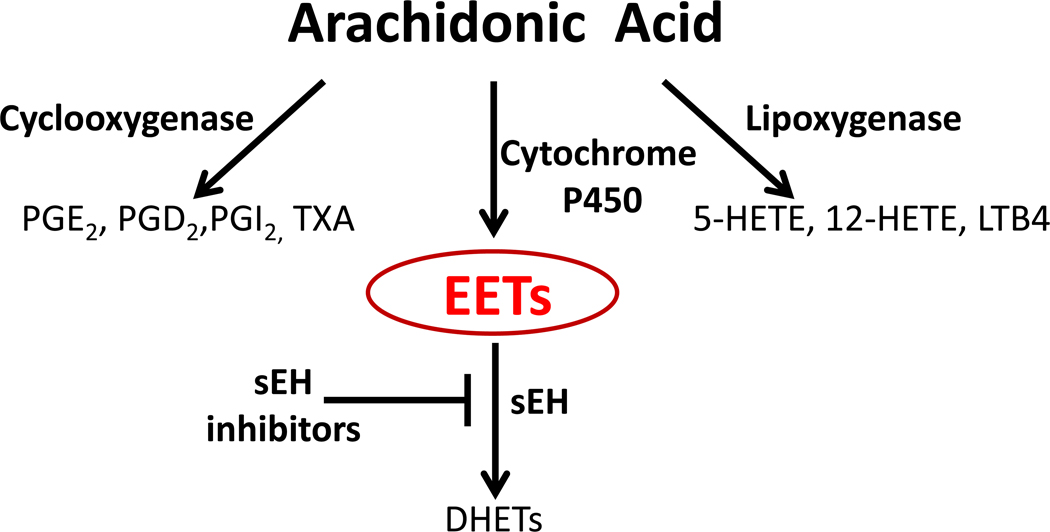
Figure 1. The Arachidonic Acid Cascade.
A simplified depiction of the formation of major metabolite families generated from arachidonic acid. AA is metabolized by cytochrome P450 enzymes into anti-inflammatory lipid mediators while the COX and LOX enzymes produce largely pro-inflammatory metabolites. The epoxyeicosatrienoic acids (EETs) are rapidly metabolized by the soluble epoxide hydrolase enzyme (sEH) to their corresponding diols the dihydroxyeicosatrienoic acids (DHETs). Inhibitors of sEH block this degradation and stabilize EET levels in vivo while reducing DHETs. The EETs suppress the transcription of the COX-2 isozyme and thus affect the AA cascade in an unexpected manner. Key cyclooxygenase metabolites include: (PGE2) prostaglandin E2, (PGD2) prostaglandin D2, (PGI2) prostaglandin I2 (prostacyclin), (TXA) thromboxane. Key lipoxygenase metabolites include: (5-HETE) 5-Hydroxyeicosatetraenoic acid, (12-HETE) 12-Hydroxyeicosatetraenoic acid, (LTB4) leukotriene B4. Other PUFAs can enter this pathway yielding among other metabolites, a variety of EFAs.
EFA metabolism and elimination
Epoxygenated fatty acid metabolites are subject to several routes of metabolism with rapid degradation by the soluble epoxide hydrolase enzyme predominant [18]. The soluble epoxide hydrolase (sEH) is an enzyme downstream of cytochrome P450 expoygenases in the AA cascade [18]. It is expressed in the brain and CNS [23, 24] although higher expression levels occur in liver and kidneys of studied species including humans [25]. The sEH is the principal epoxide hydrolase responsible for the enzymatic degradation of EETs to corresponding diol products [26, 27]. Endogenous free EETs have a short half-life given their degradation via sEH [13]. In support of the role of sEH in the metabolism of EFAs, the genetic knock out of the sEH expression in the mouse leads to approximately 3 times higher plasma levels of summed EETs compared to congenic wild type animals [28]. We recently demonstrated that epoxides of EPA and DHA are both natural substrates of the sEH and are rapidly metabolized by this enzyme [29]. The epoxides of DHA are arguably better substrates for sEH than the EETs since they are turned over more efficiently [29]. Interestingly the concentration of one regioisomer, 7,8-EpDPE that is not a preferred substrate for the sEH is about 30 times higher in the brain and the spinal cord of rats [29]. This suggests that the regioselectivity of both the P450s which make the epoxides and the sEH contribute to the relative concentration of the EFA isomers. Although in many cases the reported outcomes of sEH inhibition is attributed to EETs it is highly likely that other EFAs and their diols are involved. This aspect will be addressed in the following sections. The dihydroxyeicosatrienoic acids (DHETs), diols formed by sEH action on EETs and other EFAs are far more polar and easily conjugated. They seem to lack biological activity in most cases or have lower or different activities from the epoxides though it is difficult to draw a general conclusion for example diols of linoleate epoxides are pro-inflammatory [30, 31]. Besides the sEH mediated degradation the free EFAs may also undergo spontaneous hydration, chain elongation or β-oxidation [9, 11]. Glutathione conjugation of these relatively stable epoxides is generally slow. The epoxy-fatty acids can also be acted on by further P450 oxidative reactions as well as fatty acid binding protein (FABP) uptake [18]. Lastly the EFAs are efficiently incorporated into lipid bilayers though it is not yet fully clear if this eliminates their biological activity because there are proposed actions of membrane bound EETs [13]. Nevertheless, the tight regulation of EFAs reveals their importance as essential signaling molecules.
EFA action
In the CNS, key parent PUFAs are available at high concentrations, the P450s that generate EFAs, and the sEH that degrades them are selectively expressed in the glial and neuronal cells. This demonstrates the capacity for de novo synthesis, action on neuronal cells and rapid degradation via sEH [24, 25, 32, 33]. In parallel to genetic knockout in mice, chemical inhibition of sEH in rodents using potent urea inhibitors (sEHIs) stabilizes the EETs and other EFAs in vivo leading to significant increases in their plasma and tissue levels and often decreases the corresponding diols. Extensive use of sEHIs in the past 5 years has allowed observation of numerous biological functions of EFAs, most prominently their anti-nociceptive effects [34]. Interestingly we recently found that neuronal sEH expression shows a bias towards axonal regions as opposed to dendrites [35]. If the presynaptic degradation of EFAs is more efficient than post synaptic degradation this may indicate a more prominent postsynaptic function.
EFAs modulate pain signaling
The contrasting pro-inflammatory and pro-nociceptive roles of bioactive lipid mediators are well established [36, 37]. The COX branch of the AA cascade leads to prostanoids which generate pain directly in addition to sensitizing nociceptors [38]. The LOX branch leads to leukotrienes which are thought mostly to sensitize nociceptors via transient receptor potential vanilloid type I channels (TRPV1) [39]. The anti-inflammatory and anti-nociceptive effects of the EETs, products of the cytochrome P450, however were unexpected because the AA cascade is overall viewed as a major inflammatory pathway which increases nociception.
In contrast to other lipid signaling molecules such as prostaglandins or endocannabinoids, a receptor for EETs has not been identified [40]. Cyclooxygenase generated metabolites of AA such as PGE2 and thromboxane bind to a family of G protein coupled receptors (GPCR) including E and D prostanoid receptors 1–4 and the thromboxane receptor [41, 42]. The endocannabinoids which have similarities with eicosanoids as lipid signal messengers affecting nociception also act on identified GPCR receptors named cannabinoid receptors with subtypes 1 and 2 [43]. Identification of a specific receptor for EETs will certainly be a major step towards understanding the biological roles of these molecules. Currently with the use of recently synthesized EET mimetics [44, 45] and antagonists [46] the search for an epoxyeicosatrienoic acid receptor is underway. However given the diversity of the effects of EETs reported so far it is possible that a number of receptors exist.
In addition to the suspected GPCR, there are several additional proposed mechanisms for the bioactivity of EETs in the nervous system [40]. EETs activate TRPV4 channels [47] stimulating rapid calcium release events (calcium sparks) that activate potassium channels and the hyperpolarization of cells [47–49]. In smooth muscle cells, specific regioisomers of EETs also activate ATP-sensitive potassium channels in both a protein kinase A (PKA) and G protein related manner [50, 51]. It should be noted that the many studies providing this mechanistic evidence have been conducted in endothelial and smooth muscle cells of vascular tissues. Spector has made a strong argument that there likely are multiple GPCRs modulated by EFAs but also other regulatory systems including ion channels are regulated by these molecules (Personal communication). The effect of EETs in neural tissues is comparatively unexplored but is expected to share these mechanisms.
Anti-inflammatory effects of inhibition of sEH in animal models
Studies originally conducted by Nakashima et al. in the mid 1990’s suggested a role for EFAs in pyresis and the regulation of body temperature [52]. This led to further work supporting the anti-inflammatory effect of the 11,12-EET regioisomer and demonstration of its ability to inhibit the activation of the NFκB pathway [53]. Some subsequent workers have shown clear evidence of EFAs influencing nuclear translocation NFκB [21].
With the availability of potent sEHIs the first in vivo tests of stabilizing EETs proved to be strongly anti-inflammatory in a murine model of sepsis elicited by a high dose of LPS [54]. These initial experiments indicated a prominent mechanism of sEHI mediated anti-inflammation was a sharp reduction of levels of prostanoids, importantly PGE2. As a result we asked if inhibition of sEH would decrease inflammatory pain [55]. Consistent with the LPS induced sepsis model we used an LPS induced inflammatory pain model, which is more reflective of an infection mediated pain. Intraplantar administration of LPS to the hind paw elicits both allodynia, sensitivity to innocuous stimulation, and hyperalgesia, enhanced sensitivity to normally noxious stimuli [56]. These changes in responses are thought to be mediated by sensitization of nociceptive neurons to stimuli by a number of pro-inflammatory mediators including eicosanoids. A number of structurally different sEHIs attenuate both allodynia and thermal hyperalgesia under these conditions and the anti-nociceptive effects are highly correlated with increases in EFAs brought about by sEH inhibition [34]. These effects are dose dependent and are comparable to the effects of a moderate dose of morphine (1 mg/kg), though some sEHIs have long half-lives and are effective for a much longer period [22].
In parallel to these initial results the sEHIs similarly reduce pain-related behavior in carrageenan induced inflammatory pain, even when administered hours after intraplantar carrageenan [57]. The efficacy of sEHIs on mechanical and thermal withdrawal behavior seems to vary between models and route of administration. Whereas in the LPS model the thermal latencies are greatly improved, in the carrageenan model the allodynia is more efficiently reduced by the sEH inhibitors. Nevertheless the anti-nociception produced by sEHIs is consistently independent of the administration route whether topical, subcutaneous, or intrathecal. Systemic and even topical administration of most sEHIs so far has resulted in high enough levels in the CNS to effectively inhibit sEH. Moreover, in the carrageenan model anti-nociceptive effects with direct spinal injections of very low doses of sEHIs suggest that at least a portion of the anti-nociceptive effects are centrally mediated [22]. In support of this hypothesis of central action we demonstrated that inhibition of sEH significantly suppressed the up-regulation of spinal COX-2 mRNA. This effect is of course expected from an anti-inflammatory agent that penetrates into the CNS and prevents nuclear translocation of NFκB. Thus suppression of COX-2 expression contributes significantly to the anti-nociceptive properties of sEHIs, at least in models of inflammatory pain.
New dual sEH/COX-2 inhibitor effectively reduces inflammatory pain
Stabilization of the cytochrome P450 products by sEHIs during inflammation has unforeseen effects on the AA cascade. The sEHIs not only transcriptionally suppress COX-2 expression in the periphery and the CNS but their use also leads to significant changes in the levels of LOX produced lipid mediators [58]. These changes do not seem to be dominated by laws of mass action where one would expect to see only increased flow of substrate towards other branches when one branch is blocked. Thus a complex cross-talk among the three branches of the AA cascade exists as well as crosstalk among chemokine, cytokine, and other systems. To take advantage of the transcriptional down-regulation of the COX isozymes by sEHIs we employed a two pronged approach. On one hand we tested the effects of co-administration of a sEH inhibitor with the selective COX-2 inhibitor celecoxib and on the other hand we designed and tested a series of dual sEH/COX-2 inhibitors. The resulting dual sEH/COX-2 inhibitor is similarly potent compared to rofecoxib although less potent than celecoxib and has low nM inhibitory potency on recombinant sEH enzyme in vitro [59]. As expected from earlier work, co-administration of a low dose of sEH inhibitor with a low dose of celecoxib was highly effective in reducing pain-related behavior in a rat model of inflammatory pain (Figure 2) [59]. This is in agreement with results from the LPS induced mouse model of sepsis where co-administration of a sEHI with various COX inhibitors was synergistically anti-inflammatory [55]. However we were surprised to find that the dual sEH/COX-2 inhibitor was not only very effective but far exceeded the efficacy of either inhibitor alone or the independent larger dose co-administration of sEH inhibitor with celecoxib. A designed multiple ligand (DML) inhibitor has several advantages compared to combined therapies. These include synergistically increased bioactivity, an increased margin of safety, predictability and straightforwardness of pharmacokinetics and pharmacodynamics and ease of formulation. In the case of the sEH/COX-2 inhibitor additional advantages include reduction of side effects of COX inhibitors such as disturbance of prostacyclin to thromboxane ratio and improving safety of COX inhibitors by reducing the required dose for effectiveness. Thus these dual inhibitors may replace COX-2 selective compounds for the treatment of inflammatory conditions.
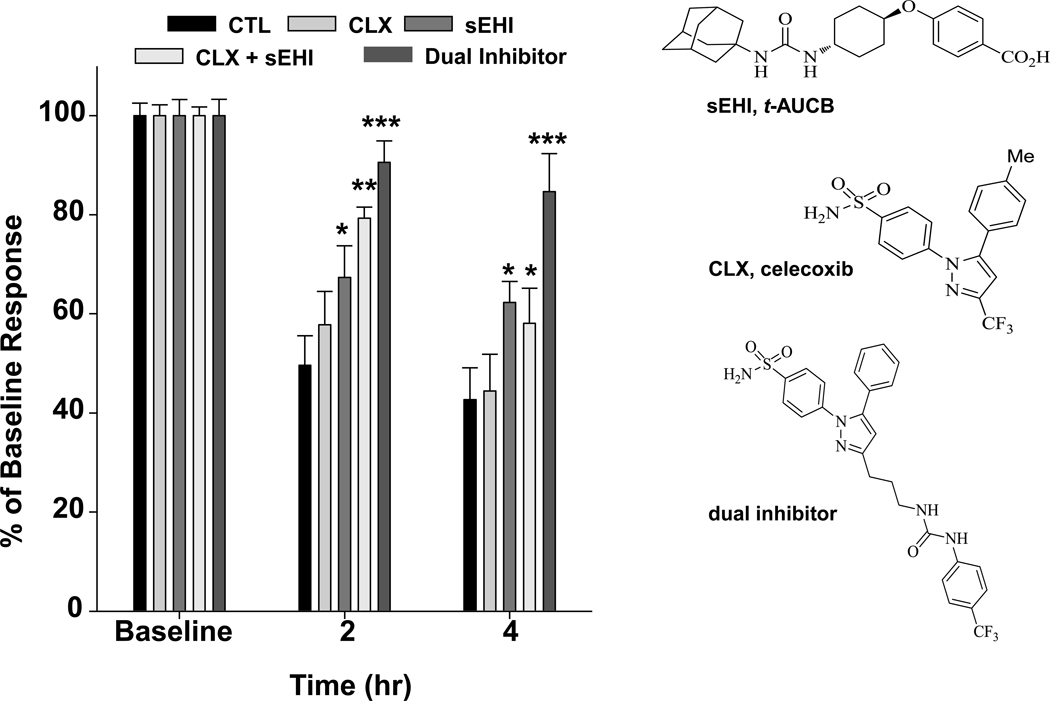
Figure 2. A dual sEH/COX-2 inhibitor efficiently blocks pain.
A potent dual sEH/COX-2 inhibitor significantly alleviates intraplantar LPS induced allodynia measured by a von Frey mechanical nociception test (n=6 per group). The sEHI and co-administration of celecoxib and sEHI also attenuate the allodynia but not as effectively. Doses of celecoxib (CLX), sEHI (t-AUCB), or the dual inhibitor (10 mg/kg each s.c.) were compared to a combination of CLX + t-AUCB and an LPS control. The sEHI and combination treatment were significantly anti-allodynic when compared to the LPS control (P=0.02 and P<0.001 respectively). However the sEH/COX-2 dual inhibitor significantly (P<0.001) attenuated allodynia at both time points and was more effective than celecoxib or sEHI alone and the combination. Results are presented as percent of baseline mechanical withdrawal threshold on the y-axis.
EETs, EpDPEs and EpETEs reduce pain related behavior
Structurally different sEHIs inhibit sEH and stabilize EFAs, and this is strongly anti-hyperalgesic and anti-allodynic. However it was not entirely clear if these effects were mediated by a non-specific interaction of the sEHIs scaffold with another target or by the EFAs themselves. Therefore we asked if the direct administration of EFAs is anti-hyperalgesic in reducing nociceptive behavior. In the LPS induced inflammatory pain model methyl esters of EETs administered topically to the site of inflammation display significant anti-hyperalgesic effects [34]. Similarly, in the carrageenan model, EETs and other EFAs reduce pain related behavior following local intraplantar injection. These effects occur at doses as low as 300ng per administration. Moreover individual regioisomers of DHA epoxides display differing anti-allodynic effects indicating the selectivity of the bioactive lipids. The differences in the ability of regioisomers to elicit anti-nociception may be related to their non-uniform presence in tissues or their ability to engage a receptor molecule [28]. However, these effects have a rapid onset beginning within 15 minutes following administration when first measurements were taken. Because sEH was not inhibited in these studies, the EFAs had a short duration of activity (<2 hours). Interestingly, while the direct administration of EFAs significantly reduced pain in these experiments the parent PUFAs at the same doses are devoid of effect. The PUFAs had to be administered at minimally 100 times higher doses to attain similar levels of pain reduction. Both the PUFAs and the EFAs had no effect on nociceptive thresholds of normal rats while they were highly efficacious in the induced pain model [29].
In addition to the peripheral anti-nociceptive effects of EFAs these molecules show central activity. Our experiments on direct spinal administration of EpDPEs to animals using the carrageenan model resulted in highly significant reduction of pain related behavior [29]. Here a dose of 3000ng of spinal EpDPEs completely reversed carrageenan induced thermal hyperalgesia while significantly reducing allodynia. These results of EFA administration are strikingly similar to our earlier studies with spinally administered sEHI where thermal hyperalgesia is reduced more effectively than mechanical allodynia. In parallel to earlier observations spinal EpDPEs had no effect on changing the thermal withdrawal latency or mechanical withdrawal thresholds on normal animals in the absence of a painful state. Overall the results indicate that the EFAs, as a class, are anti-nociceptive with both peripheral and central sites of action. These findings strongly suggest that the reduction of pain related behavior with sEHI administration is because of their ability to stabilize and elevate the levels of EFAs. Thus stabilization of the EFAs by inhibiting sEH is a potential therapeutic strategy for the treatment of pain. In fact intravenous or spinal infusion of natural EFAs with or without sEHIs could be an effective therapy for intractable and debilitating pain.
Anti-nociception beyond anti-inflammation
A potential impediment to understanding the direct anti-nociceptive roles of EFAs may in fact be their strong anti-inflammatory effects. Early on we observed a lack of correlation between the anti-hyperalgesic effects and suppression of COX-2 expression [22]. This indicated an inflammation independent mechanism. Currently, convergent lines of evidence indicate the anti-nociceptive roles of sEHIs are separate from their anti-inflammatory effects. Firstly, the sEHIs are highly effective in a model of neuropathic pain that is largely independent of COX-2 induction [22]. Allodynia elicited by streptozocin induced type I diabetes is effectively blocked by structurally different sEHIs. Preliminary assessment of this activity indicates that the anti-allodynic effects are independent from modulation of glucose homeostasis in these diabetic animals, within the time frame that pain is reduced (i.e., within minutes to hours following sEHI administration). Secondly, we demonstrated direct anti-nociceptive effects of sEHIs using a pain model involving direct intraplantar administration of PGE2 [60]. Given that PGE2 is a product of both COX isozymes, the non-selective inhibitor indomethacin displays no efficacy on this type of pain [61].Consistent with these earlier findings celecoxib and dexamethasone both of which are strongly anti-inflammatory do not affect allodynia elicited by intraplantar PGE2 [60]. Surprisingly structurally different sEHIs do block pain produced by PGE2 differentiating sEHIs from NSAIDs, selective COX inhibitors and steroids as a separate class of analgesic agents. Thus EFAs do not modulate transmission of nociceptive information under physiological conditions but their effects are switched on under pathophysiological conditions whether the pathology is inflammatory or neuropathic. Such observations indicate that EFAs influence a more fundamental aspect of pain transmission. The sEHIs are also different from narcotic agents which elevate acute pain thresholds of naïve rodents. Overall the sEHIs have a distinct profile of anti-nociceptive effects from known agents that reduce pain suggesting that they may be useful in the treatment of pain.
Pain dependent effects of sEHIs are modulated by pro-nociceptive cAMP
Importantly, all sEHIs tested to date lack a clear effect on acute pain thresholds of rodents in the absence of an induced pain state whether measured by responses to thermal or mechanical stimuli [22, 34, 55]. An unexpected property of sEHIs mediated reduction of pain emerged when injection of sequentially lower quantities of PGE2 resulted in a gradual decrease in the efficacy of a constant dose of a sEHI. Inhibitors of sEH, similar to NMDA antagonists, are effective in an activity dependent manner, requiring a baseline painful status to become anti-hyperalgesic. At high doses sEHIs induce hypoalgesia (i.e. elevation of responses above baseline measures). Based on these observations we hypothesized the presence of a pain state generated co-factor is required for the analgesic effects of sEHIs. Because PGE2 leads to the production of cAMP and activation of PKA which is a prominent molecular mechanism of inflammatory pain, we asked if cAMP would modulate the activity of sEHIs. In these experiments normal animals that did not have an underlying painful state were tested. Remarkably prevention of the degradation of cAMP by a potent phosphodiesterase inhibitor, rolipram, allowed the sEHI to become analgesic in normal animals while the sEHIs on their own do not change nociceptive thresholds in normal animals [60]. Thus, simulation of a painful state by elevating cAMP which occurs upon the algogen PGE2 binding the prostaglandin (EP) receptors [62] activated the ability of EFAs to suppress pain related behavior.
Nevertheless, the interactions between cAMP and EFAs are multifaceted. The functional effects of EETs acting through a cAMP pathway are extensively studied in the vasculature [40]. EETs are endothelium-derived hyperpolarizing factors (EDHF) in smooth muscle cells where the activity is mediated via Gαs stimulation, adenylyl cyclase (AC) activation, and subsequent increased cAMP [63]. The elevated cAMP results in activated BKCA channels which induce relaxation of coronary arteries. Similarly, in renal vasculature an EET mediated Gαs ADP ribosylation leads to AC activation, cAMP increases and by activating PKA prompts the dilation of microvessels [64]. Furthermore, EETs also mediate TRP channel translocation to plasma membranes [49]. It is unclear if these findings in the vasculature directly apply to neurons.
Another level of complexity between the interaction of cAMP and EFAs is our recent observation that phosphodiesterase inhibitors (PDEIs) as a class can modulate the levels of EFAs as dramatically as sEHIs. Various PDEIs increase the plasma levels of EFAs along with elevating cAMP levels [60]. There seems to be a great deal of selectivity in this regard since PDE4 and PDE5 inhibitors have the most remarkable effects. These effects interestingly are regioisomer selective and specific for EFAs of LA, AA and DHA but not of EPA origin. Though PDEIs elevate the plasma levels of EFAs, unlike the sEHIs they do not seem to influence the levels of dihydroxy-fatty acid degradation products. Inhibitors of sEH not only elevate EFAs but they quite efficiently decrease the levels of their degradation products. Lipolysis that is activated by high concentrations of cAMP seems to be responsible for the elevation of EFAs by PDEIs although this hypothesis needs to be further evaluated.
Given that EETs elevate cAMP [57], and that stabilization of cAMP elevates EETs we asked if the analgesic activity of rolipram, a PDE4 inhibitor, can be differentiated from that of a sEHI+ rolipram combination. Based on our previous results suggesting the involvement of neurosteroids in the mechanism of action of sEHIs [22] we used picrotoxin, an antagonist of neurosteroid action. Picrotoxin strongly antagonized the effects of sEHI+ PDEI combination but not the effects of the PDEI4 rolipram. Picrotoxin at this dose was ineffective by itself in producing changes in nociceptive thresholds. Remarkably, picrotoxin had different effects on thermal versus mechanical withdrawal responses produced by the sEHI+ PDEI combination. These findings suggest the involvement of the GABA system in the analgesic effects of the EFAs and sEHIs.
Analgesic effects of EFAs in non-inflammatory models of pain
In support of our work on inflammatory models of pain and analgesic effects of EFAs in non-inflammatory models, recent data from Terashvili et al. corroborate direct anti-nociceptive roles of EFAs, specifically EETs [65]. These studies demonstrated that site specific administration of EETs into the ventrolateral periaqueductal gray of the brain led to elevation of the thermal tail flick latency in rats and that these anti-nociceptive effects were highly correlated with an increase in the release of endogenous opioid peptides. The analgesia produced by EETs was antagonized by both β-endorphin and met-enkephalin antisera. In accordance with these results more recent work from Conroy et al. suggests that the cytochrome P450 products directly contribute to the action of opioid agonists [66]. These authors elegantly demonstrated that both inhibition of cytochrome P450s or genetic knock out of cytochrome reductase in the brain, which essentially reduces all cytochrome P450 activity, interfere with bulbospinal analgesia mediated by opioid agonists [66, 67]. The mechanism of action of these findings is still under investigation but is hypothesized to take place via P450 inhibition modulating a pathway that relates presynaptic opioid receptor activation to GABA activity [68]. The EETs do not bind to opioid receptors, though they seem to contribute to opioid mediated analgesia [65, 66]. Thus in addition to the crosstalk mediated by the EFAs that occurs between the different branches within the arachidonic acid cascade there seems to be a crosstalk among multiple analgesic pathways, with EFAs contributing to the descending analgesic pathway.
At least part of this crosstalk between the analgesic pathways may be mediated by the overlap in the structural features of endocannabinoids and EFAs (Figure 3). There are two cannabinoid receptors, CB1 expressed in the brain and CB2 which is more prevalent in the immune system and in microglial cells of the CNS although they were historically viewed as absent from this tissue [69]. The two major ligands of the cannabinoid receptors are arachidonylethanolamine (AEA known as anandamide) and 2-arachidonylglycerol (2-AG). These are an amide and an ester of arachidonic acid. The endocannabinoids are rapidly hydrolyzed by the actions of the fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) [70]. Early on endocannabinoid epoxidation was suggested to be an activation pathway leading to epoxide metabolites with greater stability than the parent amides or to the formation of unique oxygenated metabolites with potent signaling capacities [71]. More importantly, epoxygenated endocannabinoids have recently been identified and their potential biological roles characterized [72, 73]. Epoxyeicosatrienoic acid ethanolamides (EET-AE) are endogenously produced by select isozymes of P450 in the brain [74]. The 5,6-EET-EA is one of four regioisomer EET-EA formed by these P450 enzymes [74]. 5,6-EET-EA is a potent agonist of CB2 receptors exceeding the binding affinity of the parent anandamide by 1000 fold and with greater stability [72]. While the metabolism of these fatty amides has not been completely described there is evidence of epoxide hydrolase metabolism of 5,6-EET-EA [72]. A different regioisomer 14,15-EET-EA has been found to bind CB1 receptors in rat brain albeit more weakly that anandamide. Interestingly the EETs themselves can directly act on the CB2 receptor [57]. We found that the regioisomeric mixture of EETs can displace CB2 agonist WIN 55212-2 but not the CB1 agonist CP 55940 in radioligand displacement assays. The activity is mostly mediated by the 5,6EET regioisomer, in accordance with results from Snider et al. demonstrating the potency of 5,6-EET-EA [72]. Though the affinity of 19µM for CB2 is weak we tested if either a CB1 or CB2 antagonist would block sEHI mediated anti-hyperalgesia. Predictably, the CB2 but not CB1 antagonist blocked the sEHI anti-hyperalgesia (Figure 4).
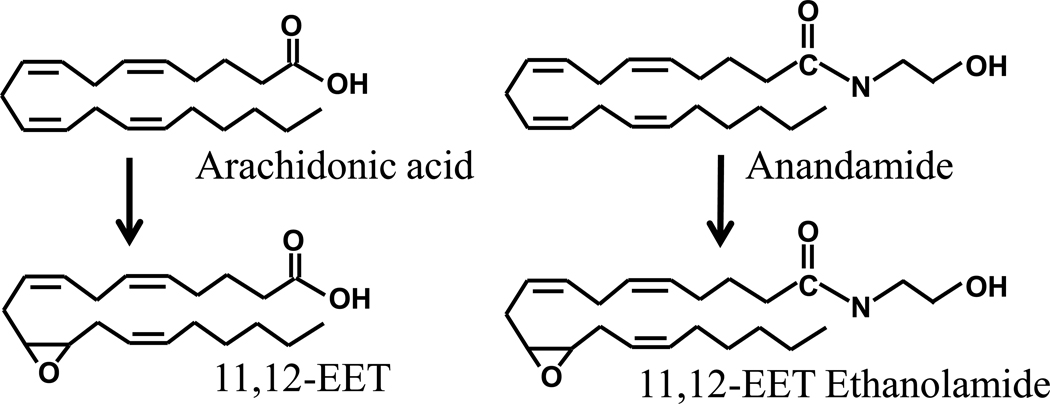
Figure 3. EFAs and epoxyeicosatrienoic acid ethanolamides (EET-EAs) share structural features.
Arachidonic acid is enzymatically converted to four regioisomers of epoxyeicosatrienoic acids (EETs) by cytochrome P450s. Here one regioisomer 11,12-EET is depicted. Arachidonic acid is a precursor in the synthesis of endogenous cannabinoids anandamide and 2-arachidonyl glycerol (not shown). Once synthesized from N-arachidonoyl phosphatidylethanolamine (NAPE) anandamide can be epoxygenated by cytochrome P450s to form regioisomers of EET-EAs which have similar functional properties with anandamide. Anandamide or 2-arachidonyl glycerol upon degradation by FAAH or MAGL releases arachidonic acid as a product which can act as substrate for cytochrome P450s to be converted to EETs.
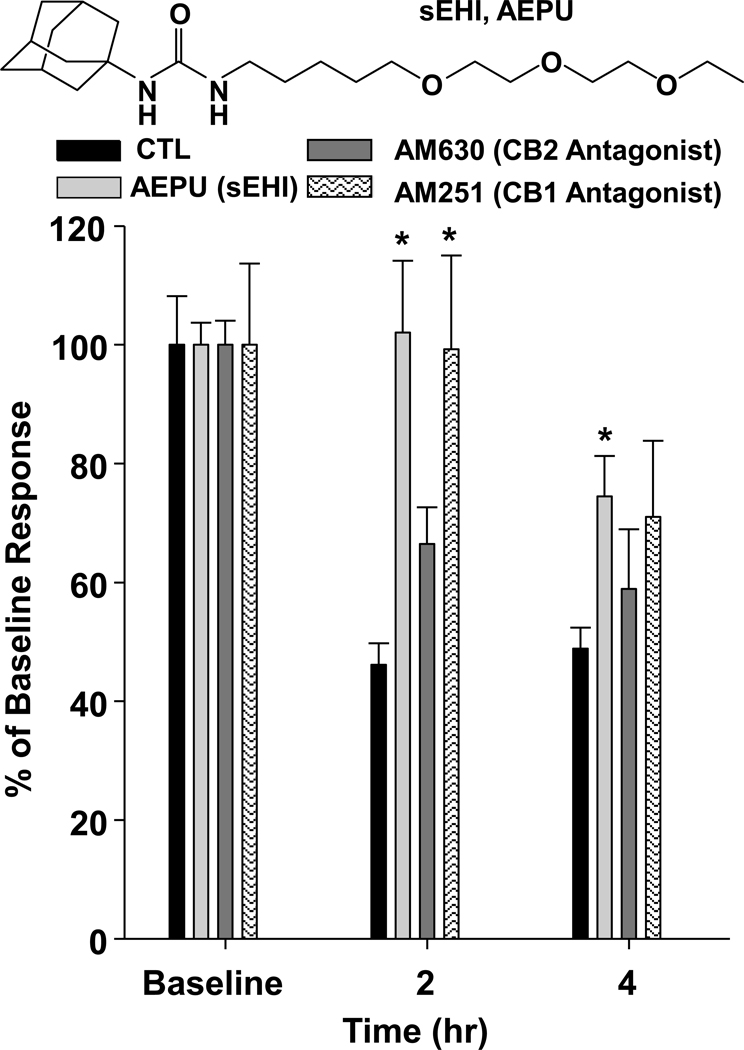
Figure 4. Cannabinoid receptors play a role in sEHI mediated anti-nociception.
The soluble epoxide hydrolase inhibitor AEPU administered topically significantly attenuates thermal hyperalgesia when compared to control (CTL) LPS treated rats. Male Sprague Dawley rats (n=4 per group) were administered intraplantar 10ug/paw LPS in one hind paw followed by 10mg/rat AEPU and monitored using the modified Hargreaves’ thermal hyperalgesia test. This anti-hyperalgesic effect of the sEHI AEPU is blocked by a 2mg/rat topical administration of the selective CB2 receptor antagonist AM 630. However, the CB1 selective antagonist AM 251 displays no effect on sEHI mediated anti-hyperalgesia. Results are presented as percent of baseline thermal withdrawal latency on the y-axis.
The CB2 receptor agonists mediate analgesia without CNS side effects observed for CB1 agonists [75]. EFAs share structural similarities with agonists of CB receptors that induce anti-nociception. In addition the activity profiles of sEHI administration and EFAs have several similarities with CB2 agonists. Inhibitors of sEH do not have activity in the absence of pain, they are not motor impairing and they do not affect open field activity. They are both effective in inflammatory and neuropathic pain models. Cannabinoid and EFA anti-nociceptive systems both have cAMP mediated mechanisms. Synthesis of the endocannabinoid precursor N-acylphosphatidylethanolamine (NAPE) is driven by cAMP in neurons [76] and there is evidence of cAMP synergizing sEHI anti-nociception [60]. Overall there seems to be a great deal of similarity in the ways that EFAs and endocannabinoids affect the nociceptive system.
Conclusion
Taken as a whole, EFAs whether exogenously applied or increased endogenously attenuate pain related behavior. The past decade has seen a number of rapid developments in this area including the availability of orally active potent small molecule inhibitors of the sEH. EFAs are tightly regulated via reincorporation in to membranes and enzymatic degradation. In animal models they clearly attenuate inflammation and inflammatory pain but also pain not dependent on inflammation. This anti-nociception mediated by EFAs is dissimilar to that produced by most other class of therapeutics. The EFAs do not alter motor skills like analgesics and cannabinoids. They are active in inflammatory models but also in diabetic neuropathic pain and importantly other types of pain such as that produced by PGE2 that is recalcitrant to NSAIDs and steroids. The side effects profile of sEHIs is unknown, but based on data from rodent pain studies, the sEHIs seem to be highly effective. The sEHIs reduce pro-inflammatory mediators through several different mechanisms but do so without inhibiting COX or LOX enzymes and thus maintain homeostasis. While targeting the sEH in the AA cascade has great potential for treating neuropathic pain, exploring the crosstalk between the branches of the cascade and the synergistic response it offers in pain relief may yield still more novel therapeutics. It will be interesting to describe the activity of EFAs in modulating nociception mediated through the opioid and cannabinoid analgesic systems. This crosstalk between analgesic systems offers even more opportunities to improve pain therapies by potentially lowering side effects of current agents with combination therapy. Reducing pain is still an unmet need in numerous disease states and novel alternatives to current therapies with more preferable side effect profiles are necessary. The sEH inhibition mediated anti-nociception, though yet to be tested in man, offers a unique path towards this end.
Highlights.
Epoxygenated fatty acids (EFAs), endogenous signaling molecules, mediate nociception. > EFAs are tightly regulated primarily by the soluble epoxide hydrolase (sEH) enzyme. > sEH inhibitors and EFAs are anti-nociceptive in inflammatory and neuropathic pain models. > EFAs mediate crosstalk among branches in the AA cascade and among analgesic systems.
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710, NIEHS Superfund Basic Research Program P42 ES004699, and NIEHS Training Grant T32ES007059 (to K.W.). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murakami M. Lipid mediators in life science. Exp Anim. 2011;60(1):7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- 2.Chalon S, et al. Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission. Lipids. 2001;36(9):937–944. doi: 10.1007/s11745-001-0804-7. [DOI] [PubMed] [Google Scholar]
- 3.Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48(3):195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 4.Wassall SR, et al. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem Phys Lipids. 2004;132(1):79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–6):251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89(3–4):112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Mayurasakorn K, et al. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr Opin Clin Nutr Metab Care. 2011;14(2):158–167. doi: 10.1097/MCO.0b013e328342cba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson GE, Lajtha A, Dienel GA. Springer reference. 3rd ed. New York: Springer; 2007. Handbook of neurochemistry and molecular neurobiology. Brain energetics : integration of molecular and cellular processes; p. 924. xv. [Google Scholar]
- 9.Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004;7(2):137–144. doi: 10.1097/00075197-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 11.Bernstrom K, et al. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267(6):3686–3690. [PubMed] [Google Scholar]
- 12.Tomita-Yamaguchi M, et al. Incorporation, distribution, and turnover of arachidonic acid within membrane phospholipids of B220+ T cells from autoimmune-prone MRL-lpr/lpr mice. J Exp Med. 1990;171(3):787–800. doi: 10.1084/jem.171.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55(1):1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 15.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814(1):210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Fer M, et al. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471(2):116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 18.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50 Suppl:S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLennan P, et al. The cardiovascular protective role of docosahexaenoic acid. Eur J Pharmacol. 1996;300(1–2):83–89. doi: 10.1016/0014-2999(95)00861-6. [DOI] [PubMed] [Google Scholar]
- 21.Chiamvimonvat N, et al. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 22.Inceoglu B, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105(48):18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marowsky A, et al. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163(2):646–661. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Bianco RA, et al. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res. 2009;1291:60–72. doi: 10.1016/j.brainres.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enayetallah AE, et al. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52(4):447–454. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- 26.Chacos N, et al. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223(2):639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 27.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44(1):1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Luria A, et al. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282(5):2891–2898. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morisseau C, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam MF, et al. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3(5):562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buczynski MW, et al. Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58(2):503–510. doi: 10.1111/j.1471-4159.1992.tb09749.x. [DOI] [PubMed] [Google Scholar]
- 34.Inceoglu B, et al. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdu E, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira SH, Lorenzetti BB, Correa FM. Central and peripheral antialgesic action of aspirin-like drugs. Eur J Pharmacol. 1978;53(1):39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- 37.Levine JD, et al. Hyperalgesic properties of 15-lipoxygenase products of arachidonic acid. Proc Natl Acad Sci U S A. 1986;83(14):5331–5334. doi: 10.1073/pnas.83.14.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KA, Vasko MR. Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol Sci. 2005;26(11):571–577. doi: 10.1016/j.tips.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Bang S, et al. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res. 2010;33(10):1509–1520. doi: 10.1007/s12272-010-1004-9. [DOI] [PubMed] [Google Scholar]
- 40.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 42.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 43.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 44.Yang W, et al. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324(3):1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 45.Falck JR, et al. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol. 2003;284(1):H337–H349. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 46.Gauthier KM, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90(9):1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 48.Earley S, et al. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 49.Fleming I, et al. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(12):2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 50.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2005;288(1):H358–H364. doi: 10.1152/ajpheart.00423.2004. [DOI] [PubMed] [Google Scholar]
- 51.Ye D, et al. Mechanism of rat mesenteric arterial KATP channel activation by 14,15-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2006;290(4):H1326–H1336. doi: 10.1152/ajpheart.00318.2005. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima T, et al. Inhibitors of cytochrome P-450 augment fever induced by interleukin-1 beta. Am J Physiol. 1996;271(5 Pt 2):R1274–R1279. doi: 10.1152/ajpregu.1996.271.5.R1274. [DOI] [PubMed] [Google Scholar]
- 53.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmelzer KR, et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102(28):9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmelzer KR, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(37):13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanaan SA, et al. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996;66(2–3):373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- 57.Inceoglu B, et al. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82(1–4):42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu JY, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79(6):880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang SH, et al. Synthesis and Structure-Activity Relationship Studies of Urea-Containing Pyrazoles as Dual Inhibitors of Cyclooxygenase-2 and Soluble Epoxide Hydrolase. J Med Chem. 2011 doi: 10.1021/jm2001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inceoglu B, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khasar SG, et al. Comparison of prostaglandin E1- and prostaglandin E2-induced hyperalgesia in the rat. Neuroscience. 1994;62(2):345–350. doi: 10.1016/0306-4522(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 62.Zeilhofer HU. Prostanoids in nociception and pain. Biochem Pharmacol. 2007;73(2):165–174. doi: 10.1016/j.bcp.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 63.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49(3):590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 64.Carroll MA, et al. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291(1):F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 65.Terashvili M, et al. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther. 2008;326(2):614–622. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conroy JL, et al. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci. 2010;13(3):284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hough LB, et al. Brain P450 epoxygenase activity is required for the antinociceptive effects of improgan, a nonopioid analgesic. Pain. 2011 doi: 10.1016/j.pain.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinricher MM, et al. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol. 2010;104(6):3222–3230. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 70.Piomelli D, et al. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21(6):218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 71.Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2–3):211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- 72.Snider NT, et al. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol. 2009;75(4):965–972. doi: 10.1124/mol.108.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sridar C, Snider NT, Hollenberg PF. Anandamide oxidation by wild type and polymorphically expressed CYP2B6 and CYP2D6. Drug Metab Dispos. 2011 doi: 10.1124/dmd.110.036707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snider NT, et al. The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther. 2008;327(2):538–545. doi: 10.1124/jpet.108.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malan TP, Jr, et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3(1):62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 76.Cadas H, et al. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16(12):3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]